Original Article
, Volume: 12( 4)Triazolyl Glycoconjugates and their Impact in Medicinal Chemistry
- *Correspondence:
- Somesh S, Department of Chemistry, H.D. Jain College, Veer Kunwar Singh University, Arrah, Bihar, India, Tel: 06182-239178; E-Mail: someshschem@gmail.com
Received: September 07, 2016; Accepted: September 20, 2016; Published: September 29, 2016
Citation: Somesh S, Madhuri K. Triazolyl Glycoconjugates and their Impact in Medicinal Chemistry. Org Chem Ind J. 2016;12(4):104.
Abstract
Triazoles, especially 1,2,3-Triazoles were known for their wide range of biological activities. Cu(I)-Catalyzed 1,3 dipolar cycloadditions of organic azide and terminal alkyne (click reaction) has been extensively used in adjoining two entirely different building blocks having azide and alkyne functionality thus enabling an easy access of simple to complex molecular architectures containing 1,4-disubstituted triazole skeleton. Carbohydrate-based triazole containing molecules in combination of standard drugs and other biologically relevant molecules are always represented itself as impressive drug candidate which show their effect with increased therapeutic efficacy. Triazole synthesis is also important for developing peptide isostere, the triazolyl components has recently gained momentum chemical biology and drug research. This review highlights the extensive application of triazolyl glycoconjugates with their potential scope in medicinal chemistry.
Keywords
Click chemistry; CuAAC; Glycoconjugates; Carbohydrate; Triazole
Introduction
Carbohydrates having complex structures also called ‘bio-molecules’ play crucial roles in many physiological as well as pathological cell functions [1-3]. They are found very important to cellular recognition events, including signal transduction, inflammation, immune response, apoptosis, tumor metastasis and viral & bacterial infections [4,5]. Involvement of carbohydrates in various vital metabolic processes such as cell-cell interaction, cell migration, pathogen defense etc., makes carbohydrate a promising candidate for the drug development.
Many microbes, including viruses, bacteria and their toxins, have evolved to bind cell surface carbohydrates, a binding that is a prerequisite for infection to occur [6]. Additionally, carbohydrates and their conjugates including biomolecules participated efficiently in the fertilization, blood clotting, hormone trafficking, cellular differentiation and development. Cell differentiation and tumor development changes carbohydrate phenotype, this creates novel targets for immunotherapeutic strategies in the treatment of malignant diseases.
Utilization of carbohydrates in drug synthesis are found to be easy due to presence of several chiral hydroxyl groups which after diverse modifications coupled with appropriate biological relevant scaffolds to generate various glycohybrids [7,8]. Multivalent nature of carbohydrates enhances their affinities for targeting different biological processes, such as the binding of bacteria, bacterial toxins [9], galectins [10], and other lectins [11].
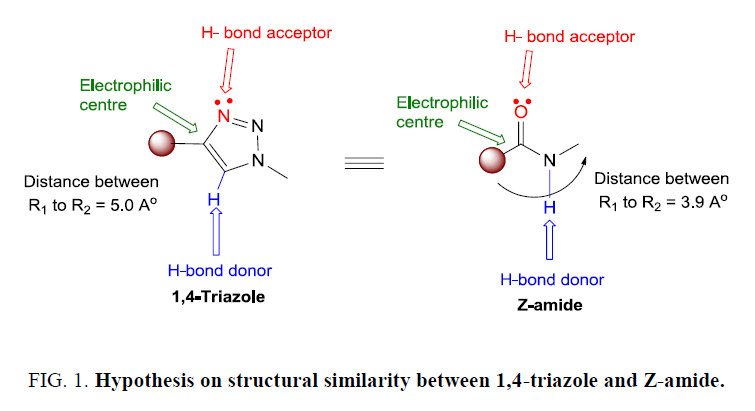
Triazole ring also known as isoster of amides and exhibiting chemical and electronic resemblance to amide bond (Figure 1). Amide bond represents the main chemical bond that links amino acid building blocks together to constitute proteins, where this functionality plays a major role in the elaboration and composition of biological systems [12].
