Research
, Volume: 19( 1) DOI: 10.37532/0974-7516.2025.19(1).003The Effect of Compressed Air Foam on the Detection of Ignitable Liquid Residues on Fire Debris Samples
- *Correspondence:
- Victor Omondi* Department of Forensic Sciences and Biochemistry, Kenya Medical Training College, Anglia Ruskin University, Nairobi County, Kenya E-mail: vik362002@gmail.com
Received: August 22, 2023, Manuscript No. TSOC-23-110947; Editor assigned: August 25, 2023, PreQC No. TSOC-23-101491 (PQ); Reviewed: September 11, 2023, QC No. TSOC-23-101491; Revised: January 09, 2025, Manuscript No. TSOC-23-101491 (R); Published: January 16, 2025, DOI:10.37532/0974-7516.2025.19(1).003
Citation:Omondi V. The Effect of Compressed Air Foam on the Detection of Ignitable Liquid Residues on Fire Debris Samples. Org Chem Ind J. 2025;19(1):003
Abstract
Compressed air foam is a substance that is used as an extinguisher delivery system for fire suppression in various fire scene case scenarios. It’s due to its high fire extinguishing efficiency, use of less water hence suitable for areas with no water such as rural areas and its range of large fire suppression has made it receive considerable attention. Therefore, this technology is widely being accepted as an alternative source to water as a fire extinguishing agent which is commonly used because of its surface tension properties making it more efficient. Although both water and compressed air foam are being used, have certain advantages and also inherent limitations that should be considered. Unfortunately, there is one study that has been done to investigate whether the introduction of foam to the seat of the fire created any problems in subsequent analyses of fire debris samples using gas chromatography-flame ionization detector. No significant interferences were found from the foam when the samples were analyzed suing activated carbon strips. The only foam component found was limonene. To date there has been no research published as to whether the foam causes any interference on subsequent analyses of accelerant analyses making this study very unique. This study main objective is to prove that no interference is brought about by the introduction of foam during fire suppression and in the analysis of ignitable liquid residues from fire debris sample. This was achieved through the use of gas chromatography-mass spectrometry which is capable of carrying out extracted ion analysis hence able to prove that no significant interferences from the foam.
Keywords
Compressed air foam; Water; Fire suppression; Gas chromatography-mass spectrometry; Extracted ion analysis
Introduction
Arsonists in the U.K are accountable for 2100 fires every week, which results into two fatalities including 55 other casualties and a cost of £40 million [1]. According to Bertsch defines the crime of arson as being the willful and malicious destruction of a building or other property through burning, and yet being one of the hardest to investigate it is considered also as one of the easiest crimes to commit. In the year June 30, 2003, through government initiatives, the number of deliberate fires declined for the first time by 3% to 107,400, however, the number of deliberate fires has steadily increased over the last decade given by Arson. Under the Criminal Damages Act 1971 Section 1, and the law for this to be considered a crime, hence for a fire to be recorded as arson, it must encompass two intrinsic elements. Destruction of property must be must be proven beyond reasonable doubt thus surface burning or discoloration through heat and that it was carried out through recklessness or on purpose/by intent as stated in Arson [2].
As opposed to other criminal acts, the evidence is mostly destroyed rather than created as the crime progresses. There may well be a legitimate reason for the presence of ignitable liquids for at a scene hence not positive proof of arson, also not all properties are deliberately set. Either as an ingredient in certain products like insecticides, or as a fuel in lamps or heaters, kerosene commonly occurs in domestic environments. Lentini describes the term petroleum derivatives, as often seen on goods labels, is most likely referring to kerosene or methylated spirits [3].
According to the statistics obtained from U.S Fire Administration, the leading cause of fire and second leading cause of deaths and injuries is arson which results in lost lives of lives and property. As a result, for the federal government, the criminal justice system, and the fire service arson investigation is of forensic significance. Criminals may use a variety of ignitable liquids to start a fire given by Keto. Consisting of hundreds of compounds these are available fuels or solvents that are typically commercially available. Tan, Hardy and Snavely, suggested that because ignitable liquids may comprise same or similar components but with different distributions identification is difficult. Further identification complication arises through combustion because volatile compounds may be evaporated from the liquid and additional pyrolysis products may be produced that can alter the composition of the residual ignitable liquids detected in fire debris as given by Wallace (Figure 1).
FIG. 1. Fire scene attended by Cambridge shire fire and rescue service.
Literature Review
Compressed air foam
In the 1970’s compressed air foam was originally developed in Texas as an innovative approach for fighting grassland fires in areas where water is extremely scarce. The Boston fire department during most of 1992 and in the early part of 1993, participated in a field test of the compressed air foam system explains Routley. In 1987 according to Lattimer et al., suggested after the first version of the Montreal Protocol was introduced, it became a beacon of international commitment to protect the earth’s ozone layer from further damage by Chlorinated Fluorocarbons (CFCs). To replace the chlorine or bromine based gaseous fire suppressants known as halons, this commitment spurred a decade of developing alternative fire suppression technologies describes Weaire and Phelan. Therefore, Gardiner, Dlugogorski and Jameson, suggest that as a potential method for halons replacement the research on compressed air foam technology is receiving considerable attention due to its high fire extinguishing efficiency and anti-reburn performance, significantly less water damage, capability for large fire suppression, and far distance of agent discharge [4-8].
International studies given by Crampton of the optimization of foam performance and suppression test of various fires have occurred, especially in Australia, Canada, and USA. For instance, Kim et al., gave a comparison between the fire suppression performance of compressed air foam with foam water sprinkler systems for class B hazards. Experimental test of fire protection for files in electrical cabinets, cable trays and housing units in remote areas were also performed as suggested by Kim, Dlugogorski, Schaefer and Kennedy, verified the consistency of effective viscosity and pressure loss data for designing foam proportioning systems. In compressed air foam technology for firefighting, important progress has been made which is used to effectively suppress various types of industrial fires at reduced cost and reduced water requirement [9-12]. However, in China there have been relatively few studies, regarding the development of improved foam agents, experimental tests of their performance and fire suppression mechanisms especially concerning compressed air form technology. Therefore, a newly developed foam agent was tested on wood crib and pool fire extinguishing concerning such conditions as foam concentration, mixing chamber structure and working pressure in order to develop environment friendly capability as suggested by Zhou, Liao and Cai.
Generation of compressed air foam
Recently, for use by the fire services several companies have developed mobile CAF systems. By injecting air under pressure into a foam solution stream CAF is generated of which properly engineered CAF systems produce superior quality foam with high momentum. However, there has not been a study to systematically evaluate the fire suppression effectiveness of the mobile CAF systems until now. The fire suppression effectiveness of the CAF systems in comparison with traditional fire suppression systems, such as hose stream application is necessary, to truly appreciate the superior fire suppression effectiveness of the CAF approach as suggested by Kim and Dlugogorski [13-15].
CAF combines two technologies that include, an agent to reduce the surface tension of water, and an injection system to produce an expanded volume of fire extinguishing agent. To make water much more efficient as an extinguishing agent, is accomplished by introducing a small percentage of foam concentrate into water due to surface tension reduction. Thus, to create a mass of foam bubbles compressed air is then injected into the solution to expand the foam, to provide a much greater volume of extinguishing agent.
The CAF system suppressed the fire and cooled down the fire compartment much quicker than the water alone or foam solution (to 200°C). Also, Kim and Dlugogorski, describe that the total amount of water used to control the test fire was much less with the CAF system than with the water alone or foam solution [16].
In industry and the military firefighting foams are widely used for the suppression of flammable liquid (Class B) fires. at fires Due to their ability to form a film over the fuel surface, the oxygen in the air and preventing contact between the fuel foams containing flourosurfactants are quite efficient. Three methods to generate firefighting foams from the surfactant solutions include the following;
• By drawing air into a stream of surfactant solution inside a specially designed nozzle which produces foam is the traditional method for foam generation. This approach relies on the energy of mixing to be derived from the flowing foam solution.
• Utilizing compressed air to create larger number of evenly sized bubbles within the foam. In this case, air is injected into the flowing foam solution from an air compressor or cylinder involving a chemical reaction to create numerous, uniformly sized bubbles of nitrogen within the foam; rather than bubbles of CO2 of chemical foams widely used in the first half of the 20th century.
• In large scale the method may be easily employed where the inclusion of one additional chemical species dissolved into the foam concentrate by generation systems, which, may be mixed with another chemical species dissolved in the water in the foam generation process as given by Laundess et al.
Using these three generation methods there are a number of differences in the characteristics of foams produced. Predominantly, the distinctions created using each method are in terms of the size and uniformity of the bubbles. In fire suppression and burn back tests such differences can lead to significant variations of foam performance describes [17].
Advantages of compressed air foams
A great deal of literature written by Boston agrees regarding CAFS advantages for structural fire-fighting applications. The Boston fire department in a field report, compressed air foam for structural firefighting, evaluated 146 fire calls where CAFS was used as the extinguishing agent. The report finds as an extinguishing agent CAFS was more effective than water, the hose line was easier to move, fire attack was initiated quicker, concealed spaces were extinguished faster, reduced need for overhaul, and steam “punch” was less than with water. Murdock explains the point regarding limited water resources and CAFS capability to expand this resource. Also, he wrote, “CAF systems do offer a mechanism to increase and enhance the effectiveness of water improving the extinguishing capabilities of limited water resources will prove a major benefit” Murdock [18-20].
In the article, The Finer Points of Foam, Cavette states that, “compressed air foam offers significant advantages in both wild land and structural incidents.” Some of the advantages which Cavette listed included:
• Uses less water.
• Uses less foam concentrate.
• Penetrates fuels more effectively.
• Gives faster knock down.
• Absorbs heat more rapidly to lessen the chance of flashover in structure fires.
• Reduced the potential for rekindle.
• Can be pumped twice as high as water under the same pressure.
• Lowers the risk of heat stress during interior attack.
• Reduces water and smoke damage.
• Aids fire investigation by lessening disruption of the scene by streams.
Many claims have been made against the increased firefighting performance of CAFS and Class A foam. In Report 083 of the U.S. Fire administration's major fires investigation project as described by Stern and Routley, surveyed several fire departments using CAFS. The reported advantages were as follows:
• Class A foam can be produced at a relatively low cost.
