Original Article
, Volume: 13( 3)Optimization of Polyhydroxyalkanoate Production by Recombinant E. coli Supplemented with Different Plant By-Products
Abstract
Polyhydroxyalkanoates (PHAs) are biodegradable polymers synthesized in cytoplasmic granules in bacteria, such as Cupriavidus necator (Ralstonia eutropha), and several other bacteria. PHAs accumulation occurs in response to stress conditions, i.e. under high carbon and low nitrogen (24:1 ratio). In this study, E. coli was genetically modified for PHA production in biofermentors. PHA was synthesized in bacteria transformed with the operon phbA/phbB/phbC. The bacteria were fed using a basal medium supplemented with three different plant by-products, potato tuber skin hydrolysate, corn hydrolysate, and banana juice supplement. The growth in biofermentor was monitored through the evaluation of consumption of sugars and quantification of PHA synthesis. A microarray scanner was used to read fluorescence intensity of Nile Blue stained bacteria. PHA production by E. coli fed on a banana juice supplement outperformed all the other fermentation media, with highest amount of PHA per dry cell weight.
Keywords
Biodegradable ; Bacteria; E. coli; Hydrolysate
Introduction
Many bacterial species, such as the chemolytotrophs Cupriavidus necator (Ralstonia eutropha), Cupriavidus metallidurans and Alcaligenes latus [1-9], Pseudomonas putida, P. aeruginosa [10-14], P. pseudoflava [15] and other Pseudomonas spp [16], Azotobacter vinelandii [17], Halomonas campisalis [18], Enterobacter spp. [19], Thermus thermophilus [20]; Bacillus subtilis [21,22] and Bacillus cereus [23] among others, can synthesise and accumulate and store polyhydroxyalkanoates (PHAs) in intracellular organelles [24], co-composed of hydroxypropionate, hydroxybutyrate and hydroxyvalerate, depending on the availability of precursors (such as octanoic acid) [11,25-27] and intermediate compounds regulating the metabolic flux toward their synthesis, such as citrate. These are biodegradable, biocompatible and useful for production of bioplastics. PHAs based bioplastics are easily moulded, possess good mechanical properties and are components of bottles and medical devices. PHAs are produced by bacteria in presence of nitrogen limitation [28], converting the carbon sources into energy storing . In the production of PHA in biofermentors, one-stage culture [29,30], two-stage batch culture [9,17,29], fed-batch [1,16,31-33] high-cell density cultures [1,34-37] and mixed cultures [38-40], as well as submerged and solid state fermentation processes [41] have shown potential to be exploited as production methods [42].
Carbohydrate stocks may be injected in continuous into the fermentors or supplied at determined time points (fed-batch), to provide the substrates for PHA production [43-46]. Bacterial cells respond to environmental stress (pressure, N or P limitation) by increasing the synthesis of PHAs [10,47].
PHA has been also produced in E. coli [48], using genetic engineering [44,45,47,49-57] and through bacterial cell factories [58]. The genetically modified E. coli system overcomes the nitrogen limitation problem [38,46,59,60] and the need to change the composition of the growth medium. Other bacterial species have been used for biotechnology application and gene engineering, such as Aeromonas hydrophila [61] Halomonas spp. [62], mutants of Pseudomonas putida [63], P. aeruginosa [13], and Bacillus spp. [64], or using other biotechnological systems [65].
Recombinant E. coli producing PHA grow rapidly, accumulate PHA for about 50% of dry weight [38,66-69] and are able to exploit various carbohydrates and intermediate compounds [53,57,70,71]. Several factors (i.e., type of feed, aeration conditions) influence the biomass growth rate and PHA production and molecular weight size. Several authors showed that PHA production using E. coli recombinant systems could be optimised by increasing the oxygen dissolved into the medium, for instance using high rate sparging and aeration [72].
PHAs costs may also vary depending on the type of application, since materials for drug delivery and medical device components have high value [73,74]. The main problem is the high cost of the feedstock, about 1 dollars/kg of PHA, in addition to the operational costs, the extraction solvents and purification costs. In the industrial production of PHAs, more than 50% of the costs are due to the carbohydrate substrates. Therefore, new methods to feed bacteria using plant by-products have been developed. Significant research was performed on agro–industrial even agro–waste streams as feedstock for fermentation. Researchers realized a high-productivity of PHA [75], and fermentation process using C1 carbon sources [76,77], sugarcane molasses [9,19,21,40,44,55,78,79], soy molasses [80], fruit pomace and agroindustrial byproducts [52,81], chicory roots [7, 50], whey, spent sulfite liquor [82], glycerol [53,54,57], grass press juice [36], animal fats and vegetable oils [14,83,3,16,8,13] microalgae [71] and lignocellulosic feedstock [26,34,35,38,53, 58,71,79 84,85].
There is need to reduce bioreactor costs by optimisation of fermentation conditions, and to optimize the PHA yield. The application of sensors in the biofermentors is mainly envisaged to monitor bacterial growth, the level of nutrients, and the effectiveness of PHA production. A typical bioreactor (i.e. BIOSTAT Q Bioreactor System) is provided with three basic sensors for the monitoring of physico-chemical parameters: a temperature probe, a sensor of pH, and a probe for oxygen tension.
Recently a metabolic/polymerization/macroscopic modelling system was developed, to assess process variables, and to control process operating variables (i.e., nutritional and aeration conditions) in order to optimize biomass production rate, PHA accumulation and molecular weight of PHA [86]. Various types of sensors can be used to determine bacterial concentration, able to quantify bacteria at high density. Biosensors for whole-cell bacterial detection have been recently described [69,87,88].
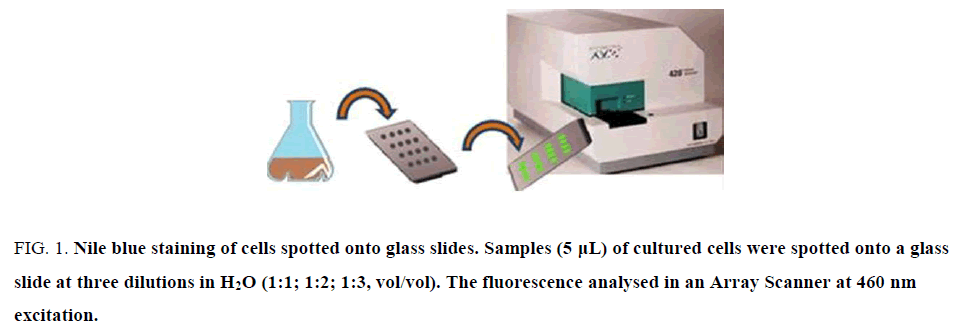
PHA production is a critical point in industrial fermentation, since keeping the process for the shortest time possible is economically advantageous. PHA screening methods for PHA determination in bacteria are based on Nile Red (λ excitation: 543 nm, λ emission: 560-710) [89] or Nile Blue staining [82,90,91]. Recently, highly sensitive and quantitative methods to read quantitatively the Nile Blue stained bacteria have been developed, based on fluorometry combined with a flow cell [2], and based on laser scanner (λ exc 460 nm/λ emiss 550 nm,) for fluorescence quantification of stained bacteria spotted on microarray slides [92].