Hence like amide triazoles also create important biological activity and interplay with suitable biological targets owing to their high dipole moment, which allows strong hydrogen-bonding interaction with targets. Moreover, this heterocycle is stable towards metabolic degradation and can improve the solubility of molecules, thereby establish triazoles as a potential pharmacophore [13]. The importance of 1,2,3-triazole derivatives as biologically important molecules having activities such as anti-HIV, antiviral [14], antibacterial [15], antifungal [16], antiparasitic [17], anti-inflammatory and antitubercular agents [18] has been known through literatures after their discovery. 1,2,3-triazoles are the special isomer of these triazole rings which are not natural occurring molecules, yet they are gifted numerous biological activities. Therefore, triazole synthesis along with adjoining process of carbohydrates with any other groups affords drug like architectures possessing a variety of biological activities and their development as a pharmacophore, is valuable area of research in the field of medicinal chemistry. The present review is aimed at recent developments in carbohydrate-based triazolyl scaffolds in medicinal chemistry.
Synthesis of 1,2,3-Triazoles via Alkyne-Azide Cycloaddition Reaction
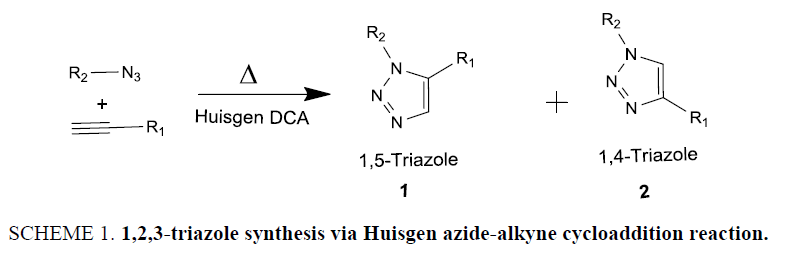
The Huisgen 1,3-dipolar cycloaddition reaction of organic azides and alkynes [19,20] giving a mixture of regioisomers 1,4- and 1,5-disubstituted 1,2,3-triazole (Scheme 1), is among extremely important transformations in organic chemistry, as evidenced by the large number and wide scope of targets that can be prepared by this methodology. The reaction was discovered at the beginning of the 20th century, but Huisgen et al. [19,20] in the 1960s were first behind the comprehensively study and unveiling the mechanism of this reaction.
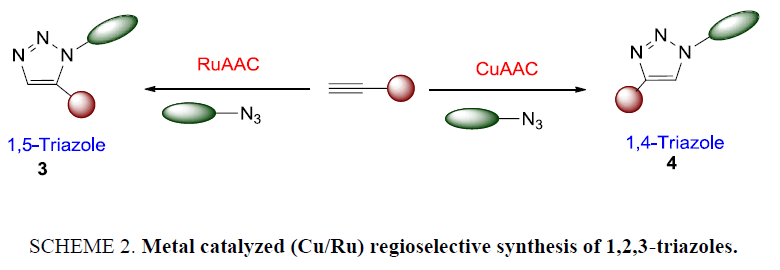
Despite the high versatility, for more than 40 years this reaction suffered from disadvantages such as requirement of heating and long reaction time for completion, lack of selectivity in product yield, and difficulty in separation of the two regioisomers using classical chromatographic techniques. In 2002, Meldal [21] and the Sharpless [22] laboratories independently reported that copper (I) salts were able to accelerate this reaction at room temperature or with only moderate heating condition furnishing exclusively the 1,4-regioisomer with minimal work-up and purification (Scheme 1). Likewise, Cu(I) catalyze click reaction, opposite result was obtained in ruthenium-catalyzed alkyne-azide cycloaddition reactions which afforded the next regioisomer of 1,2,3-triazoles in 1,5-disubstitution manner solely [23,24].