• Class A foam forms a protective blanket.
• Foam is visible during and after application.
• Class A foam can be used on flammable liquids fires.
• Foam use may help to preserve evidence of fire cause.
Class A foam may provide long term cost savings and reduced property damage.
Disadvantages of compressed air foam.
In municipal fire services the first application of CAF’s as a fire suppression tool was in the wildland environment. In the early 1970’s Texas Forest Service began using CAF’s as written by Colleti, IFSTA, Taylor. It is gaining acceptance in structural firefighting because of advancements in technology and the efforts of proponents of CAF’s, but not without its disadvantages.
IFSTA gave a report that CAF does have some inherent limitations:
• A hose reaction can be erratic with a CAFS if foam solution is not supplied to the hose line in sufficient quantities.
• A CAFS adds expense to a vehicle and can be a high maintenance item.
• Additional training is required for personnel who are expected to make fire suppression using a CAFS or who will operate CAFS equipment.
• The compressed air accentuates the hose reaction in the event the hose ruptures.
Fire suppression by water
To British Fire Brigades the UK Home Office Fire Research and Development Group (FRDG) have several responsibilities, including the assessment of new fire-fighting techniques, the development of training material and the publication of technical reports. The use of water sprays to suppress and extinguish compartment fires of the type attended by the fire service on a daily basis is one topic of continuing interest to the FRDG. Consequently, a major research initiative entitled a study of the science of fire suppression and extinction by the University of Edinburgh sponsored by the FRDG in order to determine the current state of the art of the subject and to identify any gaps in the current knowledge base.
To date two FRDG technical reports have been published: a brief review of the actual mechanisms of fire suppression by Grants and Drysdale and a more comprehensive analysis of the important role that water plays in fire-fighting practice as written by Grants and Drysdale. The literature review on which the present paper is based has revealed, although research into fire safety science has increased greatly since the Second World War in general, the subject of fire suppression has received relatively little attention suggests Grants and Drysdale. However, over the last few years this trend has been overcame, due in large measure to the interest in water mist as a replacement for Halon gas fixed fire protection systems.
Classification fire types
As shown in Table 1 below compares the standard fire characteristics currently adopted by Britain/Europe BS EN 2, 1992 with those of the US. These fires normally of organic nature involving solid materials compounds of carbon, in which the formation of glowing embers combustion generally occurs. The most common fires are Class A and the most effective extinguishing agent is generally water in the form of a jet or spray. The definition of Class A given above covers those solids which basically form glowing embers; most thermoplastics such as PVC used in building construction do not form glowing embers. However, the situation is more complex as they liquefy before burning, they would seem to fall into the Class B category. Hydrocarbons possess a low ‘fire point’ hence are less dense than water and are not efficiently cooled by water because of the ease with which combustible vapors are released. In contrast, through the application of water thermoplastics can be cooled effectively and generally have fire points in excess of 200?, and in some cases 300?.
| Class of fire | Definition (BS EN2: 1992) | Definition (NFPA 10, 1990) |
|---|---|---|
| A | Solid materials, usually organic (e.g. coal, paper, cardboard etc.) which burn with the formation of glowing embers. | Ordinary combustibles (e.g. wood, cloth, paper, rubber and many plastics) |
| B | Liquids or liquefiable solids (e.g. petroleum products). | Flammable liquids, oils, greases, tars, oil-based paints, lacquers and flammable gases |
| C | Combustible gases. | Fires involving energized electrical equipment where the electrical non-conductivity of the extinguishing medium is of importance. |
| D | Combustible metals such as magnesium, titanium, zirconium, sodium, lithium and potassium. | Combustible metals. |
| Note: The following definition of class ‘A’ fires is taken from the training material of UK fire-fighters (Manual of firemanship, 1974). | ||
TABLE 1. Above compares the standard fire characteristics currently adopted by Britain/Europe (BS EN 2, 1992) with those of the US (NFPA 10, 1990).
Class A fire extinguishment by water
According to Fristrom, the principal action of liquid suppressants, such as water, is the removal of heat from the fire through their heat capacity and latent heat of vaporization. Although water may contribute to fuel dilution in cases of water miscible liquid fuels or fuel ‘blanketing’ through forming a barrier on the fuel surface, in cases of class A fires the most important suppression mechanisms are:
• Cooling the surface, thus reducing the pyrolysis rate and so the rate of fuel supply to the flame zone, hence reducing the heat release rate and the radiative feedback from the fuel surface.
• Cooling the flame zone which is responsible for combustion directly disrupts the chemical reactions. Less thermal energy is available in the vicinity of the reaction zone therefore some portion of the heat reaction is abstracted in heating and evaporating the liquid water.
• Volumetric displacement of the oxidant within the combusting environment through the production of (inert) water vapor is also known as flame smothering.
In addition, by providing a heat sink the pre-wetting of adjacent combustible surfaces which effectively delays ignition may also control fire spread. The ability of water to absorb thermal radiation has also been exploited as an indirect firefighting measure, in order to shield personnel or property as stated by Fristrom.
The potent cooling effect of water is due to its high latent heat of vaporization, as illustrated in Figure 2. Here, to raise the temperature of one litre of water from 0 to 100? 428 kJ of thermal energy is required, whereas to affect the phase change to water vapor without further change in temperature a further 2257 kJ are subsequently required. Moreover, given that evaporation can occur only at the liquid surface, in theory at least, it seems desirable to seek to maximize the surface area per unit volume of firefighting water.
FIG. 2. Thermal energy for heating and phase changes of 1L of water. (source: Reproduced from Water as an extinguishing agent, Herterich 1960).
In practice however, Fristrom, suggests the efficiency of water as a heat sink is usually determined by the application technique, as water that fails to reach the seat of the fire cannot contribute to its ultimate extinguishment. Only a small fraction of relatively large droplets will realize their maximum heat extraction potential through evaporation in typical firefighting sprays, whilst the majority will remain in the liquid phase and form runoff. Conversely, explains Baratov, that if the water is delivered in the form of very fine droplets with the purpose of promoting rapid evaporation, the spray may not possess the momentum required infiltrating the flame; again, the net result is that water is waste and firefighting is compromised. Thus, by both improving water dispersion and by adding various additives fire suppression effectiveness of water can be increased.
Ignitable Liquid Residues (ILR’s)
As written in T.C Forensics, in the context of a suspicious fire, an accelerant is a substance typically an ignitable fluid of some sort that has been deliberately introduced to a scene expressly for the purpose of facilitating the spread of a fire. The classifications and flammability properties of some common ignitable liquids are shown in Table 2.
| Liquid | Boiling point (°C) | Flash point (°C) | Ignition temperature (°C) |
| Kerosene | 175-260 | 38-74 | 229 |
| Gasoline | 40-190 | -43 | 257 |
| Diesel | 190-340 | 69 | 399 |
| Engine oil | N/A | 150-230 | 260-371 |
| Acetone | 57 | -20 | 465 |
| Octane | 126 | 13 | 220 |
| Pet ether | 35-60 | -18 | 288 |
| Spirit turpentine | 135-175 | 35 | 253 |
| Alcohol | 78 | 13 | 365 |
| White spirits | 150-200 | 35 | 232 |
TABLE 2. Flammability properties of some common ignitable liquid.
The American Society for Testing and Materials (ASTM) revised the classification scheme for ignitable liquids in 2001. While the older system Table 3 uses both names and numbers, the current system Table 4 uses a two-dimensional approach, with more categories being defined and each category divided into three subcategories, with the exception of gasoline.
To accommodate the evolution of the petroleum industry with many new products being developed, products that in the old system would all have been classed in the “0” (miscellaneous) category, with the result that the number of subcategories exceeded the number of classes this revision was necessary as suggested by Stauffer and Lentini.
However, with some classes of medium range distillate products the system becomes more complicated, where differentiation is based on the relative abundances of aromatic components. In this range petroleum distillates and de-aromatized distillates will appear very similar by Total Ion Chromatogram (TIC) and Flame Ionization Detection (FID), with the extracted ion profiles from a gas Chromatography-Mass Spectrometry (GC-MS) necessary to make a distinction as described by Dolan and Stauffer. For fire debris examiners this can be problematic as there are no guidelines stating the cut off limits for aromatics for each of these groups of liquids, making it difficult to ascribe a definite identity as given by Dolan and Stauffer.
Café and Stern, suggest the most commonly used accelerants are liquids for example petrol, diesel, and kerosene, complex mixtures of hydrocarbons obtained from the fractional distillation of crude oil and popular due to their ready availability. Most of these commonly used ignitable liquids have similar chemical properties but differ in the boiling point ranges of their respective components as stated. Often hard to detect and identify they are complex substances, as they do contain many different components. Common household items like plastics and carpets pyrolysis products the analyses for these ignitable liquids are problematic by the presence of many of these components.
Commonly used accelerants
Petrol: This comprises of a much lighter hydrocarbon component than kerosene or diesel. According to T.C Forensic, it has a much greater volatility and will readily form an explosive air vapor mix to cause an extensive damage upon ignition. Unlike many other accelerants’ petrol has a very distinctive chromatographic pattern and is not characterized by equidistant n-alkane peaks as stated by Bertsch. Instead, a typical petrol chromatogram as shown in Figure 3 has many different peaks in varying proportions. Whilst pure gasoline may have an identifiable distinctive pattern, although it weathers quickly, and in its evaporated state is more difficult to recognize and can be mistaken for white spirit. A study by Bertsch et al., showed that out of 120 laboratories tested, only 70% correctly identified a 95% weathered gasoline sample using capillary gas chromatography flame ionization detector (GC-FID) the standard method at the time.
FIG. 3. A typical petrol chromatogram by gas chromatography-mass spectrometry.
To determine whether or not unique markers may be obtained for petrol from different sources, studies have been carried out as an aid to establishing a link between a suspect and an arson scene where gasoline has been detected. This has been carried out, successfully, via tetra-alkyl lead content or by examining the profiles of the more volatile components present in gasoline described by Hirz. However, according to Mann, with the banning of leaded gasoline in the case of the former, and in the latter the inability to differentiate samples after more than 50% evaporation due to loss of the target compounds both of these have become less feasible.