In the project TRANSBIO of the EU commission [92] aimed to the production of PHA and organic acids from microorganisms, we tested the effectiveness of use of plant by-products in the reduction of costs of industrial fermentation and PHA recovery.
In industrial fermentation for production of PHA, three devices are envisaged for monitoring the following parameters: a) bacterial biomass determination, b) quantification of carbohydrates feedstock, c) monitoring of the level of PHA produced during time. A combination of these three parameters has been set up to optimize the use of bioreactors for: time of use (bacterial biomass), and maximum synthesis of PHA in fermentors at short operating time.
The growth of bacteria on three different plant by-products was monitored, i.e. potato enzymatic hydrolysate, corn enzymatic hydrolysate, and banana juice, a by-product of the infant food industry. The PHA production in fermentors was optimised in three days (after reaching the desired biomass) using the banana by-product feed. We monitored bacteria growth in biofermentors for media acidification, sugar consumption, bacterial cell density, and the timing and the amount of PHA synthesised: this parameter, together with the type of feed used, influences the economy of the process for the correlation between costs of instrumentation and running time of operations.
Material and Methods
All reagents were of high grade purity (Sigma-Aldrich, Merck Millipore, Darmstadt, Germany).
Microorganisms and plasmid preparation for E. coli transformation
A series of Ralstonia eutropha environmental isolates were selected for their ability to produce high amounts of PHA, and used as controls and comparison to establish the maximum yield of product under bioreactor conditions.
Cupriavidus necator (Ralstonia eutropha) was used to amplify phaCAB operon. Escherichia coli TOP10 chemically competent and Escherichia coli BL21 (DE3) chemically competent (Invitrogen) were used. Linear cloning pUC19 vector (Invitrogen, ThermoFisher, Waltham, MA, US) was used for cloning of the genes through homologous recombination. Expression vector pET24b characterized by strong hybrid T7/LacO promoter (Novagen, Merck Millipore, Darmstadt, Germany) was used for inducible phaCAB genes expression. DNA was extracted from an overnight cell culture of Cupriavidus necator ATCC 17699 using DNA Purification Kit (Promega, Fitchburg, WI, US).
After PCR amplification of phaCAB operon devoid of native promoter, the PCR product was purified using PureLink PCR Purification Kit (Invitrogen) and used for the homologous recombination by GENEART Seamless Cloning and Assembly Kit (Invitrogen). The 4100 bp amplified phaCAB operon devoid of its promoter was cloned in pUC19 vector by homologous recombination, as these primers shared terminal ends of linearized vector. Chemically competent E. coli TOP10 cells were transformed and plated in LB plates containing 100 μg/mL Ampicillin and 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-Gal) for blue/white screening. The recombinant pUC19/phaCAB vector (6700 bp) was extracted by PureLink Hipure Plasmid Miniprep kit (Invitrogen), digested by EcoRI and HindIII restriction enzymes (37°C for 2 hours) and purified fragment ligated in the pET24b vector (5310 bp), previously linearized by same restriction enzymes. E.coli BL21 (DE3) cells were transformed and plated in LB plates containing 50 μg/mL kanamycin. Colonies of E. coli expressing the recombinant vector were selected by detection of phaCAB gene by PCR after plasmid linearization. Several methods of transformation of E. coli have been described previously, but is is difficult to reproduce the same results obtained or the same yield of PHA produced.
Culture Media and Conditions
Recombinant E. coli BL21(DE3) harbouring heterologous phaCAB operon from Cupriavidus necator ATCC 17699 was cultivated at 30°C and 150 rpm in fed-batch conditions, both in 500 mL shake flasks with a starting volume of 150 mL and in 20 L Biostat bioreactor (Sartorius, Goettingen, Germany) with a starting volume of 15 L. Optical density (OD) was used to monitor the bacteria biomass, achieved by maintaining a 40% oxygen saturation with a constant flow of compressed air (2 vvm) and with a cascade control speed. pH was monitored automatically at 6.9 ± 0.1 through addition of a stock 15% v/v H2SO4 and NH4OH (20% v/v). The induction phase was conducted adding galactose 10 mM or lactose 30 mM at 25°C, pH 6.9 and air flow 3 L/min after 24 h, when the bacteria reached their stationary phase. Feeding solution was added at 4 mL/min for 48 h.
The fed-batch hydrolysate media containing Sweet Corn Enzymatic Hydrolysate 10%, Banana juice 5% or Potato Skin Enzymatic hydrolysate 25% were supplied from TRANSBIO Consortium [27]. The sugar content in Sweet Corn Enzymatic Hydrolysate, Potato Skin waste and Banana juice hydrolysate is shown in Table 1. A buffer Na2HPO4/Na2PO4 × H2O (pH 7) was used in the initial growth culture. To maintain plasmid stability, kanamycin (50μg/mL stock solution) was added 1:100 to the medium. A Trace Element Solution (TES) stock was prepared as follows (g/L): 10 FeSO4 × 7H2O, 2 CaCl2 × 2H2O, 2,2 ZnSO4 × 7H2O, 0,5 MnSO4 × 4H2O, 1 CuSO4 × 5H2O, 0,02 Na2B4O7 × 10H2O and 1% of TES solution was added to hydrolysate medium. The feeding solution consisted of only hydrolysate medium; without additional sugar added.
| Composition | Sweet Corn (g/L) | Potato skin (g/L) | Banana juice (g/L) |
|---|---|---|---|
| Glucose | 104 | 45 | 90 |
| Fructose | 10 | ≤5 | 82 |
| Maltose | ≤5 | 10 | nd |
| Sucrose | ≤5 | ≤5 | nd |
| Nitrogen | 3,6 | 4 | 6 |
| Citric acid | - | - | 3,5 |
| Magnesium | - | - | 0,3 |
| Phosphate | - | - | 0,25 |
Table 1: Composition of three enzymatic hydrolysates used in this study (values by TRANSBIO Consortium partners).
Quantification of Reducing Sugars
The carbohydrates content in the bacterial medium was daily measured by Sucrose, D-Fructose and D-Glucose kit (Megazyme, Wicklow, Ireland), through which NADH is quantified by its absorbance. This was made using microwell plates and spectrofluorometer reads at 340 nm in an Infinite 200 Pro instrument (Tecan, Männedorf, Switzerland). Glucose and fructose have been quantified every 12 h. The enzymatic reactions allowed to determine the levels of consumed sugars. Depending on sugar availability, the bacteria stopped producing PHA, therefore additional sugar stock solution was injected into the biofermentor. Based on the volumes to be added as sugar stock solution to sustain bacterial growth for 5 days, it was estimated that the optical density of cells before inducing them to synthesise PHA is 25 or higher.