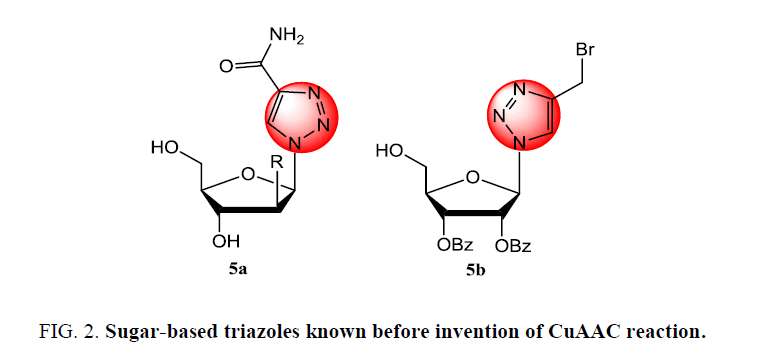
Especially in case of carbohydrate chemistry, the CuAAC reaction finds wide application in the preparation of carbohydrate derivatives including the generation of N-glycosyl triazoles from simple acetylenes or derivatives containing the acetylenic functional groups. This approach is a potent tool in accessing diverse carbohydrate mimetics with improved affinity stabilized by the glycosylation steps. Therefore, click chemistry has emerged as powerful conjugation strategy for the synthesis of 1,2,3- triazole-containing glycoconjugates of biomedical interest [25-39]. Mimic of naturally occurring glycoconjugates has been achieved in a large scale using carbohydrate chemistry in combination of click reaction provides a potentially more reliable path to artificial glycoconjugate which retain the geometric and spatial characteristics of the native glycoform with sufficient hydrolytic stability toward glycosidases and glycosyltrasferases. These may and also inhibit these enzymes to some extent, so increasing the potential for a wide array of in vivo applications [40]. Hence, the 1,4-disubstituted 1-H-1,2,3-triazoles have been considered significant for diverse range of in vivo applications since before the discovery of CuAAC reaction (Figure 2).
Mechanism of Cu-Catalyzed Click Reaction
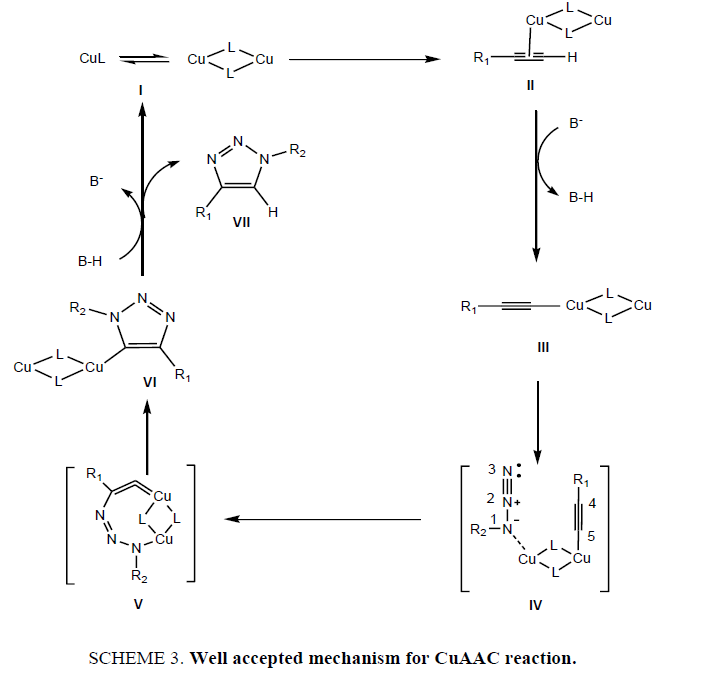
The cycloadditions generally proceed through a concerted mechanism. However, the Huisgen cycloaddition was suggested to be stepwise process [23,41,42] but based on available experimental evidences, it was found that first, Cu(I) coordinated with alkyne II π-electron system. It decreases the Pka value of acetylene proton and there is exothermal formation of Cu-acetylide III. Each terminal alkyne invites two coopers for coordination to generate Cu-acetylide where Cu(I) present in dimeric or polymeric cluster form having bond angles 130 Å and 140 Å respectively. In subsequent step, azido group underwent complexation with Cu(I) metal where most commonly it is considered that N-1 of azide functionality participated in coordination and form Cu-acetylide complex IV, which in turn activates N(3) of azide for nucleophilic attack at C(5) to give metallocycle V [43]. After the ring contraction where a lone pair electrons of N(1) attacks on C(5) to give the respective intermediate VI, reaction proceeded for elimination of Cu ligand and subsequent protonation C-5 carbon releases 1,4 disubstituted 1,2,3 triazole VII product (Scheme 3).