Recently, a study as suggested by Sandercock and Pasquier, has shown more promise. This involved taking 35 randomly selected un-evaporated gasoline of different grades i.e. regular unleaded, premium unleaded, and lead replacement, and then focusing on the high boiling components so that the technique may be subsequently applied to a weathered sample. Taylor et al., suggests that the target polar compounds and Poly Aromatic Hydrocarbons (PAHs) were isolated via a novel Solid-Phase Micro-Extraction (SPME) process incorporating activated alumina, and then analysing by GC-MS with Selected Ion Monitoring (SIM).
According to Ioppolo-armanios, Alexander and Kagi, as previous studies have reported that C0–C4 alkylphenols exist in crude oil in varying concentrations, and have been used successfully as markers to characterize oil spills in aquatic environments due to the solubility of the phenols in water the polar compounds were selected. Furthermore, a study done by Mach, into the determination of PAHs in evaporated and burned samples of gasoline concluded that certain PAHs are unique to gasoline, while a later study by Hennig, demonstrated that the levels of these compounds in a sample were dependent on the refinery from which it was produced.
In instances such as petrol, where samples have been weathered to the extent that only heavy, low-volatility fractions remain; readily ignitable liquids, may become difficult to identify. Coulombe, nevertheless, verified weathered gasoline samples using diphenyl disulfide compounds.
Using comparative GC-MS, Coulombe, analysed samples of heavily weathered petrol, evaporated diesel, creosote, evaporated gasoline from an incendiary device, and fire debris. These tests were positive showing that the marker diphenyl disulfides were present only in the gasoline and debris samples but were not found in pyrolysate, suggesting they were particular to gasoline, and their presence in residues as such is characteristic; however, their origin is unknown at present.
Kerosene: According to Lentini, this is the second most popular accelerant after petrol. Although more difficult to ignite due to a lower volatility, and given adequate ventilation it will burn longer, hotter. Similar to diesel, kerosene but contains a greater proportion of lighter hydrocarbon components, giving it a greater volatility, and due to its relativity high boiling range (175- 260°C), Table 2 is more likely to leave a detectable residue after a fire than petrol as stated by Bertsch and Ren. A chromatogram of un-evaporated kerosene displays eight evenly spaced characteristic peaks (Figure 4), with many of these identifying components still visible in the weathered or burned sample described in T.C forensic.
Unlike petrol, which is almost exclusively used as motor fuel, however, from charcoal kerosene is legitimately found in a number of common household products, lighters to paint thinner, and is thus the most common incidental accelerant describes Lentini. Consideration must be given to other evidence at the scene to determine how it may have come to be there as it is not necessarily indicative of a criminal act therefore detection of a kerosene residue at a scene must be treated with caution.
FIG. 4. A typical kerosene chromatogram by gas chromatography-mass spectrometry.
Diesel: In T.C Forensic states that it comprises the heavier components of crude oil, thus having a high boiling point low volatility, it can be difficult to ignite. A diesel chromatogram has similar eight peaks characteristic of kerosene, although in different proportions, plus a further eight from higher boiling components (Figure 5). Care should be taken during recovery owing to its high boiling range that these higher boiling components are not lost as the resultant chromatogram can resemble that of the weathered kerosene as given by T.C Forensic. Studies have found that however, the chromatograms of burned and weathered ignitable liquids vary in a predictable way, and hence there now exists libraries of degraded ignitable liquid patterns for comparative purposes suggests Henderson.
FIG. 5. A typical diesel chromatogram by gas chromatography-mass spectrometry.
ASTM method standards
Since 1990 the American Society for Testing and Materials (ASTM) has provided standards for fire debris analysis. In the two main standards for the analysis of ignitable liquid residues, ASTM E1387 and ASTM E 1618 for Gas Chromatography (GC) and Gas Chromatography-Mass Spectrometry (GC-MS), respectively important changes have been made. The following standards were changed in 2001:
• ASTM E 1387-01 standard test method for ignitable liquid residues in extracts from fire debris samples by gas chromatography.
• ASTM E 1618-01 standard test method for ignitable liquid residues in extracts from fire debris samples by gas chromatography-mass spectrometry.
It is of importance to note that ASTME E1618 is no longer a standard guide, but a standard test method. This means that the method is no longer directly related to E1387, but serves as the only standard method. In the revised version of the ASTM E 1387 and E 1618 standards the main change introduced is the classification system of the ignitable liquids. The earlier classes had both numbers and names as shown in Table 3. On this topic, an excellent description of the changes that occurred in the last several years was written by DeHaan.
| Class number | Class name |
|---|---|
| 1 | Light Petroleum Distillates (LPD) |
| 2 | Gasoline |
| 3 | Medium Petroleum Distillates (MPD) |
| 4 | Kerosene |
| 5 | Heavy Petroleum Distillate (HPD) |
| 0 | Miscellaneous |
| 0.1 | Oxygenated solvents |
| 0.2 | Isoparaffins |
| 0.3 | Normal alkanes |
| 0.4 | Aromatic solvents |
| 0.5 | Napthenic/paraffinic solvents |
TABLE 3. Old ASTM classification systems of ignitable liquids.
A two-dimensional classification is developed in the new system as presented in Table 4. More categories have been defined, and each category is divided in three subcategories light, medium and heavy, with the exception of the gasoline category. Light means a carbon range from C4-C9, medium from C8-C13, and heavy from C8-C20 and above.
Criteria to interpret and identify ignitable liquid residues are not as specific in E 1387 as they are in E 1618, since the latter includes mass spectral characteristics. Table 4 below presents a summary of the main classes with their characteristics which should help the fire debris analyst in the choice of proper class. The frame light, medium, heavy is not rigid and may be necessary to characterize a product as light to medium, or medium to heavy, when the carbon number range does not fit neatly into one of the above categories.
| Class | Light | Medium |
Heavy |
|---|---|---|---|
| Gasoline | Fresh gasoline typically falls in the range of C4-C12 |
|
|
| Petroleum distillates | Petroleum ether, some cigarette lighter fluids, some camping fuels | Some charcoal starters, some paint thinners, some dry-cleaning solvents |
Kerosene, diesel fuel, some jet fuels, some charcoal starters |
| Isoparaffinic products | Aviation gas, specialty solvents | Some charcoal starters, some paint thinners, some copier toners |
Some commercial specialty solvents |
| Aromatic products | Some paint and varnish removers, some automotive parts cleaners, xylenes, toluene-based products | Some automotive parts cleaners, specialty cleaning solvents, some insecticide vehicles, fuel additives |
Some insecticide vehicles, industrial cleaning solvents |
| Napthenic paraffinic products | Cyclohexane based solvents/products | Some charcoal starters, some insecticide vehicles, some lamp oils |
Some insecticide vehicles, some lamp oils, industrial solvents |
| n-Alkanes products | Solvents, pentane, hexane, heptane | Some candle oils, copier toners |
Some candle oils, carbonless forms, copier toners |
| De-aromatized distillates | Some camping fuels | Some charcoal starters, some paint thinners |
Some charcoal starters, odorless kerosene |
| Oxygenated Solvents | Alcohols, ketones, some lacquer thinners, fuel additives, surface preparation solvents | Some lacquer thinners, some industrial solvents, metal cleaners/gloss removers |
Some charcoal starters, odorless kerosene |
| Other miscellaneous | Single component products, some blended products, some enamel reducers | Turpentine products, some blended products, various specialty products |
Some blended products, various specialty products |
TABLE 4. New ASTM classification system.
When distinguishing between categories such as distillates, isoparaffinic products and naphthenic-paraffinic products extracted ion profiles are most useful. As an example, a quick overlay of extracted ions 55, 57, 83, and 105 the analyst should quickly differentiate between petroleum distillates, naphthenic-paraffinic, isoparaffinic products, and de-aromatized distillates. Such distinctions are usually not possible using GC alone as suggested by Stauffer and Lentini.
Gas chromatography–Mass spectrometry (GC-MS)
The method of choice for the characterization of accelerants from suspected arson scene has been Gas chromatography as given by Lucas. According to ASTM E1618-01, GC-MS is applied as the standard American Society for Testing and Materials (ASTM) method for ignitable liquid analysis. National Centre for Forensic Science website, states a large database of GC-MS data of ignitable liquids is available on the Internet.
Parker, Rajaswaran and Kirk suggested that typically petroleum products such as gasoline, lighter fluid, kerosene, etc. to determine their presence, the technique involves visual comparison of chromatograms of fire debris extracts with those of known accelerants. According to Midkiff and Washington, in many instances, the presence of an accelerant can neither be established nor be disproved due to co-eluting substances obscuring the chromatographic pattern. Pyrolysis of common floor coverings such as carpet and vinyl flooring during a fire can interfere with low level detection of common accelerants as described by Holzer and Bertsch.
Juhala describes sample clean up prior to GC analysis by acid stripping or selective adsorption as suggested by Aldridge and Oates, because of the requisite structural differences between the contaminants and analyte are minimal is time consuming and not always successful. Moreover, many frequently observed pyrolysis products are hydrocarbons of the type present in petroleum-based accelerants as stated by Bertsch et al.
Kelly and Martz, suggested that replacement of the nonspecific GC detector by a mass spectrometer provided a means of greatly reducing these interferences. According to Wineman to identify and quantitate a significant number of compounds known to be components of accelerant fuels, the mass spectrometer can be used as a GC detector. Hence semi-quantitative peak areas can then be used to generate a pattern referred to as target compound chromatogram or TCC. This pattern showing the retention time and relative amount of each target compound is essentially a reconstructed chromatogram. To confirm the fit to TCC’s of known accelerants for the identification of unknown, visual pattern recognition is used.
Another approach as suggested by Hites and Biemann, used in this study as an adjunct to target compound analysis is the generation of extracted ion profiles or mass chromatograms. Generally limited to weak samples where the base ion is all that is available for the identification of a particular target compound or class of compounds mass chromatography is not used on a routine basis. Because the number of different mass chromatograms generated is burdensome, and the result are still subject to interpretation when interfering compounds also contain the extracted ion of interest. Preferred is target compound analysis because it displays all of the accelerant related compounds on a single reconstructed chromatogram, therefore facilitating comparison to known accelerant patterns.