The cell growth in shaking flasks and 20 L bioreactor was monitored by measuring the DO600nm of washed aliquots, using a spectrophotometer (Shimadzu, Kyoto, Japan).
Monitoring the Production of PHA by Nile Blue Staining and Fluorescence Quantification
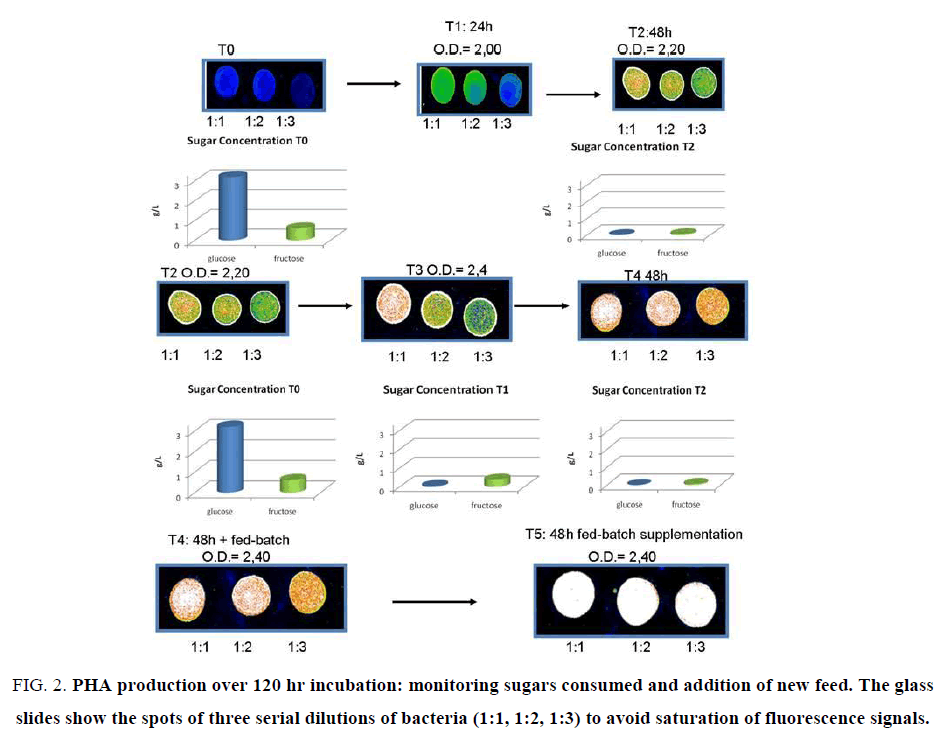
Determination of PHA based on Nile Blue involves several steps, fixing with alcohol or acetone, for dye permeability through the membrane, requiring few hours and transfer of bacteria from aliquot sample to eppendorf tubes. After induction of PHA synthase after 24 h of bacterial growth by the addition of lactose 30 mM or galactose 10 mM; production of was monitored daily by Nile blue staining. An aliquot of bacterial culture was washed twice with MilliR H2O and 5 μL were pipetted onto clean glass slide, air-dried, heat fixed and stained with a Nile blue solution for 10 minutes at 55°C. Three aliquots were done for each sampling, with two serial dilutions of the sample (1:2, 1:3). Then, the slide was washed and treated with 8% acetic acid for 30 seconds to remove the stain excess. The glass slide was washed, air-dried and analysed by Affymetrix 428 array Scanner, at excitation 460 nm/550 nm emission (Figure. 1). When the signal reached maximal fluorescence (white signal, Figure. 2), the fermentation was stopped, the suspension centrifuged and the pellet dried for PHA extraction.
Figure 2: PHA production over 120 hr incubation: monitoring sugars consumed and addition of new feed. The glass slides show the spots of three serial dilutions of bacteria (1:1, 1:2, 1:3) to avoid saturation of fluorescence signals.
PHA Extraction
To measure intracellular , direct cellular digestion in sulphuric acid was used. In particular, 50 μL of medium were added in 500 μL 96% sulphuric acid into a water bath at 95°C to 98°C for 30 min as PHA was converted to crotonic acid by heating in concentrated sulphuric acid. Spectrophotometric assay at 235 nm was conducted by spectrophotometer (Shimadzu). Pure polyhydroxybutyrate (PHB) (Sigma-Aldrich, Merck Millipore, Darmstadt, Germany) was used for calibration curve. The PHA concentration was defined as gram of polymer per litre of culture broth.
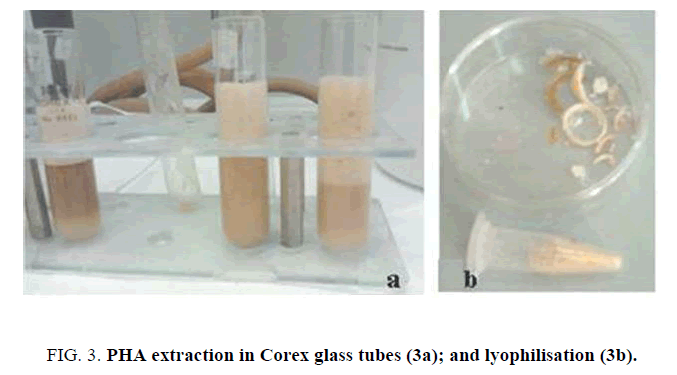
To extract PHA , cellular lysis was performed using enzymatic digestion. 50 μL lysozyme (50 mg/mL stock, added 1:100) were added and incubated for 1 h and 30 min followed by proteinase K (1 mg/ml stock addition (1:100), left for 3 hours. Then, the digested cellular material was transferred in a 30 ml Corex glass tube. Hot chloroform was added and samples were kept in a boiling water bath for 2 h (vortexing every 10 min) (Figure. 3a). For each mg of pellet, 12,5 μl of chloroform and 12,5 μl of 6% Na hypochlorite solution are added to the pellet and incubated at 37°C for 15 min. A centrifugation at 3000 g for 25 minutes was conducted to remove non-PHA cell material and to recover the chloroform lower phase, containing PHA . Finally, solid PHA was achieved by adding methanol (7:3 v/v of methanol and water) (5 volumes in respect to chloroform) and filtration (Figure. 3b).
Cell Dry Weight (CDW)
The Cell Dry Weight (CDW) of a sampled volume of culture broth (5 mL) or the entire fermentation was obtained by centrifugation at 8000 rpm for 5 minutes, and washed, followed by bacteria lyophilisation. The weight of the dry pellet was expressed in g/L.
