Research
, Volume: 15( 6) DOI: 10.37532/tsbt.2019.15(6).196Integrated Biodiesel and Biogas Production from Chlorella sorokiniana: Towards a Sustainable Closed-Loop through Residual Waste Biodegradation
- *Correspondence:
- Ahmed MA Mohamed
Department of Life Sciences and Chemistry, Jacobs University Bremen, Campus Ring 1, Bremen, Germany
E-Mail: dr.a.mohamed789@gmail.com
Received: December 04, 2019; Accepted: December 16, 2019; Published: December 26, 2019
Citation: Mohamed AMA, Salem OMA, Klock G, et al. Integrated Biodiesel and Biogas Production from Chlorella Sorokiniana: Towards a Sustainable Closed-Loop through Residual Waste Biodegradation. Biotechnol Ind J. 2019;15(6):196
Abstract
To date, more effort has been put towards biodiesel production using algal lipid, while less attention has been paid on biogas production. In this paper, we used the accumulated biomass of Chlorella sorokiniana cultivated under nitrogen stress in columns bioreactors with 120 L working volume to produce biodiesel from the extracted neutral lipids and biogas from the residual biomass. The accumulated neutral lipids were 14%, 17% and 21% for cultures containing 100%, 50% and 0.0% NaNO3 concentration respectively. The highest net accumulated methane yield was 315 ml g-1 volatile solids from the residual biomass after lipid extraction of the 50% NaNO3 culture. The fatty acid profile shows a significant increase of saturated fatty acids by 40.5% and 54.1% in nitrogen-deficient cultures 50% NaNO3 and 0.0% NaNO3 respectively and sharply decrease in poly-unsaturated fatty acids by 54.2% under complete deficiency of nitrogen (0.0% NaNO3). The percent of the produced biodiesel through trans esterification was 95% with high cetane value 61 and a high percentage of palmitic acid 37% which reflect high efficiency and stability of the produced biodiesel. The biomethane potential for microalgae (Chlorella sorokiniana) and macroalgae (Laminaria japonica) were 255 and 173 mL g-1 VS respectively under the same conditions.
Keywords
Biodiesel; Chlorella sorokiniana; Biogas; Algae; Biomethane
Introduction
Algae are the most promising biological feedstock to address future bioenergy challenges [1]. Third-generation biofuels, typically based on microalgae can overcome the drawbacks of first and second-generation biofuels and seems more productive and sustainable. Microalgae can be produced in non-arable land, furthermore can be grown in wastewater as their culture medium, reducing the use of freshwater and nutrients [2]. According to Oncel [3], Microalgae have faster growth rates than other crops and thus higher yields per unit area; the cornerstone in the bioenergy process from microalgae is biomass production which has a very wide range of yields.
Microalgal biomass productivity can vary highly, depending on species and the cultivation conditions. Recently [4], underlined the sturdy influence of the climate on microalgae productivity. A growth model was used to determine the current near-term lipid productivity potential of microalgae around the world and the maximum biomass yield of 13-15 gm-2d-1 was assumed. The high variability of the available published data makes it somewhat difficult to accurately compare the productivities per unit area between different algal species. Considering the theoretical potential and challenges that currently stand in the way of improving the economics towards a cost-competitive algae-based fuel scenario, there is a need to maximize the yield and composition of any given algae strain [1]. The potential of native algal species is enormous by virtue of their large metabolic and physiological plasticity and native diversity. Recent advances in metabolic engineering have opened up new opportunities to improve upon native algal properties such as productivity and oil content [5,6].
Several studies have reported increases in lipid contents by nitrogen or phosphorus starvation [7-9]. Nitrogen is one of the most important growth factors as it is a major component of biological macromolecules like DNA, chlorophyll, and protein [10]. Although continuous nitrogen starvation increases lipid and carbohydrate contents of microalgae, it decreases their growth rate, consequently reducing their overall productivities [11]. Nitrogen starvation is considered the most widely used effect to induce lipid accumulation; however, the optimum conditions to enhance lipid productivity will depend specifically on the strain, cultivation system and environmental conditions [12]. Neutral lipids which are almost in a form of TAGs are synthesized as a defense under unfavorable conditions to act as energy storage sources and can be easily detected as green droplets by fluorescence spectrophotometer using Nile red stain as reported by Ahmed MAM [13]. Microalgae under nutrient deprivation have the capability to accumulate large amounts (20%-50% dry weight) of triacylglycerides (TAGs) which are the main compounds for biodiesel production [14,15].
The extraction of lipids from algal biomass is a complex process in biodiesel production that can significantly increase production costs. Lipid extraction methods such as organic solvent extraction [9] or supercritical fluid extraction [16] require drying of the biomass. Dewatering is a complicated process that increases production costs and reduces algal biofuel profitability. Additionally, lipid recovery is not 100% efficient. In order to improve lipid recovery percentages, and benefit the subsequent anaerobic digestion processes, several pretreatments of the biomass have been developed. Pretreatments provoke cell disruption and liberate the lipids inside the cells. In order to reduce lipid extraction costs, pre-treatments on wet biomass are preferable, these include hydrothermal liquefaction [17,18], microwave-assisted extraction and enzymatic extraction [19], osmotic shock [20] oxidative stress [21], ultrasound-assisted extraction [19,22] and pulsed electric field technology [23].
Anaerobic digestion is a widely utilized process for the treatment of organic wastes that leads to the production of methane-rich biogas. This is a complex process in which specialized microorganisms (hydrolyzing, fermentative, acetogenic, homoacetogenic, sulfate-reducing and methanogenic archaea) decompose organic compounds in an oxygen-free environment [24]. The microbial consortia work together to decompose complex organic substances into simple and chemically-stable compounds, such as methane and carbon dioxide through a series of biochemical reactions, including hydrolysis, acidification, acetogenesis and methanogenesis [24-26]. Key variables that affect the performance of an anaerobic digestion process are substrate composition, water content, temperature, pH, alkalinity, organic loading rate and hydraulic retention time [25]. Biogas composition is impacted by both the substrate composition (which impacts methane and CO2 ratios) and pH (which regulates the speciation of the carbonate system and CO2 release) [27].
Hydrolysis is the first phase in the process of anaerobic digestion. Fermentative microorganisms are active in this phase, breaking down complex polymers (proteins, carbohydrates, and lipids) into soluble organic compounds (C10-C100, e.g. glucose, fatty acids, and amino acids). Due to the size of the polymers, fermentative bacteria cannot perform intracellular metabolism directly, and thus extracellular enzymes are used in the degradation [28].