As a target any compound known to be present in the type of accelerant used can be sought. Those that remain detectable when the accelerant is highly evaporated, diluted and contaminated with high levels of co-eluting substances are desirable targets. Current targets as shown in Tables 5-7 are the best choice of accelerant identification.
Analysis using GC-MS
| Compound | CAS Number |
|---|---|
| 1,3,5-Trimethylbenzene | 108-67-8 |
| 1,2,4-Trimethylbenzene | 95-36-3 |
| 1,2,3-Trimethylbenzene | 526-73-8 |
| Indane | 496-11-7 |
| 1,2,4,5-Tetramethylbenzene | 95-93-2 |
| 1,2,3,5-Tetramethylbenzene | 527-53-7 |
| 5-Methylindane | 874-35-1 |
| 4-Methylindane | 824-22-6 |
| Dodecane | 112-40-3 |
| 4,7-Dimethylindane | 6682-71-9 |
| 2-Methylnaphthalene | 91-57-6 |
| 1,1-Methylnaphthalene | 90-12-0 |
| Ethylnaphthalenes (mixed) | 1127-76-0 |
| 1,3-Dimethylnaphthalene | 575-41-7 |
| 2,3-Dimethylnaphthalene | 581-40-8 |
| Note: ASTM E1618-06 Standard method for ignitable liquid residues in extracts from fire debris samples by gas chromatography-mass spectrometry. | |
TABLE 5. Gasoline target compounds.
| Compound | CAS Number |
|---|---|
| Nonane | 111-84-2 |
| Propylcyclohexane | 1678-92-8 |
| 1,3,5-Trimethylbenzene | 108-67-8 |
| 1,2,4-Trimethylbenzene | 95-36-3 |
| Decane | 124-18-5 |
| 1,2,3-Trimethylbenzene | 526-7-8 |
| n-Butylcyclohexane | 1678-93-9 |
| Trans-Decalin | 493-02-7 |
| Undecane | 1120-21-4 |
| 1,2,3,5-Tetramethylbenzene | 527-53-7 |
| n-Pentylcyclohexane | 4292-92-6 |
| Dodecane | 112-40-3 |
| n-Hexylcyclohexane | 4292-75-5 |
| Note: ASTM E1618-06 Standard method for ignitable liquid residues in extracts from fire debris samples by gas chromatography-mass spectrometry. | |
TABLE 6. Medium Petroleum Distillate (MPD) target compounds.
| Compound | CAS Number |
| Decane | 124-18-5 |
| n-Butylcyclohexane | 1678-93-9 |
| Trans-Decalin | 493-02-7 |
| Undecane | 1120-21-4 |
| 1,2,3,5-Tetramethylbenzene | 527-53-7 |
| n-Pentylcyclohexane | 4292-92-6 |
| Dodecane | 112-40-3 |
| n-Hexycyclohexane | 4292-75-5 |
| 2-Methylnaphthalene | 91-57-6 |
| 1,1-Methylnaphthalene | 90-12-0 |
| Tridecane | 629-50-5 |
| n-Heptylcyclohexane | 005617-41-4 |
| 1,3-Dimethylnaphthalene | 575-41-7 |
| Tetradecane | 629-59-4 |
| n-Octylcyclohexane | 1795-15-9 |
| 2,3,5-Trimethylnaphthalene | 2245-38-7 |
| Pentadecane | 629-62-9 |
| n-Nonylcyclohexane | 2/5/2883 |
| Hexadecane | 544-76-3 |
| Heptadecane | 629-78-7 |
| Pristane | 1921-70-6 |
| Octadecane | 593-45-3 |
| Phytane | 638-36-8 |
| Nonadecane | 629-92-5 |
| Eicosane | 112-95-8 |
| Heneicosane | 629-94-7 |
TABLE 7. Heavy Petroleum Distillate (HPD) target compound (ASTM E1618-06, 2006).
Analysis using GC-MS
For use in fire debris analysis as a technique GC-MS has been available for more than twenty years however, only recently with the development of cheap, compact, and user-friendly models it has become more popular for routine use as described by Bertsch, with around 90% of laboratories now regularly using the technique according to Frysinger and Gaines, and a prescribed ASTM method. The Figure 6 below shows the instrument that was used to carry out this study.
FIG. 6. Shimadzu GCMS-QP2010 SE Plus gas chromatography mass spectrometer.
A Mass Spectrometer (MS) device can display data either as mass chromatogram extracted from the Total Ion Chromatogram (TIC) data, as specific characteristic ions via Selective Ion Method (SIM) or the more in-depth Target Compound (TCC) analysis. Even in highly complex matrices interferents and background noise can be greatly reduced, enabling a high degree of sensitivity to be achieved using this latter approach.
In overlapping GC peaks result in elevated baselines, this is particularly useful in fire debris samples and complex ignitable liquids. The co-eluting peaks as suggested by Frysinger and Gaines, can be almost entirely separated by mass, as individual component species having unique molecular fragmentation patterns that can be used as a fingerprint for that class of ignitable liquid. The data from the TIC are screened for specific parent/daughter fragmentation ions characteristic of the ignitable liquid within a defined retention window, in a TCC analysis, and their abundances are used to provide confirmation by comparison with known data for the substance as given by Bertsch and Ren. The Figure 7 below shows the various components of GC-MS apparatus quite similar to the one used to carry out this study.
FIG. 7. Schematic diagram of GC-MS apparatus.
Encompassing the key components from all target groups for a specific substance a composite chromatogram of no more than 30 components is produced. By reducing the amount identifying chromatograms obtained from complex debris samples describes Lennard and Rochiax.
According to Bertsch and Ren, petroleum based ignitable liquids are ideal for analysis by GC-MS, as the compounds that comprise the majority of petroleum distillates namely alkanes, cycloalkanes, and aromatic hydrocarbons produce several species of characteristic ions for each of the major classes see Table 8.
Extraction of the ion fragments specific to the aromatics present in gasoline enables an identifying chromatographic fingerprint to be generated as suggested by Newman and Gilberts. These fragments include the C3 and C4 alkyl-benzenes m/z 105 and 119 respectively, toluene and xylenes m/z 91, plus naphthalene and the alkyl naphthalenes (m/z 128, 142, 156).
Although not necessarily the most abundant, particularly at the elevated temperatures experienced during a fire these components are concentrated and subsequently more persistent, and are thus more likely to become concentrated and subsequently more readily detectable as described by Rella et al.
| Compound | m/z |
|---|---|
| Alkane | 43, 57, 71, 85, 99 |
| Cycloalkane and alkene | 55, 69 |
| n-Alkylcycloalkanes | 82, 83 |
| Aromatic-alkylbenzenes | 91, 105, 119; 92, 106, 120 |
| Indanes | 117, 118; 131, 132 |
| Alkylnaphathalenes (Condensed ring aromatics) | 128, 142, 156, 170, |
| Alkylstyrenes | 104, 117, 118, 132, 146 |
| Alkylanthracenes | 178, 192, 206 |
| Alkylbiphenyls/acenaphthalenes | 154, 168, 182, 196 |
| Monoterpenes | 93, 136 |
| Ketones | 43, 58, 72, 86 |
| Alcohols | 31, 45 |
| Note: ASTM E1618-06 Standard method for ignitable liquid residues in extracts from fire debris samples by gas chromatography-mass spectrometry. | |
TABLE 8. Major ions present in mass spectra of common ignitable liquids.
For commonly encountered ignitable liquids identification is carried out on the basis of the fragments at m/z 57 and 71 comprising mainly aliphatic species such as diesel, kerosene, aviation fuel. The liquid is determined to be kerosene or diesel, when the ratio of m/z 57 to m/z 71 falls between 1.5 and 1.6 specific classifications is being based on the differing carbon chain lengths. The sample is determined to be aviation fuel when the ratio is higher than this or falls below 1, and has a chain length of C8–C9 as stated by Rella et al.
Frysinger and Gaines suggested that one major problem with GC-MS arises when the target compounds and background materials contain ions with the same mass. This most commonly occurs when the sample matrix consists of petroleum-based products, particularly burned carpet in fire debris analyses. Carpet pyrolysates can include large amounts of alkylbenzenes such as ethyl-, butylbenzene, and propyl- all of which are found in, and used diagnostically for petroleum derived ignitable liquids. Their presence thus makes positive detection and identification of the target compound problematic if not impossible.
Interference problems and development in MS detection
Almirall and Furton suggest that interfering compounds are recognised problem in the analysis of ignitable liquid residues, and may be attributable to one or more sources naturally from the background of a substrate material, its manufacture, or contamination; from substrate combustion products; or from pyrolysates generated during its combustion.
In both burned and unburned samples of vinyl flooring the presence of ignitable liquid products containing straight chain alkane isopar H and Nopar has been determined by GC-MS study by Wells to derive from plasticizers used during the vinyl manufacture process as suggested by Rodgers et al. Isopar H and Norpar products are also commercially available in products such as indicative of ignitable liquid use and should be treated with caution as this can lead to complications for fire investigators.
According to DeHaan, Brien and Large, suggested that under combustion both animal and human subcutaneous fat have been demonstrated to produce significant quantities of volatiles that can be detected in subsequent smoke and fire debris. The naldehydes in the C5-C10 range were found to predominate, with n-alkanes, alkenes, and other aromatics also detected, which may be misconstrued as being of ignitable liquid origin.
Almirall and Furton, examined the pyrolysis of 35 products commonly ranging from varieties of carpet and flooring and determined that many of the identifying compounds used to determine the presence of ignitable liquid residues, such as 2- methylnaphthalene, are frequently detected.
Co-elution of matrix and pyrolysis volatiles, and from microbial degradation of samples complications with GC can arise. Existing methods are not always successful to counter these and cannot be used on a micro-scale. Mass chromatograms can be extremely difficult, with TCC not only requiring prior determination of the optimal target ions but also that these persist in the sample at detectable levels states Rodgers et al., where samples have been exposed to harsh environmental conditions this a particular problem for fire debris.