Results
Biomass growth for PHA production
Recombinant E. coli BL21(DE3) cells, harbouring a heterologous phaCAB operon, were grown in scaling up experiments before reaching the biofermentor scale in 20 L bioreactors, using media containing plant carbohydrates, such as Sweet corn enzymatic hydrolysate 10%, Banana juice 5% and Potato skin enzymatic hydrolysate 25%. No glucose was added, and lactose was used as inducer of expression of PHB synthesis operon. After 24 h, when bacteria reached a high density, phaCAB operon was induced and expressed, under the strong hybrid T7/LacO promoter of pET system, by addiction of lactose or galactose (Figure. 2). Previously several authors used lactose as inducer.
Moreover, we tested the same hydrolysate media using a natural strain, Ralstonia taiwanensis. A limited basal expression in the first hours for the recombinant pET/CAB E.coli strains was present (green signal, Figure. 2b). After addition of lactose, the PHA synthesis was detected (white signal, Figure. 2d). To evaluate the polymer synthesis, 50 μL of medium were directly digested in 500 μL 96% sulphuric acid into hot water bath for 30 minutes; poly-β-hydroxybutyric acid was converted to crotonic acid by heating and spectrophotometric assay was conducted at 235 nm. After centrifugation to remove non-PHA cell material, PHA were recovered by filtration and methanol precipitation (Figure. 3b).
When testing the three different agro-industrial wastes as feed, the best result in bacterial growth and PHA production was observed with banana juice hydrolysate and the recombinant strain (Tables 2 and 3). The natural strain Ralstonia taiwanensis, usually good PHA producer when tested in two-stage batch production (nutrient medium followed by limiting medium), when grown exclusively in agro-industrial wastes showed very low yield (Table 2).
| Sweet Corn Enzymatic hydrolysate 10% | CDW (g l-1) | PHA (g l-1) |
|---|---|---|
| Recombinant E. coliBL21(DE3) EmptyE. coliBL21(DE3) Ralstoniataiwanensis |
8,2 7,9 4,3 |
1,7 / / |
| Potatoskin enzymatichydrolysate25% | CDW (g l-1) | PHA (g l-1) |
| Recombinant E. coliBL21(DE3) EmptyE. coliBL21(DE3) Ralstoniataiwanensis |
15,3 14,9 9,5 |
2,9 / 0,4 |
| Banana juice hydrolysate 5% | CDW (g l-1) | PHA (g l-1) |
| Recombinant E. coliBL21(DE3) E. coliBL21(DE3 without plasmid Ralstoniataiwanensis |
20,6 20,2 15,8 |
3,9 / 0,2 |
Table 2: Ralstonia spp. and E. coli PHA accumulation in three fed-batch hydrolysate media, after 72h. The reported values were means of triplicate experiments. Cell dry weight (CDW).
| PHA production in biofermentor | ||
|---|---|---|
| (g/L) | % DW | |
| Total Biomass | 3,15 | |
| PHA | 0,252 | 50% |
Table 3: PHA synthesised by E. coli fed with banana juice stock in biofermentors.
Although in the literature natural strains are reported as the best PHA producers, the results depend on the medium composition. While recombinant strains do not require carbon/nitrogen imbalance in the bacterial medium, PHA synthesis in natural strains depends on a precise C:N ratio. E. coli pET/CAB is able to produce PHA using plant by-product hydrolysate. Banana juice, 5% (v/v) of the final medium, particularly rich in glucose and fructose (89.80 ± 0.5 g/L and 82.24 ± 0.4g/L, respectively) and nitrogen (6 g/L); was shown to be an excellent medium for recombinant E. coli for PHA production using the optimised protocol and fermentors incubation for three days (Table 3).
To overcome the C:N imbalance, glucose syrup or molasses are used as carbon feed, with an increase of the production costs. Therefore new strains, mutants or engineered for PHA production, have been studied worldwide. The recent application of agricultural by-products, lignocellulosic biomass, oils and C1 carbons have widened the potential to produce PHAs at lower costs. In this study, three different by-products were tested to supplement the bacteria with carbohydrates. However, as shown in Table 4, the first two hydrolizates required higher energy costs, and enzymes for the breakdown of polysaccharides. Potato hydrolysate showed to contain the highest content of glucose, while fructose is probably required in the high synthesis of PHAs. Therfore, banana juice by-product affected the growth of E. coli due to a well-balanced content of reducing sugars (Table 4). An additional advantage in the use of banana juice is related to its citric acid content. In fact, the utilisation of the available sugars in the medium is divided into two steps. Acetyl-CoA, intermediate of the central carbon metabolism, is required for bacterial growth, but is used to produce PHA when the biomass has reached the maximum density. PHA is produced starting from acetyl-CoA, with phbA dependent conversion of two acetyl-CoA molecules into acetoacetyl-CoA; the presence of citrate inhibits the tricarboxylic acids (TCA) cycle, causing a shift of most of the acetyl-CoA generated in glycolytic pathway towards PHA synthesis.
| Inputs | Outputs | ||||||
|---|---|---|---|---|---|---|---|
| Pretreatedbyproduct | byproduct mass (g) | CH3COONa 0.05 M (ml) |
enzymes (ml) | Incubation time |
Energy (kWh) cutting, drying, milling |
Reducing sugar (g/L) | Glucose (g/L) |
| sweet-corn | 125 | 375 | 5.3 | 24h 50°C 600 rpm | 2.45 | 129-148 | 52.4-61.5 |
| endive | 105 | 330 | 4.4 | 48h 50°C 600 rpm | 7.3 | 107-119 | 32.1-47.6 |
| potato | 105 | 300 | 0.3 | 100°C+48h 70°C 600 rpm | 12.7 | 194-198 | 163.2-182.4 |
| Banana | 100 | - | - | - | 172 | 90 | |
Table 4: Requirement of energy and output of available reducing sugars for banana juice stock compared to other plant by-products. Enzymes mixture hydrolysis protocols and incubation times are unique for each reaction.
The pH was maintained stable in the range of 6.9 ± 0.1 by the addition of NH4OH (20% v/v). Another parameter that needs to be optimised for bacterial growth is the amount of oxygen in the medium. Previously, researchers used high aeration rate to promote cell growth in the first fermentation step, and lower aeration rate in the second stage, to promote PHA production [17]. However, various strategies were proposed to increase biomass and to produce PHA [29]. Based on fed-batch culture, drainage system in the bioreactor would be a good strategy as it removes the supernatant depleted of nutrient and it would concentrate the bacterial culture, reducing an excessive increase of the volume for a longer and more efficient feeding.
The advantages of using recombinant E. coli are: fast growth and high cell density cultures; high levels of PHA production. These factors are dependent on medium composition: a nutrient medium rich in carbon sources impacts PHA yield as well as production costs. Today, costs of production of PHA are around US$ 4-6/kg, including costs for polymer extraction and recovery [5,38]. PHA granules being intraorganellar, mechanical or chemical methods are required for cell disruption. W developed an extraction process based on enzymatic lysis of bacteria followed by repeated extractions, as described in material and methods.