Carbohydrate Containing Triazoles in Medicinal Chemistry
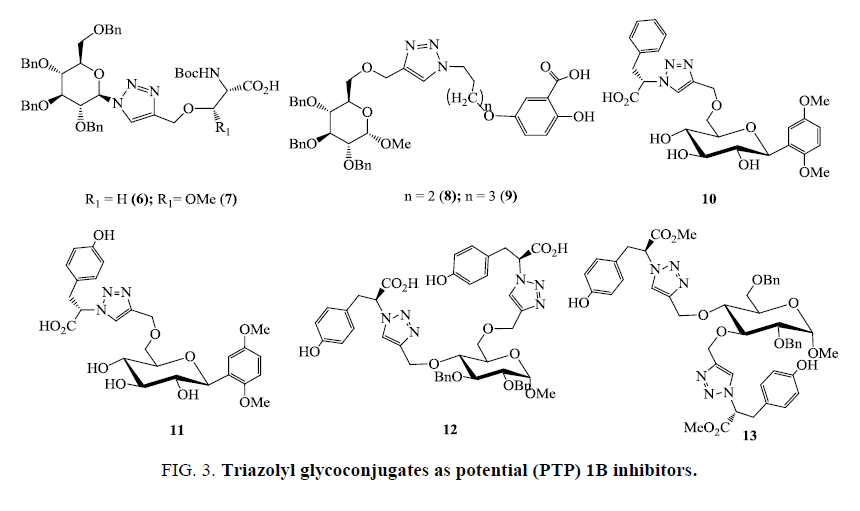
Now a day’s Cu(I) catalyzed click reaction has emerged as an important strategy for the discovery and optimization of lead along with its exploration as an effective drug candidate against various therapeutic strains. Involvement of carbohydrates in various vital metabolic processes such as cell-cell interaction, cell migration, pathogen defense etc., makes carbohydrate a promising candidate for the drug development [44]. However, complexity associated with its synthesis, especially concerning anomeric stereochemistry, poor pharmacological properties, and modest affinity for various receptors limits its development as drug lead. But, compatibility of Cu(I)-catalyzed coupling of carbohydrate based azides and alkynes to pre-appended protecting groups, as well as relative inertness of triazole ring towards string of protection/deprotection and glycosylation steps establish this approach as a potent tool in accessing diverse carbohydrate mimetics with improved affinity. A large number of biologically active glycosylated 1,2,3-triazole moieties have been designed which showed good pharmacokinetic properties. Protein tyrosine phosphatases (PTPs) are well-validated therapeutic targets for many human diseases. Zhang et al. [24] reported PTP inhibitor entities by simply “clicking” alkynyl amino acids onto diverse azido sugar templates. Triazolyl glucosyl, galactosyl, and mannosyl serine and threonine derivatives were efficiently synthesized via click reaction, one of which (compounds 6 and 7, Figure 3.) was identified as a potent and selective PTP1B inhibitor against a panel of homologous PTPs with IC50=5.9 ± 0.4 μm for R1=H (6) against PTP1B and IC50=7.1 ± 1.0 μm for R1=Me (7) against PTP1B [45].