Other analytical methods
A number of other techniques exist besides GC that can provide useful information although it should be noted these are recommended as complimentary secondary analyses to a routine GC method.
According to DeHaan traditional measurements of physical properties like refractive index and flash point can provide some useful information on a substance where liquid samples are submitted in appreciable volumes or if distillation has been performed to isolate the neat volatile. Although in terms of actually characterizing ignitable liquids they are generally of little use. Some information on the chemical structure of liquid samples obtained by the distillation of fire debris can be provided by Infrared (IR) spectroscopy, although typically the procedure requires around 20 μL of sample.
As demonstrated in the work by Alexander et al., shows that everal successful studies have been performed into the application of the ultraviolet/visible fluorescence technique to the analysis of petroleum distillates. Advances in this field have provided detailed characterizations of ignitable liquids, when used in conjunction with modern High-Performance Liquid Chromatography (HPLC) technology.
Already a well-established procedure for the analysis of volatile compounds as suggested by Matsen, Robertson and Chuoke, vapour phase ultraviolet spectroscopy has been applied. Particularly in cases where one sample component in high excess masks another to the determination of aromatic ignitable liquids, and has the potential to provide additional information to that obtainable by GC methods. This technique while also providing an acceptable degree of sensitivity and discrimination for use in casework has a number of advantages, being simple, cheap and rapid. However, it cannot be used to detect polycyclic aromatic hydrocarbons or alkanes due to their predominantly alkane composition, ruling out its use where diesel or paraffin are suspected.
Use of CAF and interference with analysis
Compressed Air Foam (CAF) systems for the use in fire suppression has been introduced into a number of firefighting, brigades in New Zealand. To reduce the amount of water needed to extinguish a fire by reducing the surface tension of the water is the aim of the foam. This makes the use of foam systems attractive for areas where there is reduced water accessibility, such as rural areas. The foam is generated at the fire scenes by mixing the foam with water on the discharge side of the water pump. The solution is then aspirated through a nozzle where it is aerated forming foam. There has been no research published to whether thefoam causes any interference on subsequent accelerant analyses to date given by Newsman, Dietz and Lothridge.
Objective of the study
The main objective is to understand and describe the use of compressed air foams in fire suppression and their relative effect in the detection of Ignitable Liquid Residues (ILRs) in fire debris samples and post-analysis of accelerants using GC-MS. The objective of this new study is: -
• To prove that compressed air foams do not cause any interference on subsequent ILR analyses.
• To determine the effectiveness of fire suppression between compressed air foam compared to water.
Accelerant detection at scenes
Evidence of accelerant use is often important to the investigation in determining whether or not a scene is the result of a deliberately accelerated fire. Odour for many years was the standard test for is a key tool in detecting ignitable liquid residues at a fire scene to both human and canine investigators alike. At trace levels, even the human nose can be trained to identify and differentiate between ignitable liquid residues. Even at trace levels, the human nose can be trained to identify and differentiate between ignitable liquids, with a sensitivity of around 20 ppm as suggested by DeHaan. While there is also an inherent risk involved in sniffing unknown materials stipulates T.C. Forensics sense of smell is subjective, varies widely with climate, however, deteriorates through fatigue over the course of a day. Nevertheless, as the primary detector when selecting samples for subsequent analysis many investigators still rely on their own sense of smell T.C Forensic. Table 9 below indicates some of the field methods used by scene investigators to locate traces of ignitable liquids.
| Detection method | Advantages | Disadvantages |
| Canine teams | Highly sensitive Rapid scanning of large areas. Can be trained to discriminate between ignitable liquid residue and other vapors |
Expensive. Suffer from olfactory fatigue. Cannot always discriminate between ignitable liquids and pyrolysates |
| Metal Oxide Sensor (MOS) | Cheap, small and robust. Detects a wide spectrum of contaminants |
Inaccurate. Non-specific. Reacts to moisture. Prone to poisoning |
| Photo-Ionization Detector (PID) | Highly sensitive. Wide operational range (10,000 ppm<1 ppb). Robust, unresponsive to moisture and inorganic vapors |
Cannot distinguish between ignitable liquid residues and plastic pyrolysis products |
| Portable gas chromatograph | Can discriminate between ignitable liquids and burned plastic. Capable of trace detection Versatile |
Early models suffered from poor resolution |
| Portable mass spectrometer | Provides rapid at-scene information (c.30’s) | Early models somewhat bulky and heavy |
| Chemical tests | Quantitative Versatile sampling (air, soil, water) |
Expensive Non-discriminative Single use |
TABLE 9. The below indicates some of the field methods used by scene investigators to locate traces of ignitable liquids.
Investigation of a scene
What to sample: It is important to select those materials that have the highest probability appreciable levels of identifiable ignitable liquid residue when collecting debris samples from a fire scene. According to T.C Forensic, the amount that can be recovered is governed by a number of things, particularly the substrate to which the ignitable liquid was applied. Most of the common accelerants are hydrophobic and hence tend not to be washed away when the fire is extinguished exceptions are acetone,ethanol, and methylated spirits. Instead they become sealed into porous substances by water and can potentially be recovered intact up to 3 months later, where they are protected from evaporation. On the other hand, water miscible ignitable liquids, tend to be washed away as the fire is extinguished they do not become sealed into porous substrates, and those residues that do remain are rapidly lost by evaporation due to exposure hence are more difficult to detect. The Figure 8 below gives an indication of the type of sample materials commonly submitted to laboratories for analysis.
FIG. 8. Composition of commonly submitted fire debris samples (Bertsch and Zhang, 1990).
Generally, the type of debris sampled will depend on what is available at the scene as suggested by T.C Forensic. Mainly, the samples should be porous with a high surface area and composed of absorbents materials so as to retain liquid accelerants residues. Thus, materials such as soil, paper/cardboard, cloth, carpet, and to a lesser extent concrete are popular for debris sampling as given by T.C Forensic.
According to Bertsch some sample matrices either through pyrolysis or degradation may produce interfering compounds, which can hinder subsequent identification. This is problematic with synthetic polymeric materials and the increasing abundance of petroleum-based products in everyday domestic use, ranging from insecticides to tile glue. However, while these materials can present an analytical challenge, their ability to thermally distort and encapsulate ignitable liquid residues can make them good samples.
Carpet materials: Carpet and carpet padding by far are the most commonly sampled material; however, it does have some inherent disadvantages as a sample matrix. Modern carpets are supported on a polypropylene copolymer and are typically made from synthetic fibers like nylon as suggested by Bertsch. On the other hand, carpet padding usually comprises synthetic rubber or polyurethane with strengthening fibers.
When the synthetic materials of their construct become pyrolysed during a fire, the resultant products contain a number of diagnostic indicators for certain ignitable liquids, like alkylbenzenes and naphthalenes, which can trigger electronic sniffers and provoke a positive reaction from canine detection teams as stated by Tranthim-Fryer and DeHaan.
Bertsch stipulates that there is also no characteristic profile for charred carpet, as it is very much dependent on the make-up and the conditions of pyrolysis. Neither the carpet itself nor the carpet padding produce volatiles that could be misidentified as being petroleum based as shown by studies, it is the carpet backing that is the prime source of interfering compounds. However, it was confirmed that the distribution of these compounds in the pyrolysis products differed greatly from the pattern produced by gasoline. Hence, samples of gasoline in the test matrices could still be identified in the presence of these interferents by the use of GC-MS analysis and careful pattern observation by an experienced analyst, showing the necessity for subsequent laboratorybased analysis of samples.
Sampling
It is essential that loss and contamination be avoided during the transfer to the laboratory and subsequent storage once samples have been collected, as this can not only affect the outcome of the analysis but may also have legal consequences if presented as evidence in court as suggested by Bertsch and Ren.
For this reason, DeHaan states that in order to maintain sample integrity and chain of custody the correct sampling procedure must be followed at all times with the proper documentation and as part of this process, the selection of the container to be used is far from complex matter.
Tan, Hardy and Snavely described there are many types of containers available, from bags to jars and metal cans, made from a variety of materials each with their own advantages and disadvantages, as shown below in Table 10.
| Container | Advantages | Disadvantages |
|---|---|---|
| Plastic bags | Flexible Hold large awkward samples Convenient to carry in bulk Quickly heat sealed |
Sample loss and contamination by diffusion of volatile gases through the bag Not sturdy (easily pierced) |
| Glass jars | Resistant to puncturing Long lifespan |
Breakable. Not suitable for heating during sample preparation. Screw tops not particularly airtight. |
| Plastic jars | Resistant to puncturing Long lifespan |
Not suitable for heating during sample preparation. Screw tops not particularly airtight. |
| Unlined metal cans | Lack organic matter that can cause interference analyses Very robust excellent sealing capabilities |
Tendency to rust through, whose rate is dependent upon the sample and storage conditions. |
| Lined metal cans | Avoids rusting Very robust Excellent sealing capabilities |
Some linings can cause interference during analyses. |
TABLE. 10. A variety of materials each with their own advantages and disadvantages.
• Plastic bags: Typically made from nylon, their use may be better suited to collection and transportation of samples, before transferring them to better containers for storage.
• Rubber sealed screw top jars.
• Cans: The preferred containers of choice for most scene investigators.
Container integrity: It is very important that the container used does not provide a source of cross-contamination and is clean. Café and Stern suggest that for screw top jars’, the cleanliness of the lids should be assured. Tests have shown that many types of plastic bag contain interfering compounds as given by Bertsch and Ren. Containers of unknown quality should be avoided for these reasons without first performing background checks, or instead use commercially available certified containers.
Laboratory sampling: Once the material has been obtained from the scene, in order to isolate the volatiles of interest that may be indicative of an ignitable liquid from the matrix, a laboratory- based sample preparation is required. This should be done in such a way as to minimize or screen out any possible background contaminants, while avoiding sample loss and maximizing detection limits. However, there is no one method that can be universally applied due to the wide range of chemical and physical properties attributable to different ignitable liquids.