The possibility to use plant byproducts such as sugar cane molasses as growth media made the use of naturally producing strains economically convenient [19,52]. Several authors used agroindustrial wastes for PHA production by adding them as supplementary carbon source in synthetic media such as LB or PCA, together with additional fructose, glucose or ammonium sulphate. In this work, we showed that optimization of cell biomass growth and PHA production may be achieved by implementing the sensing devices integrated with a biofermentor, using three sensing units able to quantify the sugar availability, the bacterial biomass, and the PHA production level. This allowed to individuate the critical sugar needs able to sustain bacterial PHA production, especially favoured by the presence of banana juice sugars.
The growth of bacteria on three different plant by-products was monitored, i.e. potato enzymatic hydrolysate, corn enzymatic hydrolysate, and banana juice, a by-product of the infant food industry. The PHA production in fermentors was optimised in three days of PHA synthesis induction (72 hr) using the banana by-product feed (Figure. 2). We monitored bacteria growth in biofermentors for sugar availability and level of PHA synthesis. The rapid consumption of sugars in the medium (either during exponential phase growth as well as during PHA synthesis induction) led to the need to fed-batch addition of increasing amounts of banana juice during the three day fermentation, until the staining of the slides showed the saturation of PHA signal (Figure. 2).
To further reduce the production costs and avoid the use of antibiotics for selection plasmid containing E. coli, it is envisaged in the future to integrate the recombinant system into E. coli chromosome. T7 promoter allows using lactose, a by-product from whey and dairy productions, as inducing agent for expression in recombinant E. coli [93].
Researchers previously integrated the phaCAB operon with a 5CPtacs promoter cluster into E. coli chromosome, to create a system of repetitive promoters for high and stable overexpression; the resulting engineered bacteria accumulated 23.7% PHA of the cell dry weight in batch fermentation [61]. Others produced pure (R)-3-hydroxybutyric acid (R3HB) from glucose, with a yield of 49.5% (85.6% of the maximum theoretical yield), by integration of the PHA biosynthesis genes into the chromosome of E. coli [94].
Interestingly, researchers produced poly (hydroxybutyrate-co-hydroxyvalerate) (PHBV) [95] inserting one copy of methylmalonyl-CoA mutase and methylmalonyl-CoA decarboxylase genes into the porin site in Halomonas TD08 2-methylcitrate synthase (prpCΔ) deletion mutant. They observed that active transcription start site and gene copy number made Halomonas TD08 better suited for chromosome engineering, compared with E. coli, for PHA production.
Recently, sensor detection was set up for in-line measurement of bio-based chemicals, using a Geobacter spp. microbial electrode for the determination of acetate [96]. The bacteria were shown to oxidize acetate making the electrode a terminal electron acceptor. When acetate is oxidized by the bacteria, a current signal is produced. The microbial electroactive films as sensing elements are promising sensor components. The organic acids that can be measured are propionate, butyrate, and other volatile fatty acids (VFA). Thus, in different settings and conditions, real-time inline monitoring of VFA concentration has been shown to be feasible [97,98].
Currently, VFA are measured using off-line, gas or liquid chromatography methods. In-line measurements may be achieved by integration of a sensor directly into the process. Electrochemical methods in general are a promising tool for real-time measurements, as the current or the measured potential give instantaneous signals. However, a sensor element is needed, selective for VFA. In fermentors, the determination of short chain fatty acids, for instance isovalerate, may be useful in the quantification of supplemented VFA for the synthesis of mixed-type PHA. In addition, advances in nutrient requirement of complex carbohydrates from plant by-products in recombinant E. coli cells producing poly-lactate-poly-butyrate in bioreactors, have been described [30].
Discussion
In this work, we showed the differential requirement of three plant byproducts by recombinant E. coli in biofermentors, as feed for producion of PHAs, as highly needed biobased biochemicals. The data of experiments comparing Ralstonia spp. and E. coli grown on three hydrolysate feeds, shown in Table 4, indicate that banana juice based feed was the best performing feed in PHA production by E. coli, and was also the best feed for Ralstonia spp. Among the by-products tested, banana juice showed to contain the nutrient required for PHA synthesis, supplying the carbohydrates and the intermediate carbons. The real time monitoring of the culture conditions allowed to optimize the growth of bacteria and the operation time for PHA synthesis, exploiting the full potential of bioreactors and minimising the operation costs. Recently, another publication reported on the feasibility to use banana by-products for PHA production [99] and for other processes such as biogas production, two thematic topics in the bio-economy era. Therefore, these efforts may lead to more affordable and less costly methods of PHA production based on suitable plant by-products and efficiently engineered bacteria [100-103].
The use of bacterial PHA producers and cheap agro-industrial residues have attracted commercial interest worldwide aiming to industrial sustainability, especially using cheap carbohydrates as feed. This study showed the feasibility of recombinant E. coli culturing in bioreactors for PHA synthesis, through the monitoring of sugars consumed, determination of PHA synthesis and quantification of PHA. The use of recombinant E. coli cells growing on banana juice by-products at the site of banana juice transformation, such as in Costa Rica, may lower the costs of fermentation for PHA production, and decrease the operational costs of the banana juice industry.
Compliance and Ethics
The authors declare there is no conflict of interest.
Acknowledgements
This work was supported by the European Commission, Framework Programme 7 (EU FP7) TRANSBIO project “Biotransformation of by-products from fruit and vegetable processing industry into valuable BIO products”, under grant 289603.
References
- Ryu HW, Hahn SK, Chang YK, et al. Production of poly(3-hydroxybutyrate) by high cell density fed-batch culture of Alcaligeneseutrophus with phosphate limitation. BiotechnolBioeng. 1997;55:28-32.
- Kacmar J, Carlson R, Balogh SJ, et al. Staining and quantification of poly-3-hydroxybutyrate in Saccharomyces cerevisiae and Cupriavidusnecator cell populations using automated flow cytometry. Cytometry, Part A. 2005;69A:27-35.
- Verlinden RAJ, Hill DJ, Kenward MA, et al. Production of polyhydroxyalkanoates from waste frying oil by Cupriavidusnecator. AMB Express. 2011;1:11.
- Park DH, Kim BS. Production of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-4-hydroxybutyrate) by Ralstoniaeutropha from soybean oil. New Biotechnol. 2011;28:719-24.
- Heinrich D, Madkour MH, Al-Ghamdi MA, et al. Large scale extraction of poly(3-hydroxybutyrate) from Ralstoniaeutropha H16 using sodium hypochlorite. AMB Express. 2012;2:59.
- Volova T, Kiselev E, Vinogradova O, et al. A glucose-utilizing strain, Cupriaviduseuthrophus B-10646: growth kinetics, characterization and synthesis of multicomponent PHAs. PLoS One. 2014;9(2):e87551.
- Haas C, Steinwandter V, Diaz De Apodaca E, et al. Production of PHB from chicory roots: Comparison of three Cupriavidusnecatorstrains. ChemBiochem Engineer Quarterly. 2015;29:99-112.