Acidogenesis is the phase which is followed by hydrolysis. Fermentative bacteria also perform the acidogenesis, where they metabolize organic compounds from the hydrolysis. Fermentation products are volatile fatty acids such as acetate, propionate, and butyrate, but also alcohols, lactate, and CO2+H2 [29]. In the third phase (acetogenesis), intermediary compounds (fatty acids and alcohols, etc) resulting from acidogenesis are converted to acetate, H2 and CO2 by proton reducing acetogenic bacteria. The low partial pressure of H2 is favorable in this process [30].
Methanogenesis is the last phase of anaerobic digestion. Methanogenesis occurs mainly by two pathways: one is called acetotrophic where acetate is converted to CH4 by aceticlastic methanogens, the other is called hydrogenotrophic where H2 and CO2 are used to produce CH4 by CO2-reducing methanogens [30]. Every pretreatment method has its advantages and limitations, a combination of thermal and alkali treatment is the best-known method for enhancing the biodegradation of complex materials [31].
Maximizing production and reduction of inputs could be achieved through the development of an integrated system for biodiesel and biogas production, using anaerobic digestion that is because, besides biogas production, anaerobic digestion leads to the production of an effluent that can be used as fertilizer for algae cultivation, reducing the need of costly nutrients [32]. Thus, the integration of biodiesel and biogas production through anaerobic digestion of algae debris after lipid extraction is a promising way to significantly enhance methane production [33], while the recycling of nutrients from anaerobic digestion is a key step to make microalgal biodiesel production sustainable and reduce overall production costs [34]. In fact, Sialve et al. [35] concluded that coupling anaerobic digestion with biodiesel production is essential for microalgal fuels to be viable. Additionally, if biogas is used for the production of heat or electricity, CO2 will be available for algae cultivation while reducing production costs and the integrated system has the potential to reduce energy consumption and reduce up to 71% greenhouse gas emissions compared to petroleum fuel [34].
Materials and Methods
1. Chlorella sorokiniana isolation, identification and biomass production
Isolation, identification and biomass production under different nitrogen concentrations regimes (100%, 50%, and 0.0%) were done in column bioreactors with working capacity 120 L [13].
2. Biomass harvesting
Harvesting of microalgae biomass was done by centrifugation at 6000 rpm (SORVALL RC 5C PLUS, Germany) for 6 minutes to remove the water. Dewatered algae biomass subjected to air dry for 72 hours, afterward milled to a fine powder using a mortar and stored in dark bottles at 4°C in the refrigerator till further analysis.
3. Biodiesel production
3.1 Neutral lipid extraction: Extraction of neutral lipids was done by using Hexane (n-Hexane ROTIPURAN® ≥ 99, p.a., ACS) in Soxhlet (heating mantle LG2, Soxhlet WESTERN 100 ml BISTABII NS 45/40, cooling chamber LAUDA, ECO-LINE star edition RE104, extraction thimbles of inner diameter × height 33 × 94 mm, Macherey-Nagel, Germany) at temperature 65°C for 12 hours [36]. The hexane was evaporated using an evaporation pump for 20 min (rotavap, HEIDOLPH; Germany)
3.2 Fatty acid analysis: The fatty acids (FAs) profiles of algal neutral lipid through our study was determined using a Gas-Chromatography (GC) after converting FAs in the oil to Fatty Acid Methyl Esters (FAMEs) by dissolving 200 mg oil in 25 mL of solution (5% HCL in methanol), and then refluxed for two hours at 60ºC. The solution was diluted with 25 mL distilled water and extracted with successive portions of diethyl ether in separating funnel for several times. The extracted fatty acid methyl esters were washed several times with distillate water to free from acidity then dried under vacuum at 35°C [37]. Gas-Chromatography (GC) provided with a split automatic injector and silica capillary column DB-5 (length: 60 m; ID: 0.32 mm. Helium is used as carrier gas at a flow rate of 1 mL/min. The column was held at 150ºC for 1 min and ramped to 240ºC, at a rate of 30ºC/min, and held at 240ºC, for 30 min. Standards are used to give rise to well-individualized peaks that allow the identification of the fatty acids composition.
3.3 Trans-esterification of algal lipid: After lipid extraction, the trans esterification reaction is carried out using H2SO4 (98% concentration) acid as a catalyst (100% in relation to the mass of lipid). Add methanol at the ratio of methanol/lipid 30:1 (Volume, ml alc/weight g. lipid), Noting that half of the methanol volume is mixed with the lipid before adding the acid. The reaction is performed at 60ºC for 4 hours under constant stirring in a water bath under reflux. After complete reaction, the excess alcohol is removed by evaporation using a rotary evaporator. The hexane solvent is then added to the reaction mixture as a non-polar solvent. The mixture is transferred to a separating funnel and after settling, the mixture is partitioned into two distinct phases: a top hexane layer containing mostly fatty acid methyl ester FAME and a bottom layer containing the glycerol and pigment.
Finally, the hexane phase was collected in a pre-weighed flask and evaporated using a rotary evaporator. The fatty esters are weighed and tested for purity by G.C. with internal standard and optionally can be purified on a silica gel/Al2O3 column to obtain the pure FAME [38].
The reaction yield calculated according to the following formula:
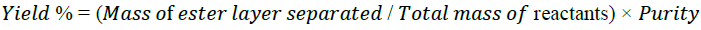 (1)
(1)
Where purity is a fraction of esters in the biodiesel layer obtained by GC analysis according to the method SRPS EN 14103. A standard mixture of methyl ester was used for qualitative analysis and methyl heptadecanoate (above 99%, Fluka) was used as the internal standard for quantification purposes [39].
The percentage of purity of obtained biodiesel was determined where; the fatty acid methyl ester content in the final product was calculated by taking the ratio of total peak areas of FAME to the peak area of the internal standard.
Cetane Number (CN) is widely used as a diesel fuel quality parameter related to the ignition delay time and combustion quality. Some equations correlate cetane number with the composition of biodiesel. In this case, it was necessary to use literature data for the fatty acids composition of the fuel used in testing the property equation [40]. In this work, the correlation formulated by Clements [41] was used for obtaining a good correlation between reported and predicted biodiesel cetane numbers using the following equation (2):
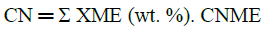 (2)
(2)
• Where CN, is the cetane number of biodiesel.
• XME is the weight percentage of each methyl ester
• CNME is a cetane number of individual methyl ester
4. Biogas production from biomass residue
4.1 Substrate and inoculums: Three types of Chlorella sorokiniana biomass were tested. The first substrate was the whole harvested biomass which grown in normal BG11 medium with normal NaNO3 concentration (control or untreated substrate). The other two substrates were the biomass residues after neutral lipid extraction (algal cake) of two different cultivation systems, which grow under 50% and 0.0% nitrogen limitation conditions (pretreated substrates).
Laminaria japonica (Lj)-Macroalgae waste from industry: The dried and shredded macroalgae biomass was obtained from Qingdao CoDo International Limited, China. Prior to experimental application the biomass was milled in a Grindomix GM 200 and sieved to a particle size of <0.5 cm.