Traditional methods
Solvent extraction: Although generally not used despite of superior methods, it is still approved by ASTM method E1386-00 as stated by ASTM E1386-00, and useful for extracting empty containers; or under circumstances where the matrix holds too strong an affinity for the sample, for very small samples; preventing the effective use of other methods as suggested by Bertsch and Ren, as may be the case with heavily charred substrates or high boiling range sample components.
A second form of solvent extraction utilizing Supercritical Fluids (SFE) has been investigated more recently, a procedure that has the advantage of avoiding subjecting samples to potentially detrimental thermal stress as given by Huang and Hsieh. The recovery efficiency of various ignitable liquid residues from carpet was found to be in excess of 80%, over a 25 min extraction, with a low degree of interference co-extraction. Although this procedure does tend to co-extract unwanted components of the matrix, resulting in complex chromatographic profiles, suggesting the potential of this technique in future analyses.
Steam distillation: Steam distillation although it is still approved by 2001 ASTM method E1385-00 given by (ASTM E1385-00, 2001), and is still used when large amounts of ignitable liquid residue are expected in a sample as suggested by Café and Stern is merely used today.
In a comparative study of distillation and adsorption techniques as described by Frontela, Pozas and Picabea, this technique displayed superior recovery of low to medium boiling range ignitable liquids in an analysis specifically gasoline and gas oil using a modern protocol distillation method with n-hexane as the extractor solvent, proven to have high recovery efficiency.
Modern methods
According to Bertsch and Ren suggested a procedure known as direct headspace analysis may also be used on occasion, although it is generally not used. It involves heating a contained sample, and then drawing of an aliquot of the vapor headspace and injecting this directly into the GC. However, it has been effective in analyses involving highly polar substances like ethanol, if sufficiently abundant in the sample hence more useful as a screening method.
Nevertheless, as technique it does not concentrate the sample like other methods, with sensitivity limited to the low injection volume and dependent on the container size hence suffers from poor sensitivity.
Dynamic headspace sampling: Chrostowski and Holmes describe this as the older of the two headspace techniques and was adapted from methods utilized in the field of environmental sampling. Also referred to as purge and trap, the basic technique involves using an inert gas to remove continuously the heated headspace of a sample so as to induce the complete removal of all the volatile components as given by Ettre. The subsequent sample retaining the volatile components, gas stream then passes through a cooled and/or sorbent packed trap. These can be then released by heating or solvent extraction and the quantified by GC.
According to Bertsch and Ren, the currently favoured method uses the sorbent trap approach, due mainly to the popularity and ease of use of porous sorbents such as Tenax GC followed by rapid thermal desorption, which do not require the implementation of an additional solvent extraction stage. Jones, describes modern methods that allow the process of dynamic headspace to be fully automated, using an Automatic Thermal Desorption system (ATD) controlled by microprocessors.
Brown and Purnell demonstrated that lower temperatures enhance Tenax retention capacity, approximately doubling per 10°C decline in temperature. The trap is quickly heated at 1500°C/min, desorbing the analytes as a sharp band.
Thermal desorption is not usually possible, owing to the strong sample interactions, and solvent extraction is normally required, either with carbon disulfide or diethyl ether as stated by Ren and Bertsch. Bertsch and Holzer suggest that current techniques now enable this to be carried out successfully with a highly concentrated final extract volume of a few microliters that may be directly injected into the GC on a micro-scale using just 5 mg of charcoal.
Waters and Palmer stipulate nevertheless, dynamic headspace methods are still less popular than passive equilibrium methods as they are inherently labour intensive and prone to contamination via the vacuum or gas supply.
Passive headspace concentration: Waters and Palmer define passive or static headspace analysis as a non-destructive and far simpler technique to perform than dynamic methods, and thus generally favored.
The procedure involves suspending an adsorbent material, either a porous polymer or carbon, in the heated headspace of a sample as described by Waters and Palmer, and then allowing the volatiles in the vapor to absorb onto the surface as suggested by ASTM E1388-00, before extraction by solvent or thermal desorption for subsequent analysis as suggested by Caddy, Smith and Macy. Jackowski, suggested as with the dynamic process, the analysis of samples obtained by passive enrichment may also be automated using the same ATD technology, although with some slight modifications, so ideal for rapid and batch sampling. A typical set up for passive headspace concentration is shown below Figure 9.
FIG. 9. Passive headspace concentration in a metal container.
The original debris sample from which samples are taken can be sorted for re-analysis, as unlike in the dynamic procedure, hence an advantage this method is performed in a closed system and avoids total depletion of the sample or introducing any potential sources of contamination given by Waters and Palmer.
Recovery by passive headspace is dependent largely on temperature and time as suggested by Bertsch and Ren. According to Newman and Dietz and Lothridge excessive heating, as well as longer sampling times, will result in poorer yields of the more volatile components, which become replaced by the water molecules.
SPME (Solid Phase Micro-Extraction): As a development of the passive headspace approach over the last decade, a technique known as SPME has emerged. The Adsorbent Carbon Strip (ACS) method using much the same techniques as, it benefits from greatly reduced sampling times in the order of 10-20 min cf. up to 16 h for ACS as suggested by Harris and Wheeler, far greater sensitivity, managing to detect gasoline in a study at levels where other passive methods failed describes Steffen and Pawlisyzn, and the elimination of expensive, toxic solvents like carbon disulfide from the process given by Ren and Bertsch.
Furton, Almirall and Wang explain furthermore, studies have shown that the process is unaffected by interferences from the pyrolysis products of wood and plastics. As opposed to a sorbent strip in the headspace as with ACS methods, the SPME technology utilizes a sorbent coated silica fiber retracted within a hypodermic syringe as described by Ren and Bertsch Figure 10.
FIG. 10. Solid phase micro-extraction assembly.
The procedure followed is according to ASTM E2154-01. An advantage of SPME is enabling sampling of aqueous fuels and water miscible volatiles such as ethanol, methanol, and acetone thus can be performed either in a heated headspace or by insertion into an aqueous matrix, as stated by Harris and Wheeler.
Extraction solvents
Camp states that traditionally, carbon disulfide (CS2) has been the solvent of choice in the field of forensic fire investigation, due in part to its high solubility and efficiency at displacing organic molecules from charcoal, and its unresponsiveness to FID detection.
According to Harris and Wheeler CS2 is not a particularly desirable substance for analysts to work with due to its cost, flammability as well as its biological and environmental toxicity. CS2 has been replaced with safer solvents with the increased use of GC-MS.
Clausen suggests that methanol; methylene chloride, diethyl ether, and pentane have all been used with varying degrees of success, although with greatly reduced solubility properties compared with CS2. Methanol has the highest solubility next to CS2 of these, while a study by Lentini and Armstrong cited the use of diethyl ether as being a suitable alternative if using a mass selective detector.
According to Furton, Almirall and Bruna previous reports have shown that headspace SPME for the extraction of spiked accelerants from fire debris using the GC-FID is comparable to conventional techniques and has the potential to be an excellent screening technique.
Sorbents
Carbon strips: In the field of fire investigation carbon strip technique is currently the method most commonly applied technique as stated by ASTM E1412-00. The technology involved can be applied to virtually any ignitable liquid, as given by Bertsch and Ren as the strips do not absorb water or nitrogen while having a high affinity for hydrocarbons and being resistant to oxidation states Café and Stern.
According to Bertsch and Ren, the nature of the strip, absorbent carbon impregnated upon pliable polymer substrates allows samples to be archived cutting the strips in half and storing one piece for later use. Limitation of this method is that with resultant complex GC-MS data displaying all the compounds present, the strips do not distinguish between target compounds and background compounds of pyrolysates as suggested by Almirall and Furton.
Methodology
ASTM method standards were used in this study for the extraction of fire debris samples, detection of ignitable liquid residues and the analysis of ignitable liquid residues using gas chromatography-mass spectrometry. This study was carried out in a certified, accredited and controlled laboratory environment. Each sample was analyzed using activated carbon strips, as is commonly used in laboratory techniques. The samples were analyzed in their collection sampling container, in this case sealed paint tins conditioned at 180°C overnight in a Thermo Scientific Heraeus Oven were used as shown in the Figures 11 and 12 below.
FIG. 11. Thermo scientific heraeus oven.
FIG. 12. Sealed paint tin.
Analysis involved suspending a carbon strip (~ 8 mm ?? 8 mm) in the headspace of the sample container at 70°C for ~16 hrs left in the oven. The strips were suspended using a paper clip attached to a length of black cotton string tied to lurches on the side of the tins. Each carbon strip was extracted with pentane 1.00 mL into sample vials which were labelled and Para-filmed. This samples were then stored in a cooling freezer for analysis using GC-MS instrumentation techniques.
• The instrumentation consisted of a Shimadzu GCMS-QP2010 SE Plus Gas Chromatograph (GC) with a Mass Spectrometer (MS).
• The parameters used for the pentane extracts were as follows:
• Gas chromatograph data acquisition parameters included (Table 11);
Inj. Port : SPL1
Inj. Heat Port : INJ1
Column Oven Temp : 50.0°C
Injection Temp : 250.0°C
Injection Mode : Split
Carrier Gas : He
Prim. Pressure : 300-500
Flow Control Mode : Pressure
Pressure : 54.0 kPa
Total Flow : 24.1 mL/min
Column Flow : 1.01 mL/min
Linear Velocity : 36.4 cm/sec
Purge Flow : 3.0 mL/min
Split Ratio : 20.0
| Rate | Final Temperature | Hold Time | |
|---|---|---|---|
| 0 | - | 50.0 | 3.00 |
| 1 | 15.00 | 300.0 | 5.83 |
| 2 | 0.00 | 0.0 | 0.00 |
| 3 | 0.00 | 0.0 | 0.00 |
TABLE 11. Column oven temperature program.