Li et al. [46] reported a library of benzyl 6-triazolo(hydroxy)benzoic glucosides as tyrosine phosphatase (PTP) 1B inhibitors via Cu(I)-catalyzed azide-alkyne 1,3-dipolar cycloaddition. These glycoconjugates bearing alkyl chain, sugar, and (hydroxy)- benzoic derivatives (compounds 8 and 9, Figure 3) (IC50=8.7 ± 1.4 μm for n=2 and IC50=6.7 ± 0.5 μm for n=3) were identified as new PTP1B inhibitors with selectivity over T-cell PTP (TCPTP), SH2-containing PTP-1 (SHP-1), SHP-2, and leukocyte antigenrelated tyrosine phosphatase (LAR). Triazole-linked phenylalanine and tyrosine-aryl C-glycoside hybrids were synthesized via microwave-assisted click reaction in high yields. The successive PTP1B biological assay found from the glycoconjugates that contain carboxylic acid and benzyl moieties as more active PTP1B inhibitors is the most potentially successive specific PTP1B inhibitor, which possess at least several-fold selectivity over other homologous PTPs tested with IC50=5.6 μm for 10 against PTP1B and IC50=5.5 μm for 11 against PTP1B (Figure 3) [47].
Recently click chemistry has been successfully explored for the identification of mono- and bis-phenylalaninyl and tyrosinyl glucoside derivatives as novel PTP1B inhibitors [47]. The designed compounds bearing one or two phenylalanine or tyrosine derivatives on the 6-, 2,3-, 2,6-, 3,4-, and 4,6-positions of the glucosyl scaffolds were efficiently constructed via the microwaveassisted Cu(I)-catalyzed azide−alkyne cycloaddition in moderate-to-excellent yields. Successive biological assays identified these compounds as novel PTP1B inhibitors, with the 4,6-disubstituted tyrosinyl glucoside 12 being the most potent. A kinetic study established that both mono- and bis-triazole linked glycosyl acids act as typical competitive inhibitors, whereas the 3,4- disubstituted tyrosinyl glucoside bis-triazolyl ester 13 exhibited a mixed-type inhibition pattern against PTPs1B and TCPTP (T cell protein tyrosine phosphatase). Compounds 12 displayed inhibition values Ki=14.4 μm, IC50=10.8 μm and 13 showed Ki=8.7 μm, IC50=9.6 μm against PTPs1B while value IC50=31.0 ± 9.6 μm against TCPTP (Figure 3) [48].
A diverse range of physiological and pathological processes that involve the breaking or construction of glycosidic linkages can be regulated by identifying inhibitors of glycosidases and glycosyl transferases that catalyze these processes. Therefore, they may serve as highly promising therapeutic agents for the treatment of diseases such as diabetes [49,50], viral infections [51,52] or cancer metastasis [53]. The 1,2,3-triazoles are poorly basic in nature and thus remains non-protonated under physiological pH. Studies reveal that non-protonated sp2-hybridized nitrogen atoms of 1,2,3-triazoles may better mimic the partial positive charge at the anomeric carbon in the transition state of the glucosidase-catalyzed reaction, thereby may actually behave as more specific glycosidase inhibitors.
A series of saccharidyl-triazoles based on anti-diabetic drug acarbose structure were synthesized by Perion et al. [54] using click chemistry. The bioassay of the synthesized triazoles significantly inhibited the α-glycosidase enzyme. Dedola et al. [55] synthesized a library of glucosidase inhibitors by the reaction of α- and β-glucopyranosyl azides with diverse alkynes carrying various functional groups, which were supposed to interact differently with the active site of enzyme [55].