Microcrystalline Cellulose (MCC) was used as a positive control in all batch setup.
Inoculum sludge: The inoculum sludge was obtained from the digester tower of the wastewater treatment plant in Farge, Lower Saxony, Germany (operation temperature: 35°C). In order to reduce the endogenous methane production by inoculum, the sludge was pre-incubated at 38 ± 0.2°C during one week prior to feeding. Hydraulic Retention Time (HRT) for each batch trial was equaled 21 days.
4.2 Total solids (TS) and Volatile solids (VS) determination
Total solids (TS): To determine the water content of the sludge and the substrate water is removed from the given sample. At a constant temperature of 105°C, the samples are dried for about 3-4 hours according to their amount of sampling substance to ensure complete water content removal. The percentage of TS is measured according to the sample’s wet or dry weight. By subtraction of the empty bowls’ weight and the weight after drying the net weight of the sample can be calculated.
Dry Matter [%] = (Final weight/Initial weight) × 100
Volatile solids (VS): VS measurements always succeed in the TS measurements. The previously dried sample contains only the remaining organic and inorganic matter. An annealing furnace is utilized to remove the organic matter from the sample (at 550°C temperature). The calcinated leftover in the bowl refers to the inorganic matter residual. By subtracting the weight of the calcinated residual from the TS weight of the sample the VS value can be calculated.
Organic Dry Matter (%) = 100 ˗ (V3-V1)/(V2-V1) × 100
V1=weight of the empty crucible (g)
V2=weight of dry residue and crucible (g)
V3=weight of ash and crucible (after cooling) (g)
The amount of VS (%) of the inoculum sludge should be <2% and the ratio of

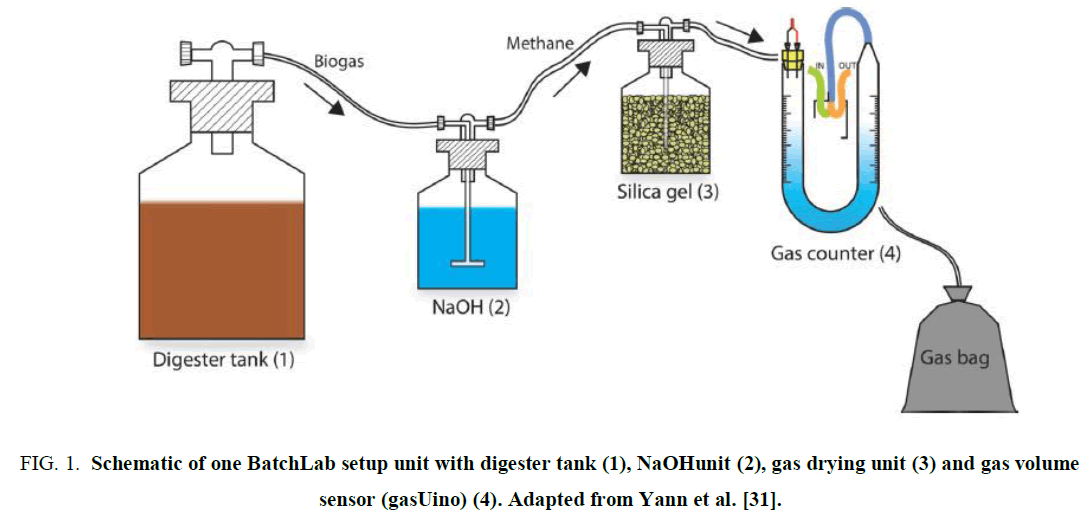
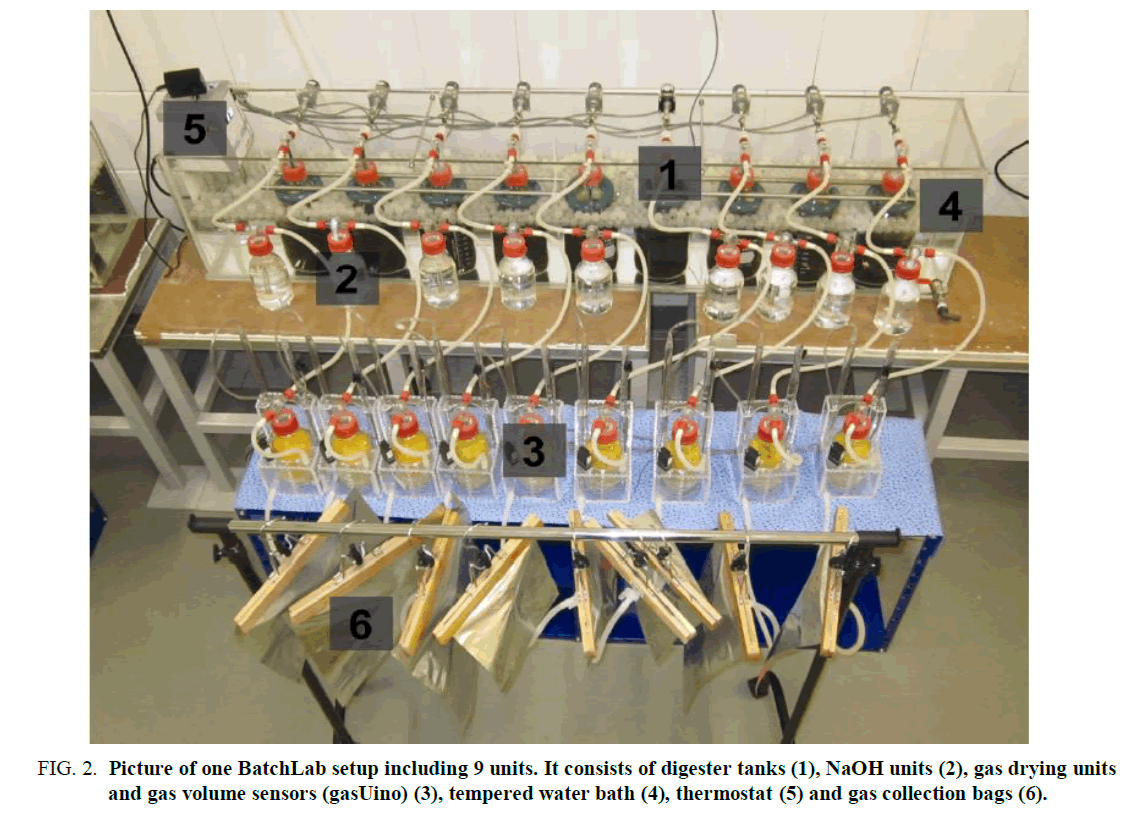
4.4 Batch-lab setup: The setup consists of nine single batch fermenter units. 2l flasks with a gastight gas scrubbing lid are utilized as fermenting chambers (F1). Each unit lid contains a gas exhaust. The flasks are residing in a water bath tempered by a thermostat (FIG. 1).