Total Program Time : 25.50 min
Column : 30.0 m ZB1-HT Inferno
Thickness : 0.25 um
Diameter : 0.25 mm Mass Spectrometer data acquisition parameters:
Ion Source Temp : 200°C
Interface Temp : 280°C
Solvent Cut Time : 2 min
Micro Scan Width : 0 u
Results and Discussion
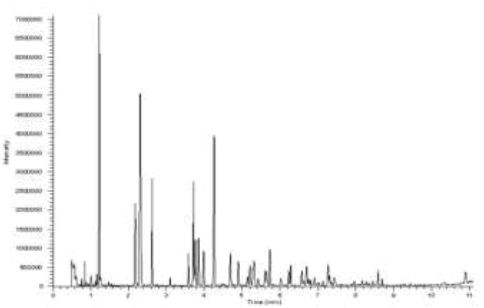
Forexpan S, a class A foam 0.1-1% class A (Angus Fire), is the brand of foam that was used to carry out this study. A sample 12 mL diluted 0.6% of the foam concentrate was obtained and the Table 12 below describes the list of components of the foam. A sample of only foam concentrate was extracted in a sampling container with activated carbon strip and then was desorbed with 5 mL pentane to determine which components were able to be detected by GC-MS during analysis. Figure 13 shows the resulting chromatogram with the major peaks assigned.
| Ingredients | Synonyms | CAS# | %(weight) |
|---|---|---|---|
| Diethylene glycol monobutyl ether | 2-(2-Butoxyethoxy) ethanol | 112-34-5 | 10-30 |
| Lauryl alcohol | 112-53-8 | 1-5 | |
| Salt of C-isodecyl ester | 37294-49-8 | 7-15 | |
| Salts of C10-16-alkyl ethers | 68585-34-2 | 10-30 | |
| Water | 7732-18-5 | Balance |
TABLE 12. Components of forexpan s as listed in the material safety data sheet.
The Figure 13 below shows the major peaks that were assigned, through the implementation of major ions present in most common ignitable liquids.
FIG. 13. The implementation of major ions presents in most common ignitable liquids.
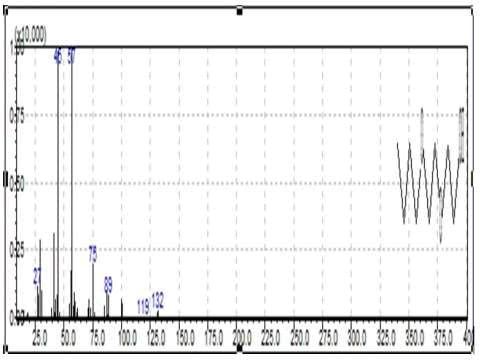
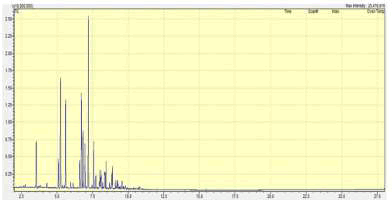
Table 8 Using the Extracted Ion Chromatogram (EIC) technique where, the dominant peak (Ethanol, 2-(2-butoxyethoxy)-) was selected Figure 14 below illustrates the TIC, then a similarity search was performed Figure 15 showing the mass spectra with the major ion of the peak, thus the mass spectra nearly similar to the peak was obtained illustrating the compound as shown in Figure 16, the following peaks were obtained from the National Institute of Standards and Technology (NIST) library software in the GC-MS through a peak integrate of all TIC groups in this case the peaks were identified.
FIG. 14. TIC for the dominant peak of the CAF sample.
FIG. 15. Mass spectra with the major ion assigned for major peak of the CAF sample.
FIG. 16. Similarity compound search of the dominant peak (Ethanol, 2-(2-butoxyethoxy)-) of the CAF sample.
Fire debris samples were prepared using a range of substrates and accelerants. The substrates included carpet and paper. All of the substrates were known to have had no contact with any accelerants prior to the experiment. The substrates were then burnt with a cigarette lighter of which prior to this were immersed with an accelerant, of which were petrol, diesel, barbeque lighter fluid and acetone. The substrates and accelerants were purchased locally and from a local Gas/Petrol Station respectively. The range of substrates and accelerants that were used reflects the type of samples that are commonly encountered in casework.
To each substrate 5.0 mL of accelerant was added, then ignited using cigarette lighter and extinguished with (~5 mL) of water and (~5 mL) of foam solution. This was done repeatedly three times for each sample also for each substrate a control burn was performed without an accelerant and both water and foam suppression techniques were used to extinguish these fires.
The foam applied was at a concentration of 0.6% however, a slightly higher concentration could be used to enhance any possible interference due to the foam. A sample of each substrate with no added accelerant was also analysed to ensure that there were no significant interactions between the foam solution and the substrate. A blank of pentane was run between each sample as a quality measure precaution. As a result, no contamination from the ignitable liquids that were used was observed or visually seen within the blank chromatograms.
Each sample was placed in their sampling containers with activated carbon strips attached to a paper clip was left for ~16hrs in the oven, to allow for equilibration. Prior to analysis, each sample activated carbon strip was desorbed in 5.00 mL pentane into a vial which was then sealed, Para-filmed and stored in the freezer as the extract analytical samples are highly volatile.
During analysis, each sample was transferred onto a GC vial 100 μL and prepared for GC-MS analytical run. Table 13 below shows the results of each sample burnt with an accelerant where the identification of accelerants was carried out by visual comparison using pattern recognition methods between the sample chromatogram and accelerant standards as a confirmatory process.
| Accelerants | Carpet control | Paper control | Carpet | Paper | |
|---|---|---|---|---|---|
| Petrol | -ve | -ve | +ve | +ve | |
| Diesel | -ve | -ve | +ve | +ve | |
| Barbeque lighter fluid | -ve | -ve | +ve | +ve | |
| Acetone | -ve | -ve | +ve | +ve |
TABLE 13. Results of sample burnt with an accelerant.
The added accelerant was correctly identified in each sample as for the controls there was no significant amount of accelerants identified. Although a significant amount of accelerants was able to be detected, carpet samples gave a more distinctive clean chromatogram as compared to paper, and acetone was merely detected because it is highly volatile and would have evaporated during extraction, desorption, and analysis thus no distinctive peaks were able to be clearly detected.
The foam did not contribute to any peak to the chromatograms that were obtained for each accelerant sample that was carried out; hence no significant interference due to the foam was encountered. This was visually confirmed by looking at the comparison between the CAF chromatogram and the matrix sample chromatograms. Hence this was verified through GC-MS extracted ion analysis similar to the method described above for the identification of the dominant peak of the CAF sample. Thus, the peaks obtained were cross referenced using this method to all the matrix sample chromatograms that were obtained and no peak from the foam was detected. Cross referencing was done by using the m/z ratio of the peaks as described using the EIC technique above, and also looking at the Retention Times (Rt.) as they appear in the chromatogram.
The presence of alcohol components as shown in Table 14 below, in the foam concentrate did not affect the detection and isolation of water-soluble accelerants, barbeque lighter fluid and acetone. The higher molecular weight and lower volatility of the alcohols present in the foam prevented these components from being present in the headspace and therefore being extracted by the carbon strips.
| Peak Name | Ret. Time | Area | Area % | Height | Height % | A/H |
| Nickel Tetracarbonyl | 2.027 | 17239223 | 8 | 4784125 | 7.54 | 3.6 |
| Nickel Tetracarbonyl | 2.12 | 817820 | 0.38 | 464780 | 0.73 | 1.76 |
| Manganese, acetylpentacarbonyl-, (OC-6-21)- | 2.761 | 237975 | 0.11 | 174496 | 0.27 | 1.36 |
| Ethanol, 2-(2-butoxyethoxy)- | 9.609 | 175981700 | 81.66 | 44217956 | 69.67 | 3.98 |
| Acetic acid, octyl ester | 9.81 | 548348 | 0.25 | 193338 | 0.3 | 2.84 |
| Undecane | 9.94 | 860085 | 0.4 | 289356 | 0.46 | 2.97 |
| 6-Dodecanol | 10.079 | 518601 | 0.24 | 184705 | 0.29 | 2.81 |
| 1-Dodecanol | 12.524 | 14161291 | 6.57 | 9800429 | 15.44 | 1.44 |
| 1-Tetradecanol | 14.269 | 4679862 | 2.17 | 3020218 | 4.76 | 1.55 |
| Dodecyl isobutyl carbonate | 14.59 | 477053 | 0.22 | 339756 | 0.54 | 1.4 |
TABLE 14. Qualitative table of the CAF chromatogram.
The first eluent was Nickel Tetracarbonyl of which can be found as well as in the pentane blank chromatograms, can be attributed as a source from the paint tins.
Accelerant profiles were obtained and identified for each accelerant that was used, thus gave a positive result to be the actual accelerants that were used during the study. This was confirmed through visual comparison of recognised patterns between the actual accelerant standards and the matrix sample chromatograms that were obtained. For example, as shown in Figure 17 below, a sample TIC chromatogram of carpet burnt in petrol and extinguished with CAF was compared to a standard TIC chromatogram of petrol Figure 18 that was used in this study, results showed there was a similarity in the pattern.
FIG. 17. TIC for carpet burnt with petrol and extinguished with CAF.
FIG. 18. TIC for petrol standard that was used in carrying out this study.
For the other matrix sample chromatograms for diesel, barbeque lighter fluid as shown in the Figures 19 and 20 respectively and acetone the same visual comparison was made using the respective accelerant standards that were used that is diesel, barbeque lighter fluid and acetone as shown in the sample example above for the carpet burnt in petrol and extinguished CAF. Similar chromatograms were observed between the comparisons with the standards.
FIG. 19. TIC for carpet sample burnt in diesel extinguished with CAF.
FIG. 20. TIC for carpet sample burnt in barbeque lighter fluid extinguished with CAF.
Even though a TIC for acetone was also obtained, it was not as distinctive neither a clean chromatogram as the rest above but this could be due to its high volatility, evaporation during sample collection and analysis but it was present. To strongly confirm that the accelerant standards were actually used in comparison to the matrix samples that were burnt with the same accelerant, using the same EIC technique as above for the identification of CAF, this was carried out. By looking at the m/z ratio of each compound peak and the retention times at which point they appear, this was able to be confirmed. An example is given in Figures 21 and 22 below of the carpet sample burnt in petrol extinguished with CAF refer back to Figure 23 for the dominant peak in which it was identified using EIC analysis technique.
FIG. 21. TIC for the carpet sample burnt in petrol extinguished with CAF.