- Riedel SL, Jahns S, Koenig S, et al. Polyhydroxyalkanoates production with Ralstoniaeutropha from low quality waste animal fats. J Biotechnol. 2015;214:119-27.
- Singhaboot P, Kaewkannetra P. A higher in value biopolymer product of polyhydroxyalkanoates (PHAs) synthesized by Alcaligeneslatus in batch/repeated batch fermentation processes of sugar cane juice. Annals Microbiol. 2015;65;2081-9.
- Follonier S, Henes B, Panke S, et al. Putting cells under pressure: a simple and efficient way to enhance the productivity of medium-chain-length polyhydroxyalkanoate in processes with Pseudomonas putida KT2440. BiotechnolBioeng. 2012;109(2):451-61.
- Le Meur S, Zinn M, Egli T, et al. Production of medium-chain-length polyhydroxyalkanoates by sequential feeding of xylose and octanoic acid in engineered Pseudomonas putida KT2440. BMC Biotechnol. 2012;12:53.
- Agrawal T, Kotasthane AS, Kushwah R. Genotypic and phenotypic diversity of polyhydroxybutyrate (PHB) producing Pseudomonas putida isolates of Chhattisgarh region and assessment of its phosphate solubilizing ability. 3 Biotech. 2015;5(1):45-60.
- Abid S, Raza ZA, Hussain T. Production kinetics of polyhydroxyalkanoates by using Pseudomonas aeruginosa gamma ray mutant strain EBN-8 cultured on soybean oil. 3 Biotech. 2016;6(2):142.
- Allen AD, Anderson WA, Ayorinde FO, et al. Biosynthesis and characterization of copolymer poly(3HB-co-3HV) from saponifiedJatrophacurcas oil by Pseudomonas oleovorans. J IndMicrobiolBiotechnol. 2010;37(8):849-56.
- Dietrich D, Illman B, Crooks C. Differential sensitivity of polyhydroxyalkanoate producing bacteria to fermentation inhibitors and comparison of polyhydroxybutyrate production from Burkholderiacepacia and Pseudomonas pseudoflava. BMC Research Notes. 2013;6:1-4.
- Mozejko J, Wilke A, Przybylek G, et al. Mcl-PHAs produced by Pseudomonas sp. Gl01 using fed-batch cultivation with waste rapeseed oil as carbon source. J MicrobiolBiotechnol. 2012;22(3):371-7.
- Chen GQ, Page WJ. Production of poly-b-hydroxybutyrate by Azotobactervinelandii in a two-stage fermentation process. Biotechnol Tech. 1997;11:347-50.
- Kulkarni SO, Kanekar PP, Jog JP, et al. Characterisation of copolymer, poly (hydroxybutyrate-co-hydroxyvalerate) (PHB-co-PHV) produced by Halomonascampisalis (MCM B-1027), its biodegradability and potential application. Bioresource Technol. 2011;102(11):6625-8.
- Naheed N, Jamil N. Optimization of biodegradable plastic production on sugar cane molasses in Enterobacter sp. SEL2 Braz J Microbiol. 2014;45(2):417-26.
- Pantazaki AA, Papaneophytou CP, Pritsa AG, et al. Production of polyhydroxyalkanoates from whey by Thermusthermophilus HB8. Process Biochem. 2009;44:847-53.
- Singh G, Kumari A, Mittal A, et al. Poly ß-hydroxybutyrate production by Bacillus subtilis NG220 using sugar industry waste water. Biomed Res Int. 2013;952641.
- Bhagowati P, Pradhan S, Dash HR, et al. Production, optimization and characterization of polyhydroxybutyrate, a biodegradable plastic by Bacillus spp. BiosciBiotechnolBiochem. 2015;79(9):1454-63.
- Sharma P, Bajaj BK. Cost-effective-substrates for production of poly-ß-hydroxybutyrate by a newly isolated Bacillus cereus PS-10. J Environ Biol. 2015;36(6):1297-304.
- Uchino K, Saito T, Gebauer B, et al. Isolated poly(3-hydroxybutyrate) (PHB) granules are complex bacterial organelles catalyzing formation of PHB from acetyl coenzyme A (CoA) and degradation of PHB to acetyl-CoA. J. Bacteriol. 2007;189:8250-6.
- Dai Y, Lambert L, Yuan Z, et al. Characterisation of polyhydroxyalkanoate cowith controllable four-monomer composition. J Biotechnol. 2008;134:137-45.
- Peña C, Castillo T, García A, et al. Biotechnological strategies to improve production of microbial poly-(3-hydroxybutyrate): a review of recent research work. MicrobBiotechnol. 2014;7:278-93.
- Anjum A, Zuber M, Zia KM, et al. Microbial production of polyhydroxyalkanoates(PHAs) and its co: a review of recent advancements. Int J BiolMacromol. 2016;89:161-74.
- Guevara-Martínez M, SjöbergGällnö K, Sjöberg G, et al. Regulating the production of (R)-3-hydroxybutyrate in Escherichia coli by N or P limitation. Front. Microbiol.2015;6:844.
- El-sayed AA, Abdel Hafez AM, HemmatAbdelhady M, et al. (Production of Polyhydroxybutyrate (PHB) using batch and two-stage batch culture strategies. Aust J Basic Appl Sci. 2009;3:617-27.
- Choi SY, Park SJ, Kim WJ, et al. One-step fermentative production of poly(lactate-c-glycolate) from carbohydrates in Escherichia coli. Nature Biotechnol. 2016;34:435-40.
- Wang F, Lee SY. Production of poly ß(3-hydroxybutyrate) by fed-batch culture of filamentation-suppressed recombinant Escherichia coli. Appl Environ Microbiol. 1997;63:4765-9.
- Ahn J, Jang I, Lee D, et al. A comparison of lyophilized amniotic membrane with cryopreserved amniotic membrane for the reconstruction of rabbit corneal epithelium. Biotechnology and Bioprocess Engineering. 2005;10:262-9.
- Urtuvia V, Villegas P, González M, et al. Bacterial production of the biodegradable plastics polyhydroxyalkanoates. Int J BiolMacromol. 2014;70;208-13.
- Shiloach J, Fass R. Growing E. coli to high cell density: A historical perspective on method development. Biotechnology Advances. 2005;23:345-57.
- Ienczak JL, Schmidell W, De Aragão, G.M.F: High-cell-density culture strategies for polyhydroxyalkanoate production: A review. J Industrial MicrobiolBiotechnol. 2013;40:275-286.
- Cerrone F, Davis R, Kenny ST, et al. Use of a mannitol rich ensiled grass press juice (EGPJ) as a sole carbon source for polyhydroxyalkanoates (PHAs) production through high cell density cultivation. Bioresour Technol. 2015;191:45-52.
- Koller M, Braunegg G. Potential and prospects of continuous polyhydroxyalkanoate (PHA) production. Bioengineering. 2015;2:94-121.