Figure 1. Schematic of one BatchLab setup unit with digester tank (1), NaOHunit (2), gas drying unit (3) and gas volume sensor (gasUino) (4). Adapted from Yann et al. [31].
To remove the vapor from the generated biogas the gas is conducted through a vapor-removing unit (flask containing silica granules, (Carl-Roth, granulate particle size 0.2 cm). A gas counter element determines the generated gas volume respectively for each digester and the gas is captured in gas-tight bags (F2). The values obtained from the gas counters are automatically recorded in given intervals (PC) (FIG. 2).
Figure 2. Picture of one BatchLab setup including 9 units. It consists of digester tanks (1), NaOH units (2), gas drying units and gas volume sensors (gasUino) (3), tempered water bath (4), thermostat (5) and gas collection bags (6).
Sodium hydroxide solution (3M NaOH) was used to capture CO2 from the generated biogas and thymolphtalein indicator (0.4%) was added to the NaOH solution to control the CO2 absorption capacity.
4.5 Gas counter (gasUino): The biomethane was measured using a gas flow meter according to the structure presented by [42]. The device is illustrated in FIG. 3. The gas meter consists of a U-shaped pipe, a magnetically controlled cross-valve for corrosive gases and a level sensor. The tube is filled with a conductive liquid barrier solution that allows transmission of signals and avoids gas leakage.’
Figure 3. Picture of gasUino gas counter with magnetic valve (1), U-shaped pipe (2), gas drying unit (3), detection electrodes (4), gas in- (5) and outlet (6). Courtesy of Harry Michael Falk.
Through liquid displacement, generate biomethane is detected, then triggers an electronic signal for each detection event which is finally recorded and subsequently processed. The gas counter was regularly calibrated on a weekly base to ensure a precise recording of methane production.
4.6 Database and web interface: Data acquisition and storage are based on standard technologies. A web application, developed with the PHP framework CodeIgniter is running on an Apache2 web server and MySQL as the relational database. For further details see [42].
4.7 Data validation: The experimental data gathered from the biogas potential experiment in the liquid displacement system was corrected to standard conditions at standard temperature (Ts=273.15 K) and standard pressure (Ps=1.013 bar) using the following equation [42]
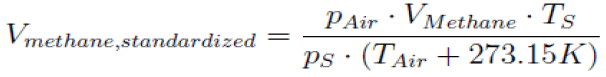
Values for ambient air pressure (PAir), temperature (TAir) and generated methane volume (VMethane) were recorded in a 3-minute interval. It was assumed that the generated methane stands environmental pressure and adopts the environmental temperature while being measured in the gas counter. The gas volume in the gas counter (VMethane) was defined by volume calibration and the information was implemented into the calculation.
Results and Discussion
1. Biomass production
As mentioned above Chlorella sorokiniana was cultivated in plastic bags photobioreactors with a working capacity 120 l in BG11 media with three different nitrogen concentrations 1.5 g, 0.75 g and 0.0 g NaNO3/L which represent 100%, 50% and 0.0% nitrogen. 6 columns were used for 2 successive cycles (4 columns for each concentration) with a working volume 10 l each, the amount of accumulated biomass shown in TABLE 1. For more details about the cultivation conditions and system setting, [13].
| Nitrogen concentration | 100% NaNO3 | 50% NaNO3 | 0.0% NaNO3 |
|---|---|---|---|
| Dry biomass in gram/40 L | 108 g | 92 g | 60 g |
| Dry biomass in gram/L | 2.7 g | 2.3 g | 1.5 g |
| Dry biomass in gram/L/day | 0.071 g | 0.06 g | 0.039 g |
| Calculated biomass in g/m3/day | 71 g | 60 g | 39 g |
TABLE 1. The amount of accumulated and calculated biomass for Chlorella sorokiniana grown under different sodium nitrate concentrations.
From our data, biomass production decreased by (14.8% and 44.4%) under 50% and complete nitrogen deficiency (0.0%) respectively as compared with the positive control (normal NaNO3 concentration 1.5 g/l). these results went parallel with our last date in [13] which depended on optical density measurements and cell count calculations and it’s also similar to Elsayed KNM et al., [43] who used the same cultivation conditions used in this work. On the other hand, in terms of biomass yield we still away from the maximum theoretical yields (196 gm-2d-1) which reported by Weyer KM et al. [44]. The differences between the theoretical case scenario and our date lie in the biomass accumulation efficiency (reflecting respiration and other metabolic losses), which is set at 100% in the theoretical case, and it is not like this in our case.
2. Lipid extraction
After biomass harvesting and drying, neutral lipids were extracted using the method mentioned above. The amount of neutral lipid was 14%, 17% and 21% for 100%, 50% and 0.0% NaNO3 cultures respectively. Hexanusedas extraction solvent because the main microalgal lipid material for biodiesel production is TAGs, which are non-polar and hence, more soluble in non-polar organic solvents [45,46]. Nitrogen replete cultures achieved the highest productivity of biomass (about 71 gm-3d-1) and lowest neutral lipid (about 14%), whereas nitrogen-starved cultures achieved the highest lipid productivity (17% and 21%) and lower biomass accumulation (60 and 39 gm-3d-1) for 50% and 0.0% NaNO3 cultures respectively. The increase in cellular lipid content with biomass degradation under nitrogen stress conditions has been reported by many scientists [9,10,13].
The percent of neutral lipids in all extracted biomass was below the normal range for Chlorella sp. even in nitrogen-free culture (21%) still very low as compared with literature (almost in range of 35%-40% for nitrogen stress cultures) and this may be explained by Ghasemi NF et al., [47] who reported that a successful extraction solvent for neutral lipids is one which can fully penetrate the biomass, making physical contact with the targeted lipids and subsequently dissolve it completely. This can be achieved by increasing the polarity of the solvent by mixing polar and non-polar solvents. This is due to the ability of the polar solvents to release the lipids from their protein-lipid complexes which facilitate their dissolving in the non-polar solvent [48].Cultivating Chlorellasorokiniana in photobioreactors using normal sunlight and fluctuated weather temperatures under different nitrogen stress conditions not only affected the amount of accumulated neutral lipid but it affected the quality of the lipids as well, TABLE 2. The most suitable biomasses for biodiesel production were those attained under nitrogen starved conditions, where saturated and monounsaturated fatty acids are largely induced [10].