FIG. 22. A mass spectra showing the ion of the dominant peak of the carpet sample burnt in petrol extinguished with CAF.
FIG. 23. Similarity search mass spectra for the dominant peak showing the compound structure.
Raw GC/MS data was qualitative using the alkane mix qualitative table as shown in Table 15 below, as appropriate for the type of accelerant product run. The resulting peak area data were then used to produce a total compound chromatogram which was used as comparison patterns for the identification of unknown samples. This was achieved through comparison techniques of the known and unknown also through EIC analysis. By calculating the mean, standard deviation and standard error in order to show the repeatability, also the alkane samples were run three times in sequence between each sample run using the GC-MS see Fig 4.11 below.
| Peak Name | Retention Time | Area | Area % | Height | Height % | A/H | Peak Height Ratio |
|---|---|---|---|---|---|---|---|
| Nonane | 5.948 | 10867461 | 9.22 | 7843572 | 9.05 | 1.39 | 0.766276447 |
| Decane | 7.46 | 12676757 | 10.75 | 9481056 | 10.94 | 1.34 | 0.926250171 |
| Undecane | 8.777 | 13262027 | 11.25 | 10074332 | 11.62 | 1.32 | 0.984210171 |
| Tridecane | 9.949 | 13220612 | 11.21 | 10235956 | 11.81 | 1.29 | 1 |
| Tridecane | 11.013 | 13165418 | 11.17 | 9496842 | 10.96 | 1.39 | 0.927792382 |
| Tetradecane | 11.999 | 10960846 | 9.3 | 8253613 | 9.52 | 1.33 | 0.806335334 |
| Pentadecane | 12.92 | 13362338 | 11.33 | 9649476 | 11.13 | 1.38 | 0.942703935 |
| Hexadecane | 13.787 | 13664971 | 11.6 | 9677892 | 11.16 | 1.41 | 0.945480031 |
| Octadecane | 14.604 | 13077534 | 11.09 | 9163526 | 10.57 | 1.43 | 0.895229132 |
| Octadecane | 15.373 | 1769477 | 1.5 | 1337857 | 1.54 | 1.32 | 0.130701715 |
| Octadecane | 16.11 | 944539 | 0.8 | 773341 | 0.89 | 1.22 | 0.075551419 |
| Tetradecane | 16.813 | 916712 | 0.78 | 699315 | 0.81 | 1.31 | 0.068319461 |
| Average 0.705737517; Standard deviation 0.376526662; Standard error 0.11352706 | |||||||
TABLE 15. Alkane mix qualitative table obtained from GC-MS.
From the calculations obtained, a low standard deviation was obtained indicating that the values were very close to the mean hence there was not much variation from the average. Therefore, the standards were close to having the same peak heights for each alkane throughout after each sample run although this cannot be assumed throughout the whole run as fluctuation in peaks heights were observed for the various alkanes. This can further be illustrated in the bar chart below Figure 24 showing the standard deviation error bars.
FIG. 24. A graph showing the standard deviation error bars in reference to qualitative.
To show the repeatability of the alkane compounds in each run during the analytical run, the graph below Figure 25 illustrates a plot of the retention time against the peak name (alkane).
FIG. 25. Illustrating the repeatability of the alkane mix against the retention times.
From the results, it is found that no interference is brought about by the introduction of CAF in the interpretation of data and the identification of ignitable liquid residues during analysis. Therefore, the objectives of this study were achieved, whereby this was all performed using the GC-MS due to the advantage that one is capable to perform EIC analysis which proved to be easy, quickand very useful in carrying out this study as well as the use of ASTM methods making it a very unique study being done for the first time Table 16.
| Target molecules standards | m/z ratio | Standards Rt. (min) | Identified using EIC |
|---|---|---|---|
| Propylcyclohexane | 83 | 6.392 | +ve |
| Phytane | 57, 71 | 15.503 | +ve |
| Indane | 117 | 7.723 | +ve |
| 2,3- Dimethylnaphthalene | 141, 156 | 12.293 | +ve |
| 2- Methylnaphthalene | 142 | 10.823 | +ve |
| 1,3- Dimethylnaphthalene | 141 | 12.098 | +ve |
| 1,2,4-Trimethylbenzene | 156 | 7.188 | +ve |
| 1,2,3- Trimethylbenze | 105 | 7.565 | +ve |
| 1,2,3,5- Tetratrimethylbenzene | 119 | 8.883 | +ve |
TABLE 16. Illustrations of the target molecule standards that were used.
Conclusion
Furthermore, looking at the standard target molecules Rt. as well as those of the ILRs, in comparison there was similarity whereby those target molecules that were found to be present in the ILRs were cross referenced to those of the standard target molecules showing minimal variation in the retention times at which the compounds seem to have appeared. This was then confirmed not only visually but also identified by the EIC analysis technique using the GC-MS as illustrated in Table above showing some of the target molecule standards that were used during this study.
In order to show that the CAF had no interference during analysis of the ILRs from the samples, once the EIC was done and the major peaks attained; these were then cross referenced throughout all the other chromatogram peaks using the same EIC analytical technique to check for any interference or contamination.
Using the parameters of retention time, m/z ratio, and components it was found that no peak was identified to have come from or was as a result of CAF. For example, referring to Fig 4.1 and looking at the dominant peak of m/z 45, with a Rt of 9.609 minutes, found to be 2-(2-Butoxyethoxy)-ethanol was cross referenced to all the other chromatograms and it was not found in any of them similarly using EIC analysis. This gives a firm confirmation that the objective of this study was achieved and CAF does not cause any interference on subsequent analyses of ignitable liquid residues.
It would be of great recommendation that further studies need to be done on the environmental impact of CAF as this has not been done yet. Also, more studies should be carried out for the different types of foams that are being used in fire suppression to give a broad range of spectrum towards the effects of CAF on analysis of ILRs. The situation where the presence of foam may cause interpretation difficulties would be if aromatherapy oils were suspected as the cause of fire. It has been found that some aromatherapy oils such as lemon and orange contain limonene. In such a case it would be necessary to establish whether a limonene containing foam had been used to extinguish the fire, as it is one of the components of foam and if so analysis for other commonly encountered components of these oils should be attempted. Witness information may also be very useful in identifying possible types of aromatherapy oils that may have been present at the scene.
Lower levels of detection are as a result of extraction of ILRs from fire debris becoming increasingly efficient. Research into new applications of existing extraction techniques have resulted in improvements in the analysis of fire debris and modification of traditional ignitable liquid extraction.
Research into other extraction methods should be carried out although GC-MS is currently being used. The goal should be to increase sensitivity and minimize the challenges associated with complex chromatograms due to co-extraction of products.
CAF should be used in the suppression of fire due to its advantages over water even though it exhibits certain limitations. Also, proper training in the use of CAF should be carried out by the fire brigade services hence minimize the destruction and loss of evidence which is key in the field of forensics.
References
- Alexander J, Masiak G, Kapitan N, et al. Fluorescence of petroleum products II. Three-dimensional fluorescence plots of gasolines. J Forensic Sci. 1987;32(1):72-86.
- Almirall J, Bruna J, and Furton K. The recovery of accelerants in aqueous samples from fire debris using SPME. Sci Justice. 1996;36(4):283-287.
- Almirall J, Furton K. Characterisation of background and pyrolysis products that may interfere with the forensic analysis of fire debris. J Anal Appl Pyrolysis. 2004;71(1):51-67.
- Bertsch W, and Zhang Q. Sample preparation for the chemical analysis of debris in suspect arson cases. Anal Chim Acta. 1990;236:183-195.
- Brown R, Purnell C. Collection and analysis of trace organic vapour pollutants in ambient atmospheres: the performance of a Tenax-GC adsorbent tube. J Chromatogr. 1979;178:79-90.
- Caddy B, Smith F, Macy J. Methods of Fire debris preparation for detection of accelerants. Forensic Sci Rev. 1991;3(1):62-67.
- Cafe T, Stern W. Is it an accidental fire or arson? Chem Aust. 1989;56(4):108-111.
- Camp M. Analytical techniques in arson investigation. Anal Chem. 1980;52(3):422A–4226A.
- Clausen C. Identification of accelerants in fire atmospheres. Arson Anal News. 1983;75(6).
- Coulombe R. Chemical markers in weathered gasoline. J Forensic Sci. 1995;40(5):867–872.
- DeHaan J, Brien D, Large R, Volatile organic compounds from the combustion of human and animal tissue. Sci Justice. 2004;44(4):223-236.
[Crossref] [Google Scholar] [PubMed]
- Dhole V, Ghosal G. Rapid detection and characterisation of petroleum products and residues by TLC: Part I. J Planar Chromatogr-Mod TLC. 1994;7(6):469-471.
- Dolan J, Stauffer E. Aromatic content in medium range distillate products-part I: an examination of various liquids. J Forensic Sci. 2004;49(5):1-13.
- Frontela L, Pozas J, Picabea, L. A comparison of extraction and adsorption methods for the recovery of accelerants from arson debris. Forensic Sci Int. 1995;75:11-23.
- Frysinger, G, Gaines R. Forensic analysis of ignitable liquids in fire debris by comprehensive two-dimensional gas chromatography. J Forensic Sci. 2002;47(3):471-482.
[Crossref] [Google Scholar] [PubMed]
- Furton KG, Almirall JR, Bruna, JC, A Novel Method for the Analysis of Gasoline from Fire Debris Using Headspace Solid-Phase Microextraction. J Forensic Sci. 1996;41:12-22.
- Gardiner BS, Dlugogorski BZ, Jameson GJ. Rheology of fire-fighting foams. Fire Safety J. 1998;31(1):61–75
- Harris A, Wheeler J. GC-MS of ignitable liquids using solvent-desorbed SPME for automated analysis. J Forensic Sci. 2003;48(1):41-46.
[Google Scholar] [PubMed]
- Hites RA, Biemann K. Computer evaluation of continuously scanned mass spectra of gas chromatographic effluents. Anal Chem. 1970;42:855-860.
- Huang C, Hsieh Y. Sample pretreatment of arson debris by supercritical fluid extraction. Huaxue. 1995;53(2):139-154.