- Wang F, Lee SY. High cell density culture of metabolically engineered Escherichia coli for the production of poly(3-hydroxybutyrate) in a defined medium. BiotechnolBioeng. 1998;58:325-8.
- Serafim LS, Lemos PC, Albuquerque MGE, et al. Strategies for PHA production by mixed cultures and renewable waste materials. ApplMicrobiolBiotechnol. 2008;81:615-28.
- Bengtsson S, Pisco AR, Johansson P, et al. Molecular weight and thermal properties of polyhydroxyalkanoates produced from fermented sugar molasses by open mixed cultures. J Biotechnol. 2010;147:172-9.
- Castilho LR, Mitchell DA, Freire DMG. Production of polyhydroxyalkanoates (PHAs) from waste materials and by-products by submerged and solid-state fermentation. Bioresour Technol. 2009;100:5996-6009.
- Chen GQ. A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. ChemSoc Rev. 2009;38(8):2434-46.
- Mahishi LH, Tripathi G, Rawal SK. Poly(3-hydroxybutyrate) (PHB) synthesis by recombinant Escherichia coli harbouring Streptomyces aureofaciens PHB biosynthesis genes: Effect of various carbon and nitrogen sources. Microbiol Res. 2003;158:19-27.
- Zhou XY, Yuan XX, Shi ZY, et al. Hyperproduction of poly(4-hydroxybutyrate) from glucose by recombinant Escherichia coli. Microb Cell Fact. 2012;11:54.
- Zhang Y, Lin Z, Liu Q, et al. Engineering of serine-deamination pathway, Entner-Doudoroff pathway and pyruvate dehydrogenase complex to improve poly(3-hydroxybutyrate) production in Escherichia coli. Microb Cell Fact. 2014;13:172.
- Lin Z,Zhang Y,Yuan Q, et al. Metabolic engineering of Escherichia coli for poly(3-hydroxybutyrate) production via threonine bypass. Microb Cell Fact. 2015;14:185.
- Guevara-Martínez M, Gällnö K, Sjöberg v, et al. Regulating the production of (R)-3-hydroxybutyrate in Escherichia coli by N or P limitation. 2015;6:844.
- Quillaguaman J, Doan-van T, Guzman H, et al. poly(3-hydroxybutyrate) production by Halomonas boliviensis in fed-batch culture. Appl Microbiol Biotechnol. 2008;78:227-32.
- Zhang H, Obias V, Gonyer K, et al. Production of polyhydroxyalkanoates in sucrose-utilizing recombinant Escherichia coli and Klebsiella strains. Appl Environ Microbiol. 1994;60:1198-1205.
- Ahn WS, Park SJ, Lee SY. Production of poly(3-hydroxybutyrate) by fed-batch culture of recombinant Escherichia coli with a highly concentrated whey solution. Appl Environ Microbiol. 2000;66:3624-7.
- Agus J, Kahar P, Abe H, et al. Molecular weight characterization of poly(R)-3-hydroxybutyrate synthesized by genetically engineered strains of Escherichia coli. PolymDegrad Stab. 2006;91:1138-46.
- Nikel PI, de Almeida A, Melillo EC, et al. New recombinant Escherichia coli strain tailored for the production of poly(3-hydroxybutyrate) from agro industrial by-products. Appl Environ Microbiol. 2006;72:3949-54.
- de Almeida A, Giordano AM, Nikel PI, et al. Effects of aeration on the synthesis of poly(3-hydroxybutyrate) from glycerol and glucose in recombinant Escherichia coli. Appl Environ Microbiol. 2010;76:2036-40.
- Andreeßen B, Lange AB, Robenek H, et al. Conversion of glycerol to poly (3-hydroxypropionate) in recombinant Escherichia coli. Appl Environ Microbiol. 2010;76:622-6.
- Saranya V, Shenbagarathai R. Production and characterization of pha from recombinant E. coli harbouring phac1 gene of indigenous Pseudomonas sp. ldc-5 using molasses. Braz J Microbiol. 2011;42:1109-18.
- Hiroe A, Tsuge K, Nomura CT, et al. Rearrangement of gene order in the phaCAB operon leads to effective production of ultrahigh-molecular-weight poly [(R)-3-hydroxybutyrate] in genetically engineered Escherichia coli. Appl Environ Microbiol. 2012;78:3177-84.
- Phithakrotchanakoon C, Champreda V, Aiba S, et al. Production of polyhydroxyalkanoates from crude glycerol using recombinant Escherichia coli. J Polym Environ. 2015;23:38-44.
- Kumar A, Srivastava JK, Mallick N, et al. Commercialization of bacterial cell factories for the sustainable production of polyhydroxyalkanoate thermoplastics: progress and prospects. Recent Pat Biotechnol. 2015;9:4-21.
- Li ZJ, Shi ZY, Jian J, et al. Production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) from unrelated carbon sources by metabolically engineered Escherichia coli.Metab Eng. 2010;12:352-9.
- Li M, Wang J, Geng Y, et al. A strategy of gene overexpression based on tandem repetitive promoters in Escherichia coli. Microb Cell Fact. 2012;11:19.
- Zhao W, Chen GQ. Production and characterization of terpolyester poly (3-hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxyhexanoate) by recombinant Aeromonashydrophila 4AK4 harboring genes phaAB. Process Biochem. 2007;42:1342-7.
- Tao W, Lv L, Chen GQ. Engineering Halomonas species TD01 for enhanced polyhydroxyalkanoates synthesis via CRISPRi. Microb Cell Fact. 2017;16(1):48.
- Mozejko-Ciesielska J, Dabrowska D, Szalewska-Palasz A, et al. Medium-chain-length polyhydroxyalkanoates synthesis by Pseudomonas putida KT2440 relA/spoT mutant: bioprocess characterization and transcriptome analysis. AMB Express. 2017;7(1):92.
- Mozejko-Ciesielska J. Proteomic analysis of Pseudomonas putida KT2440 during polyhydroxyalkanoates synthesis. New Biotechnology. 2016;33:s151.
- Suriyamongkol PF, Weselake R, Narine S, et al. Biotechnological approaches for the production of polyhydroxyalkanoates in microorganisms and plants. A review. Biotechnol Adv. 2007;25:148-75.
- Sreedevi A, Yonovitz A, Venkatesh A. Perceptual Consequences of Conductive Hearing Loss: Speech Perception in Indigenous Students Learning English as a School Language. 2008;30:1-18.
- Jacquel N, Lo CW, Wei YH, et al. Isolation and purification of bacterial poly(3-hydroxyalkanoates). Biochem Engineer J. 2008;39:15-27.
- Penloglou G, Chatzidoukas Ch, Parouti S,et al. Development of a comprehensive dynamic model for the fermentative production of poly(3-hydroxybutyrate) with tailor-made properties. 2010;150:548.