| Fatty Acids | Mass Fraction% | Mass Fraction% | Mass Fraction% | |
|---|---|---|---|---|
| Control culture (100% NaNO3) | Stressed culture (50% NaNO3) | Stressed culture (0.0% NaNO3) | ||
| Lauric Acid | C12 | 2.2 | 2.2 | 2 |
| Myristic Acid | C14 | 5.2 | 3 | 4 |
| Palmitic Acid | C16 | 21.4 | 29.7 | 49.4 |
| Palmitoleic Acid | C16-1 | 3.9 | 4.1 | 5.3 |
| Margaric Acid | C17 | 6 | 2.7 | 3.2 |
| Heptadecanoic Acid | C17-1 | - | - | 3.9 |
| Stearic Acid | C18 | 6.2 | 20 | 4.6 |
| Oleic Acid | C18:1 | 30.1 | 13.3 | 16.2 |
| Linoleic A. | C18:2 | 11 | 22.3 | 8.8 |
| Linolenic A. | C18:3 | 13.9 | 2.7 | 2.6 |
| Saturated | F. A. SFA | 41 | 57.6 | 63.2 |
| Un-Saturated | F.A. USFA | 58.9 | 42.4 | 36.8 |
| Mono-Un-Saturated | F.A.MUSFA | 34 | 17.4 | 25.4 |
| Poly-Un-Saturated | F.A. PUSFA | 24.9 | 25 | 11.4 |
| Total | F.A. TFA | 99.9 | 100 | 100 |
TABLE 2. Fatty acids composition of Chlorella sorokiniana lipids cultivated in BG11 medium under different nitrogen concentrations.
The amount of saturated fatty acids increased by about 40.5% and 54.1% in nitrogen-deficient cultures 50% NaNO3 and 0.0% NaNO3 respectively and the amount of polyunsaturated fatty acids seems to be unaffected in 50% NaNO3 culture but it sharply decreased by about 54.2% under high deficiency of nitrogen (0.0% NaNO3 ). No clear effect of nitrogen stress on monounsaturated fatty acids has been reported in our study. Although the amount of mono-unsaturated fatty acid did not induce under nitrogen stress, we still have a considerable amount (25.4%) in the culture grown under complete deficiency of nitrogen and thus increases the sustainability of Chlorella sorokiniana oil for biodiesel production [10].
Algal lipids are rich in saturated and monounsaturated fatty acids that are desirable for biodiesel production [49]. The most commonly used fatty acids for biodiesel productions in relation to quality are containing C16:0 (which is about 50% of fatty acids mass fraction in 0.0% NaNO3 culture) and C18:1 (which is about 16.2% of fatty acids mass fraction in 0.0% NaNO3 culture) fatty acids [50]. According to the American Society for Testing and Materials (ASTM) D6751 and European EN 14214 standards, monosaturated fatty acids are given preferences for the production of good quality biodiesels [50]. Therefore, Chlorellasorokiniana, which is capable of providing more saturated fatty acids than unsaturated fatty acids and has a high amount of mono-unsaturated fatty acid (34% under normal nitrogen concentration), is a good option for biofuel production.
3. Characterization of biodiesel derived from algal oil
The fatty acid methyl ester (FAME) obtained through the acidic transesterification process has a dark-green color. It’s content in the final product was 73.3% with a purity percentage of about 92.7%. The GC-analysis of derived biodiesel revealed that the major proportion of FAME was from saturated and monounsaturated fatty acid methyl esters, palmitic acid (36.91%) and oleic acid (21.43%). The high percentage of Palmitic acid proves the high stability of such biodiesel [51,52]. The low percentage of fatty acids with carbon chains more than 18, means low viscosity of produced biodiesel [53].
The calculated Cetane number (CN) of biodiesel obtained according to equation (2) was equal to 61.4. It is a high cetane number which means a shorter time between the initiation of fuel injection and the ignition [54]. So, such biodiesel has better ignition quality with higher combustion efficiency.
4. Biogas production
In this section, biogas productivity or Biomethane Potential (BMP) for different substrates under different pretreatment processes were compared.
4.1 Theoretical methane potential for macro and microalgae: Production of biogas from microalgal biomass through anaerobic digestion (AD), is considered an alternative biochemical conversion pathway for the intact algal biomass to energy [55]. Thanks to microalgal biomass high energy content, they considered as an advantageous substrate for AD as compared with macroalgae and this clearly demonstrated in the TABLE 3.
| Biogas potential (%) | ||||||
|---|---|---|---|---|---|---|
| Component | Laminaria japonica/theo.CH4 unit [31] | Chlorella sorokiniana/theo.CH4 unit [57] | ||||
| Volatile solids | 50.90% | 83.58% | ||||
| Carbohydrate | 39.2% | (145 ml/g VS) | 10.28% | (38.02 ml/g VS) | ||
| Protein | 11.4% | ( 52 ml/g VS) | 45.4% | (207.08 ml/g VS) | ||
| Lipid | 0.3% | (2.5 ml/g VS) | 27.9% | (232.5 ml/g VS) | ||
| Inorganic solids | 49.10% | 16.42% | ||||
| Total | 100% | (199 ml/g VS ) | 100% | (477 ml/g VS) | ||
TABLE 3. Composition and theoretical methane potential of Laminaria japonica and Chlorella sorokiniana.