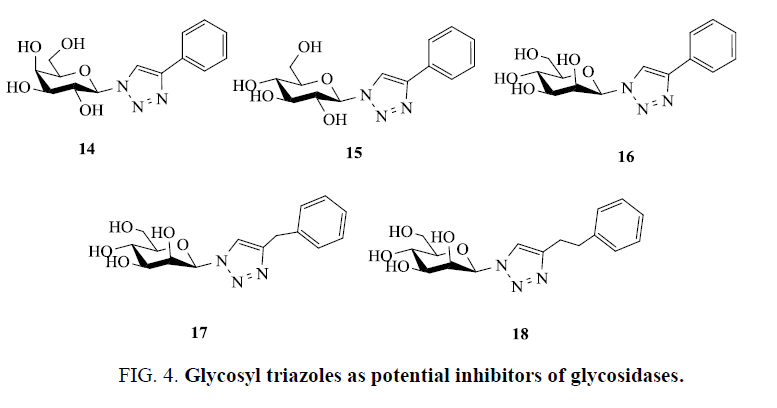
Applying Cu-catalyzed [3+2] cycloaddition reaction condition Rossi and Basu [56] prepared 1-β-D-glucosyl-4-phenyl triazole and 1-β-D-galactosyl-4-phenyl triazole (Figure 4) , which has been recognized as aglycone-modified inhibitors of β-glucosidase enzyme. Their inhibitory activity was examined by performing a percentage assay against three β-glycosidase enzymes sweet almond glucosidase (SAG), Escherichia coli galactosidase (ECG), and bovine liver galactosidase (BLG). In comparison to glycosidase inhibitors deoxygalactonojirimycin (DNJGal) and deoxynojirimycin (DNJ, gluco configuration) galactose derivative 14 showed moderate inhibition for ECG, whereas glucose triazole 15 was observed to be better inhibitor of BLG than the galactose derivative 14 and none of them showed good inhibition for SAG [57].
Polakova et al. [58] explored copper catalyzed click reaction to synthesis of new triazole conjugates derived from D-mannose and assayed in vitro assays to examine their ability to inhibit α-mannosidase enzymes from the glycoside hydrolase (GH) families 38 and 47. The developed molecules were more selective for a GH47 α-mannosidase (Aspergillus saitoi α1,2-mannosidase), exhibiting inhibition at the micromolar level (IC50 values of 50 mm to 250 mm), and less potent towards GH38 α-mannosidases (IC50 values in the range of 0.5 mm to 6 mm) towards jack bean α-mannosidase. The compounds 16-18 were found more potent and selective towards the α-mannosidase from the GH family 47 (IC50(AspMan)=0.05 mm to 0.25 mm) and weaker inhibitors of the α-mannosidases from the GH family 38 (IC50(dGMIIb, dLManII, JBMan)=0.4 mm to 6 mm) (Figure 4) [59].
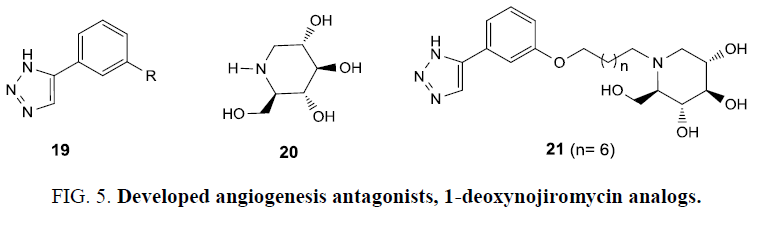
In search of more potent inhibitors for angiogenesis than 1-deoxynojiromycin (20) and aryl-1,2,3-triazole (19), which target two different pathways for angiogenesis therapy, a hybrid was prepared (Figure 5). N-alkylation of 1-DNJ followed by 1,3 dipolar cycloaddition resulted in novel bifunctional compounds, out of which compound 21 (Figure 5) showed better α-glycosidase activity (IC50=1.15 μm) than DNJ (1.67 μm) and showed improved ability to inhibit (BAEC) bovine aortic endothelial cells proliferation (IC50=0.105 mm) than 19 (IC50=0.347 mm) [58].