- Ahmed A, Rushworth JV, Hirst NA, Millner PA. Biosensors for whole-cell bacterial detection. ClinMicrobiol Rev. 2014;27:631-46.
- Lee SY. Poly(3-hydroxybutyrate) production from xylose by recombinant Escherichia coli. Bioprocess Eng. 1998;18:397-9.
- Rahman A, Putman RJ, Inan K, et al. Polyhydroxybutyrate production using wastewater microalgae based media. Algal Res. 2015;8:95-8.
- Inan K, Sal FA, Rahman A, et al. Microbubble assisted polyhydroxybutyrate production in Escherichia coli. BMC Res Notes. 2016;9:338.
- Chanprateep S. Current trends in biodegradable polyhydroxyalkanoates. J BiosciBioeng. 2010;110:621-32.
- Sreedevi S, Unni K, Sajith S, et al. Bioplastics: Advances in polyhydroxybutyrate research. In: Advances in Polymer Science. Springer, Berlin, Germany; 2015;1-30.
- Cesário MT, Raposo RS, de Almeida, et al. Enhanced bioproduction of poly-3-hydroxybutyrate from wheat straw lignocellulosic hydrolysates. New Biotechnol. 2014;31:104-13.
- Ishizaki A, Tanaka K, Taga N. Microbial production of poly-D-3-hydroxybutyrate from CO2. ApplMicrobiolBiotechnol. 2001;57:6-12.
- Khosravi-Darani K, Mokhtari ZB, Amai T, et al. Microbial production of poly(hydroxybutyrate) from C1 carbon sources. ApplMicrobiolBiotechnol. 2013;97:1407-24.
- Albuquerque MGE, Eiroa M, Torres C, et al. Strategies for the development of a side stream process for polyhydroxyalkanoates (PHA) production from sugarcane molasses. J Biotechnol. 2007;130:411-21.
- Getachew A, Woldesenbet F. Production of biodegradable plastic by polyhydroxybutyrate (PHB) accumulating bacteria using low cost agricultural waste material. BMC Res Notes. 2016;9(1):509.
- Solaiman DKY, Ashby RD, Hotchkiss AT, et al. Biosynthesis of medium-chain-length poly(hydroxyalkanoates) from soy molasses. BiotechnolLett. 2006;28:157-62.
- Follonier S, Goyder MS, Silvestri AC, et al. Fruit pomace and waste frying oil as sustainable resources for the bioproduction of medium-chain-length polyhydroxyalkanoates. Int J BiolMacromol. 2014;71:42-52.
- Weissgram M, Gstöttne RJ, Lorantfy B, et al. Generation of PHB from spent sulfite liquor using halophilic microorganisms. Microorganisms. 2015;3(2):268-89.
- Parker CT, Taylor D, Garrity GM. Exemplar Abstract for ThalassomonasagariperforansParketal. 2011 and Thalassotaleaagariperforans (Parketal. 2011) Zhang etal. 2014. The NamesforLife Abstracts. 2011.
- Jiang G, Hill DJ, Kowalczuk M, et al. Carbon sources for polyhydroxyalkanoates and an integrated biorefinery. Int J Mol Sci. 2016;17:1157.
- Penloglou G,Chatzidoukas C, Kiparissides C. Microbial production of polyhydroxybutyrate with tailor-made properties: an integrated modelling approach and experimental validation. Biotechnol Adv.2012;30(1):329-37.
- Zhao Y, Li S, Davidson A, et al. A MEMS viscometric sensor for continuous glucose monitoring. J. MicromechMicroeng. 2007;17:2528-37.
- Ghoshdastider U, Wu R, Trzaskowski B, et al. Nano-encapsulation of glucose oxidase dimer by graphene. RSC Advances 2015;5(18):13570-8.
- Elain A, Le Fellic M, Corre YM, et al. Rapid and qualitative fluorescence-based method for the assessment of PHA production in marine bacteria during batch culture. World J MicrobiolBiotechnol. 2015;31(10):1555-63.
- Spiekermann P, Rehm BHA, Kalscheuer R, et al. A sensitive, viable-colony staining method using Nile red for direct screening of bacteria that accumulate polyhydroxyalkanoic acids and other lipid storage compounds. Arch Microbiol. 1999;171:73-80.
- Oshiki M, Satoh H, Mino T. Rapid quantification of polyhydroxyalkanoates (PHA) concentration in activated sludge with the fluorescent dye Nile blue A. Water Sci Technol. 2011;64(3):747-53.
- TransBio. EU Framework Program FP7 project. Biotransformation of by-products from fruit and vegetable processing industry into valuable bioproducts. 2012.
- Lin LL, Hsu W. Lactose-induced expression of Bacillus sp. TS-23 amylase gene in E. coli regulated by a T7 promoter. LettApplMicrobiol. 1997;24(5):365-8.
- Lee Y, Lee K. Discrete-Time GeoX/G/1 Queue with Preemptive Repeat Different Priority. Queueing Systems. 2003;44:399-411.
- Yin J, Wang H, Fu XZ, et al. Effects of chromosomal gene copy number and locations on polyhydroxyalkanoate synthesis by Escherichia coli and Halomonas sp. ApplMicrobiolBiotechnol. 2015;99(13):5523-34.
- Kretzschmar J, Rosa LFM, Zosel J, et al. A microbial biosensor platform for inline quantification of acetate in anaerobic digestion: Potential and challenges.ChemEng Technol. 2016;39:637-42.
- Cui Y, Barford JP, Renneberg R. Determination of poly(3-hydroxybutyrate) using a combination of enzyme-based biosensor and alkaline hydrolysis. Anal Sci. 2006;22(10):1323-6.
- Koch K, Algar D, Searle JB. A voyage to Terra Australis: human-mediated dispersal of cats. BMC Evolutionary Biology. 2015;15:262.
- Naranjo JM, Cardona CA, Higuita JC. Use of residual banana for polyhydroxybutyrate (PHB) production: Case of study in an integrated biorefinery. Waste Management. 2014;34:2634-40.
- Kumar P, Patel SKS, Lee JK, et al. Extending the limits of Bacillus for novel biotechnological applications. Biotechnol Adv. 2013;31:1543-61.
- Leong YK, Show PL, Ooi CW, et al. Current trends in polyhydroxyalkanoates (PHAs) biosynthesis: Insights from the recombinant Escherichia coli. J Biotechnol. 2014;180:52-65.
- Ostle AG, Holt JG. Nile blue A as a fluorescent stain for poly ß-hydroxybutyrate. Appl Environ Microbiol. 1982;44:238-41.
- Singh M, Kumar P, Ray S, et al. Challenges and opportunities for customizing polyhydroxyalkanoates. Indian J Microbiol. 2015;55(3):235-49.
- Wang Y, Yin J, Chen GQ. Polyhydroxyalkanoates, challenges and opportunities. CurrOpinBiotechnol. 2014;30:59-65.