To estimate the possible methane output from Laminaria biomass in AD, firstly a theoretical calculation has been done based on methane potential values from carbohydrate, protein and lipid fractions of the substrate as proposed by Kleemann M et al., [56] and stated in the LfL Biogas Handbook, 2007. To obtain a realistic theoretical value, 7% of the maximum potential was subtracted to account for the energy consumed for biomass generation as suggested by the VDI 4630 guideline manual (2006). Organic composition for Laminaria japonica was taken from Yann B [31] and for Chlorella sorokiniana from Kobayashi N et al. [57]
From the table, we can conclude that theoretically microalgae have more biomethane potential than macroalgae and this owing to the big different of biomass composition and the high lipid and protein content in microalgae which have high biogas potential than carbohydrates which is a characteristic feature for macroalgae. High lipid contents in the biomass can be advantageous because the theoretical biogas yield from lipids is generally higher (1390 l/kg VS) than proteins (800 l/ kg VS) or carbohydrates (746 l/kg VS) (VDI 4630 2006)
4.2 Biomethane potential (BMP) for untreated Chlorella sorokiniana and Laminaria japonica:
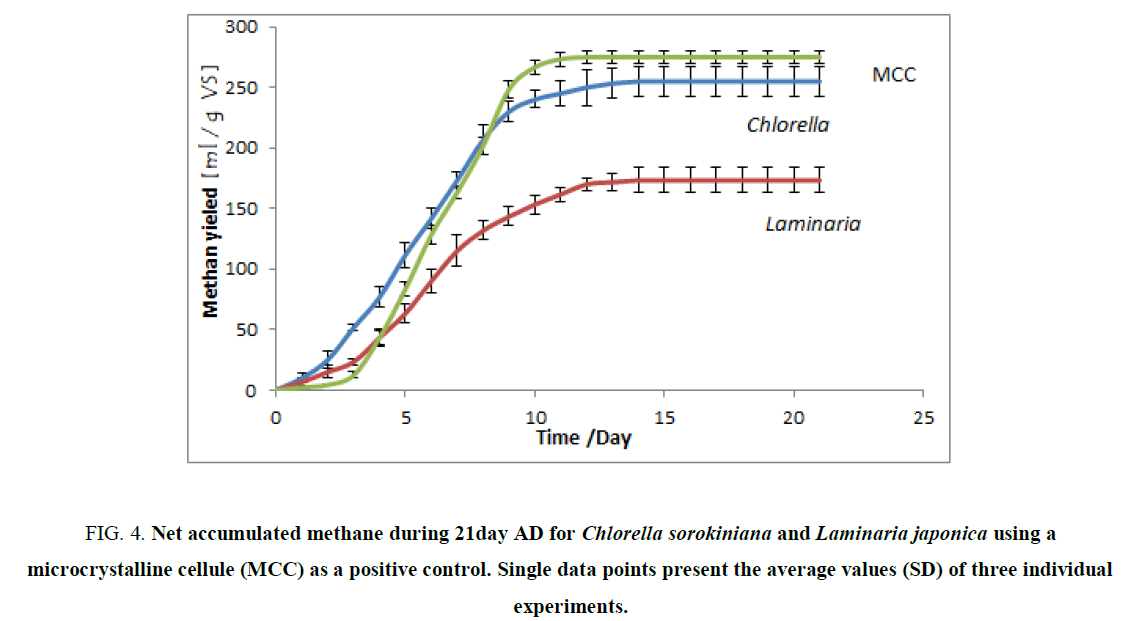
FIG. 4 shows the specific CH4 production (mLg-1 VS) for MCC, Chlorella sorokiniana and Laminaria japonica over a 21-day period with a final CH4 yield 275, 255- and 173-mLg-1 VS respectively. The BMP curve for MCC expresses a sigmoidal form (start-up (lag) phase, a subsequent exponential phase and a final stationary phase). This lag phase not encountered in the other two substrates, and this was probably due to prior hydrolysis of the substrates and the characteristics of the microbial community, which was adapted for the digestion of the given substrates but not for the digestion of MCC. The majority of the biogas was produced in the first five to seven days; the production rates during the last two weeks of the experiment remained low as illustrated by a plateau of the cumulative curve in FIG. 5 for all experimental lines. A similar biogas production pattern has also been reported by Zhang R et al. [58]. Theoretical BMP for both Laminaria japonica and Chlorella sorokiniana (100% degradation efficiency) were 199 ml/g TS (390 ml/g VS) and 477 ml/g TS (570 ml/g VS) respectively, while the total real methane yield was 173 ml/g VS and 255 ml/g VS which mean that the total degradation efficiency of the process was 51% and 44% respectively. The reason for decreased degradation efficiency could be related to the composition or molecular structure of the biomass and the subsequent challenges for microorganisms to process it [31].
Figure 4. Net accumulated methane during 21day AD for Chlorella sorokiniana and Laminaria japonica using a microcrystalline cellule (MCC) as a positive control. Single data points present the average values (SD) of three individual experiments.
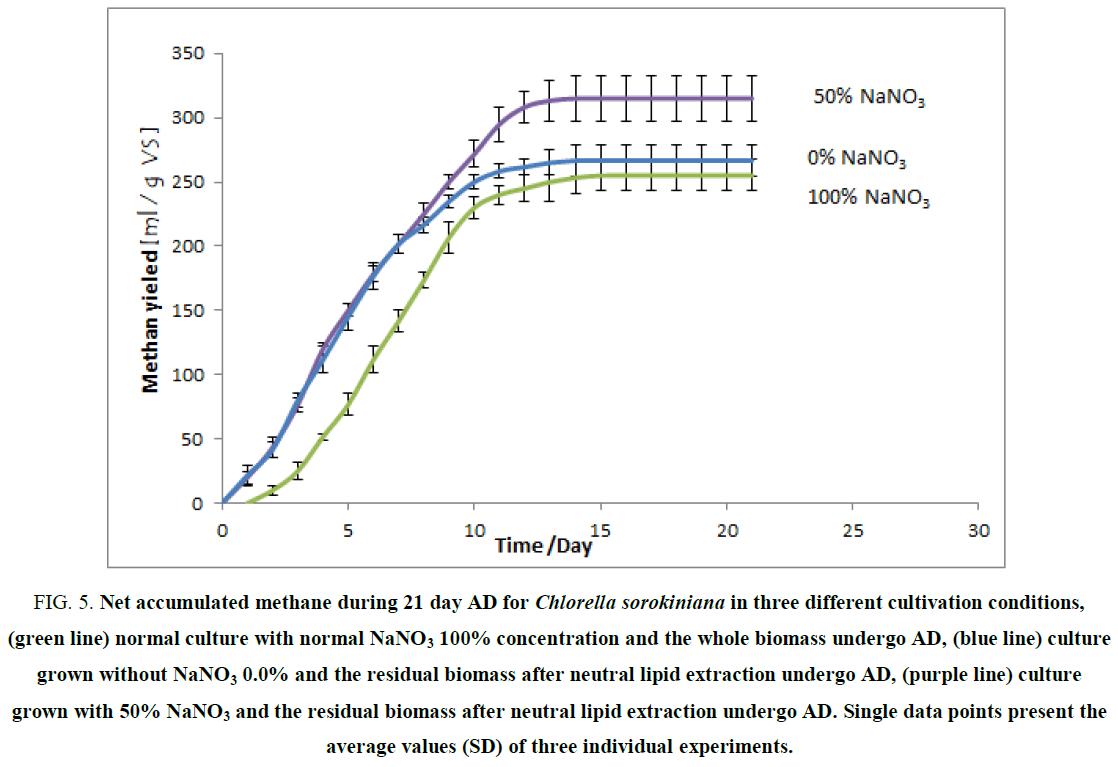
Figure 5. Net accumulated methane during 21 day AD for Chlorella sorokiniana in three different cultivation conditions, (green line) normal culture with normal NaNO3 100% concentration and the whole biomass undergo AD, (blue line) culture grown without NaNO3 0.0% and the residual biomass after neutral lipid extraction undergo AD, (purple line) culture grown with 50% NaNO3 and the residual biomass after neutral lipid extraction undergo AD. Single data points present the average values (SD) of three individual experiments.
4.3 Biomethane potential for treated Chlorella sorokiniana
To increase or enhance the degradation efficiency of the AD, pretreatment of the used substrate should undergo as some microalgae like Chlorella pyrenoidosa contain a thick cell wall (0.1 μm to 0.3 μm) which can make anaerobic digestion quite challenging [59]. Among the different types of pretreatment, thermal pretreatment is considered efficient in the case of microalgae as reported by Schwede S et al. [43,60].