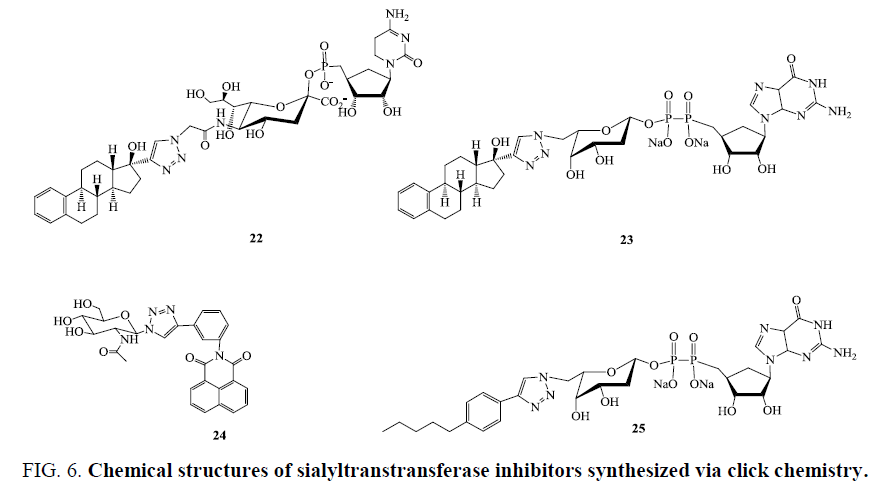
Hosoguchi et al. [60] developed novel synthetic sugar nucleotide (compound 22, Figure 6) which was identified to be the first highly specific inhibitor for rat recombinant α 2,3-(N)-sialyltransferase (α 2,3ST, IC50=8.2 μm), while compound 22 was proved to become a positive substrate for rat recombinant α 2,6-(N)-sialyltransferase (α 2,6ST, Km=125 μm, where Km is the kinetic parameter (Michaelis-Menten constant)). Versatility of this strategy was further confirmed by identification of two selective inhibitors (compounds 23 and 24, Figure 6) for human recombinant α 1,3-fucosyltransferase V (α 1,3-FucT, Ki=293 nm) and α 1,6-fucosyltransferase VIII (R1,6-FucT, Ki=13.8 μm) [59].
Cu(I)- catalyzed “click” cycloaddition reactions between glycosyl azides and alkynes were exploited to generate inhibitory therapeutic agents of O-GlcNAcase. Wang et al. [47] reported enzymatic kinetic screening which exposed that compound 25 (Figure 6) was a most potent competitive inhibitor of human OGlcNAcase (Ki=185.6 μm) among the screened compound series [60].
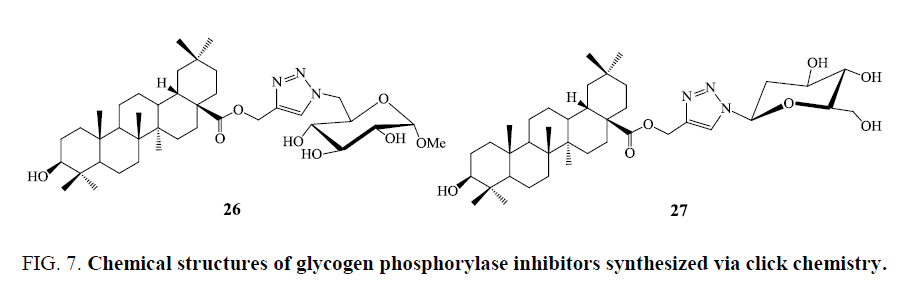
Glucoconjugates of oleanolic acid has been synthesized by applying azide-alkyne cycloaddition reaction catalyzed by Cu(I) with evaluation of biological assay. The compounds containing triazole moiety were found as potent inhibitors of glycogen phosphorylase. Numerous developed triterpene−glycoside conjugates exhibited modest inhibitory activity against RMGPa. Compound 26 (Figure 7) showed the good inhibition with an IC50 value of 1.14 μm. Possible binding modes of compound 26 by molecular docking simulations including structure activity relationship (SAR) analysis of the inhibitors were also discussed [61].
D-glucose triterpene heteroconjugates were obtained on coupling with propargyl esters of the C-28 carboxylic acids of pentacyclic triterpenes (oleanolic, ursolic, and maslinic acids) with 2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyl azide as well as N-(ω-azido-[C-2, C-6, and C-11] alkanoyl)-β-D-glucopyranosylamines under conditions of copper (I)-catalyzed click reactions. The novel developed compounds were assayed against RMGPa a or b enzymes. The heteroconjugate derivative 27 (Figure 7) showed moderate inhibition against RMGPa with IC50=26 μm [62,63].