FIG. 5 shows the methane yield of three different Chlorella sorokiniana substrates, whole biomass obtained from culture grown under normal conditions of NaNO3 (1.5 g/L) which have net methane yield about 255 ml/g VS, biomass residues after lipid extraction (biomass without neutral lipid) for culture grown under 50% NaNO3 (0.75 g/L) which have net methane yield about 315 ml/g VS, biomass residues after lipid extraction (biomass without neutral lipid) for culture grown under 0.0% NaNO3 (0.0 g/L) which have net methane yield about 280 ml/g VS.
According to the theoretical calculations of BMP for these three substrates, the normal biomass should have the highest value because it contains more total solid (whole organic components are present ie, no extracted substance), but the real value was the lowest one. This may be due to the presence of the cell wall which makes some of the total solids not free to proceed AD [43]. On the other hand, the other two substrates have undergone lipid extraction (FIG. 5) which considered as thermal pre-treatment and cell wall might be completely damaged by the effect of high temperature, thus most of the remaining total solids (whole proteins, whole carbohydrates, and the remaining lipids) were available for AD [43]. BMP of 0.0% substrate (280 ml/g VS) was lower than 50% substrate (315 ml/g VS) because the cultivated biomass under complete nitrogen depletion (0.0% NaNO3) accumulated more neutral lipids (21%) which already extracted and thus it Los high percent from the most available component (lipids) for biomethane potential during the AD process.
Conclusion
Integrating biodiesel and biogas production from microalgae biomass is a promising alternative for reducing costly inputs and energy-intensive processes, raising the overall efficiency and the cost-effectiveness of biofuel generation technologies. Using microalgal residual after lipid extraction as a proteins and carbohydrates rich substrate for anaerobic digestion will maximize the generation of energy through biomass transformation, while significantly reducing production costs. The by-products of biodiesel and biogas production are valuable inputs as fertilizers and for algae cultivation, which substantially minimizes economic and energy demands. In order to optimize the process, it is important to use process-oriented screening to choose species with high lipid and protein contents, thin cell walls that facilitate lipid extraction and algae biodegradability, as well as gravity-assisted settling abilities to reduce the harvesting cost. Using moderate doses of nitrogen starvation enables the selected strains to accumulate moderate amounts of biomass which rich in neutral lipids which intern enable us to extract the accumulated oils for biodiesel and the algal cake for biogas production.
Acknowledgments
The authors would like to gratefully thank the Egyptian Ministry of Higher Education for giving us the fund for undergoing this project. We would like also to thank prof. AnjaNoka, VerenaSiebecke (University of Applied Sciences Bremen) YannBarbot (Jacobs University) for their help along with the whole project.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- IEA Bioenergy, State of Technology Review-Algae Bioenergy, 2017.
- Correa DF, Beyer HL, Possingham HP, et al. Biodiversity impacts of bioenergy production: Microalgae vs. first generation biofuels. Renew Sustain Energy Rev. 2017;74:1131-46.
- Oncel SS. Microalgae for a macroenergy world. Renewable and Sustainable Energy Reviews. 2013;26:241-64.
- Moody JW, Mcginty CM, Quinn JC. Global evaluation of biofuel potential from microalgae. Proceedings of the National Academie of Science of the United States of America. 2014;111(23):8691-96.
- Radakovits R, Jinkerson RE, Darzins A, et al. Genetic engineering of algae for enhanced biofuel production. Eukaryot Cell. 2010;9(4):486-501.
- Gimpel JA, Henríquez V, Mayfield SP. In metabolic engineering of eukaryotic microalgae: Potential and challenges come with great diversity. Front Microbiol. 2015;6:1376.
- Zhila NO, Kalacheva GS, Volova TG. Influence of nitrogen deficiency on biochemical composition of the green alga Botryococcus. J Appl Phycol. 2005;17(4):309-15.
- Khozin-Goldberg I, Cohen Z. The effect of phosphate starvation on the lipid and fatty acid composition of the fresh water eustigmatophyte monodus subterraneus. Phytochemistry. 2006;67(7):696-701.
- Olfat MAS, Ola E, Abd El-Rahman MA. Effect of nitrogen and phosphorus concentrations in growth medium and Salt stress on growth, lipid content, and biodiesel producing ability of microalgae, Egypt J Bot. 3rd International conf.17-18 Aprl, Helwan Univ. 2013:219-33.
- Rodolfi L, Biondi N, Guccione A, et al. Oil and eicosapentaenoic acid production by the diatom phaeodactylumtricornutum cultivated outdoors in green wall panel (GWPW) reactors. Biotechnol Bioeng. 2017;114(10):2204-10.
- Pancha I, Chokshi K, George B, et al. Nitrogen stress-triggered biochemical and morphological changes in the microalgae Scenedesmussp. Bioresour Technol. 2014;156:146-54.
- Sharma KK, Schuhmann H, Schenk PM. High lipid induction in microalgae for biodiesel production. Energies. 2012;5(5):1532-53.
- Ahmed MAM, Khaled NME, Noke A, et al. Detecting the effect of nitrogen stress on Chlorella sorokiniana growing in column bioreactors using PAM fluorometer for further biofuel applications. Res J Fish Hydrobiol. 2017;2(1):21-31.
- Scott SA, Davey MP, Dennis JS, et al. Biodiesel from algae: challenges and prospects. Curr Opin Biotechnol. 2010;21(3):277-86.
- Hu Q, Sommerfeld M, Jarvis E, et al. Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J. 2008;54(4):621-39.
- Halim R, Gladman B, Danquah MK, et al. Oil extraction from microalgae for biodiesel production. Bioresource Technol. 2011;102(1):178-85.
- Gouveia L, Nobre BP, Marcelo FM, et al. Functional food oil colored by pigments extracted from microalgae with supercritical CO2. Food Chem. 2007;101(2):717-23.
- Lee JY, Yoo C, Jun SY, et al. Comparison of several methods for effective lipid extraction from microalgae. Bioresource Technol. 2010;101(1):75-77.
- Chisti Y, Moo-Young M. Review: Disruption of microbial cells for intracellular products. Enzyme Microb Technol. 1986;8:194-204.
- Yoo G, Park WK, Kim C, et al. Direct lipid extraction from wet Chlamydomonas reinhardtii biomass using osmotic shock. Bioresour Technol. 2012;123:717-22.
- Yilancioglu K, Cokol M, Pastirmaci I, et al. Oxidative stress is a mediator for increased lipid accumulation in a newly isolated Dunaliella salina strain. PloS one. 2014;9(3):e91957.
- Natarajan R, Chen X, Lau R. Ultrasound applications in lipid extractions from microalgae in ultrasound-enhanced biogas production from different substrates. Springer, Netherlands. 2014:117-39
- Kempkes Michael. Pulsed electric fields for algal extraction and predator control. In: Handbook of electroporation, Springer International Publishing. 2016.
- Horn SJ. Bioenergy from brown seaweeds. Ph.D. Thesis, Norwegian University of Science and Technology (NTNU), Norway, 2000:83
- HorriganL, Lawrence RS, Walker P. How sustainable agriculture can address the environmental and human health harms of industrial agriculture. Environ Health Perspect. 2002;110(5):445-56.
- Ampts II-automatic methane potential test system, operation and maintenance manual, Bioprocess Control Sweden AB; http://www.bioprocesscontrol.com/media/45898/Manual-AMPTS-II-version-1.6.pdf
- Howard JJ. Removal of sea lettuce, Ulva spp., in estuaries to improve the environments for invertebrates, fish, wading birds, and eelgrass, Zostera marina. Mar Fish Rev. 2005;67(4):1-8.
- Kaseng K, IbrahimK, Paneerselvam SV, et al. Extracellular enzyme and acidogen profiles of a saboratory-scale two-phase anaerobic digestion system. Process Biochemistry. 1992;27(1):43-47.
- Gujer W, Zehnder A. Conversion processes in anaerobic digestion. Water Sci Technol. 1983;15(8-9):127-67.
- Zinder SH, Koch M. Non aceticlastic methanogenesis from acetate: acetate oxidation by a thermophilic syntrophic coculture. Arch Microbiol. 1984;138(3):263-72.
- Yann B. Biogas from marine macoalgal waste. Ph.D. Thesis. 2014.
- Li Y, Horsman M, Wu N, et al. Biofuels from microalgae. Biotechnol Prog. 2008;24(4):815-20.
- Fouilland E, Vasseur C, Leboulanger C, et al. Coupling algal biomass production and anaerobic digestion: production assessment of some native temperate and tropical microalgae. Biomass Bioener. 2014;70:564-69.
- Dębowski M, Szwaja S, Zieliński M. The Influence of Anaerobic Digestion Effluents (ADEs) used as the nutrient sources for chlorella sp. cultivation on fermentative biogas production waste biomass valor. Waste Biomass Valor. 2017;8:1153-61.
- Sialve B, Bernet N, Bernard O. Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable. Biotechnol Adv. 2009;27(4):409-16.
- Zonouzi A, Auli M, Dakheli MJ,et al.Oil Extraction from microalgae dunaliella sp. by polar and non-polar solvents. Int J Biol Biomolecul Agr Food Biotechnol Eng. 2016;10(10):642-45.
- Pruvost J, Vooren VG, Cogne G et al. Investigation of biomass and lipids production with Neochlorisoleo abundans in a photobioreactor. Bioresour Technol. 2009;100(23):5988-95.
- Abdo SM, Abo El-Enin SA, El-Khatib KM, et al. Preliminary economic assessment of biofuel production from microalgae. Renewable Sustainable Ener Rev. 2016;55:1147-53
- Vujicic DJ, Comic D, ZarubicaA, et al. Kinetics of biodiesel synthesis from sunflower oil over CaO heterogeneous catalyst. Fuel. 2010; 89(8):2054-61.
- Mittelbach M, Remschmidt C. Biodiesel: The comprehensive handbook. Boerse druck Ges. M.B.H., Vienna. 2004.
- Clements LD. Blending rules for formulating biodiesel fuel, Liquid fuel industrial products from renewable resources. In: Proceedings of the Liquid Fuel Conference, 3rd, Nashville. 1996;15:1744-53.
- Falk H. Monitoring the anaerobic digestion process, Ph.D. Thesis. Jacobs Univ, 2011.
- Elsayed KNM, KolesnikovaTA, Noke A, et al. Imaging the accumulated intracellular microalgal lipids as a response to temperature stress. Biotech. 2017;7(1):41.
- Weyer KM, Bush DR, DarzinsA, et al. Theoretical maximum algal oil production. Bioenergy Res. 2009;3(2):204-13.
- Levine RB, Pinnarat T, Savage PE. Biodiesel production from wet algal biomass through in situ lipid hydrolysis and supercritical trans esterification. Energy Fuels. 2010;24(9):5235-43.
- Chen M, Liu T, Chen X, et al. Subcritical co-solvents extraction of lipid from wet microalgae pastes of Nannochloropsis sp. Eur J Lipid Sci Technol. 2012;114(2):205-12
- Ghasemi NF, González LM, Chan W, et al. Progress on lipid extraction from wet algal biomass for biodiesel production Microb Biotechnol.2016;9(6):718-26.
- Ryckebosch E, Muylaert K, Foubert I. Optimization of an analytical procedure for extraction of lipids from microalgae. J Amer Oil Chemists' Soc. 2012;89(2):189-98.
- Stansell RG, Gray MG, Stuart DS. Microalgal fatty acid composition: implications for biodiesel quality. J Appl Phycol. 2012;24(4):791-801.
- Knothe G. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Processing Technology. 2005;86(10):1059-70.
- Patil P D, Reddy H, Muppaneni T, et al. In situ ethyl ester production from wet algal biomass under microwave-mediated supercritical ethanol conditions. Bioresource Technol. 2013;139:308-15.
- Hawash SS, AboEl-EninA, ElDiwani G. Direct conversion of dry algae to biodiesel under supercritical methanolysis. Int J Agricult Innov Res. 2014;2(6):1090-95.
- Meher LC, SagarVidya D, NaikSN. Technical aspects of biodiesel production by transesterification a review. Renewable Sustainable Ener Rev. 2006;10(3):248-68.
- Singh A, Maji S, Gaurav K. Production of biodiesel from the algae: State of the art. Int J Develop Res. 2014;4(12):2685-93.
- Murphy JD, Drosg B, Allen E, et al. Perspective on algal biogas production of first and second generation biofuels: A comprehensive review. Renew Sustain Energy Rev. 2010;14:578-97.
- Kleemann M, Meliß M. Regenerative energiequellen. Springer Verlag, New York, 1993.
- Kobayashi N, Noel E A, Barnes A, et al. Characterization of three Chlorella sorokiniana strains in anaerobic digested effluent from cattle manure. Bioresour Technol. 2013;150:377-86.
- Zhang R, El-Mashad HM, Hartman K, et al. Characterization of food waste as feedstock for anaerobic digestion. Bioresource Technology. 2007;98(4):929-35.
- Northcote DH, Goulding KJ, Horne RW. The chemical composition and structure of the cell wall of Chlorella pyrenoidosa. Biochem J. 1958;70(3):391-97
- Schwede S, Rehman ZU, Geber M, et al. Effects of thermal pretreatment on anaerobic digestion of Nannochloropsissalina biomass. Bioresoure Technol. 2013;143:505-11.