Cancer is a most important public health problem worldwide. Several drugs act as anticancer agents including taxol, vinblastine, vincristine, camptothecin derivatives, opotecan and irinotecan, and etoposide developed from epipodophyllotoxin are in clinical use all over the world. Increment in cancer cases forced to synthetic chemist to search for efficient anticancer agents for the prevention and treat of disease. Cell-surface of cancerous cells is abundant in oligosaccharide antigens, which could act as markers for active or passive cancer. Also, the developed analogs of carbohydrates can severe as effective antigen to evoke antitumor immune response in body, when conjugated with some large biomolecules.
Designing of molecular architectures displaying multitude of saccharides has gained enormous attention, particularly by embracing CuAAC reaction. And especially, the glyco-cluster class is extremely valuable tool for the study of biologically relevant carbohydrate-lectin or protein recognition processes. Therefore, use of competent CuAAC reaction has made it possible to design high affinity, spatially well-defined site specific glyco-clusters that could be biomedically applicable as inhibitor of different classes of galectin [64] and lectin [65].
Galectins are a family of cytosolic β-D-galactoside binding proteins, which stalwartly modulate their expression during progressive, separation stages, and under different physiological or pathological conditions. Studies confirmed that Gal-3 is involved in colon cancer metastasis, brain tumor progression, inhibition of metastasis-associated cancer cell adhesion, and may play a key role in innate immunity. Other reports suggest that Gal-3 and Gal-1 can regulate apoptosis processes and Gal-1 acts as an insoluble host factor that promotes HIV-1 infectivity through stabilization of virus attachment to host cells [66].
Cecioni et al. [67] reported the synthesis of lactosylated glycoclusters based on porphyrin and calix (6) arene (one porphyrin and three topological conformers of calix (6) arenes) scaffolds having alkyne functionality and azido-functionalized lactoside via CuAAC under microwave activation. The glycoclusters were also evaluated and compared to a monovalent probe as ligands of two lectins: ECA from legume plant Erythrina crista-galli and recombinant human galectin-1. HIA data showed that a large increase in the inhibition of hemagglutination was observed for porphyrin 28 and calixarene cone conformer 29 (Figure 8). Topology-induced selectivity was also identified with a preference for both clusters 28 and 29 that inhibit hemagglutination in the micromolar range. Apart it, the two other calixarene conformers were identified as poor ligands of galectin-1 however these could not be confirmed using SPR analysis [66].
Carbohydrate ligands of natural occurrence exhibits weak affinity for galectins, therefore galactosides and lactosides based monovalent and multivalent triazole clusters were prepared by Giguere et al. [66] with the anticipation of better binding capacity and selective inhibition of Gal-1 over the other galectins. Evaluation of monovalent compounds showed maximum inhibitory activity for triazolyl galactoside 30 with inhibitory properties of 1250 μm for both inhibitors, whereas trivalent lactoside 31 (Figure 9) registered inhibitory properties of 20 μm for galectins.
Tejler et al. [64] prepared the acetylene derivatives of various natural and non-natural amino acids and clustered them with complex 2-azidoethyl β-D-galactopyranosyl-(1→4)-β-D-glucopyranoside via regioselective copper(I)-mediated 1,3-dipolar cycloaddition reaction to afford a panel of mono-, di- and trivalent lactoside derivatives. Among all, the divalent cluster 32 with Kd value as low as 3.2 μm was identified as a promising galectin-1 inhibitor (Figure 9) having a relative potency of 30 per lactose unit as compared to the natural disaccharide ligand lactose [67].
Peterson and Blagg [68] successfully explored click reaction for the synthesis of triazole-containing novobiocin analogues. These compounds contain a triazole ring in lieu of the amide moiety present in the natural product. The developed compounds were checked for antiproliferative effects against two breast cancer cell lines (SKBr-3 and MCF-7), and results were similar to those of their amide-containing counterparts. In addition, Hsp90-dependent client protein degradation was observed via Western blot analyses, supporting a common mode of Hsp90 inhibition for both structural classes. Compounds 33 and 34 (Figure 10) displayed most potent inhibition against SKBr-3 and MCF-7 among homologous series with IC50=13.16 ± 3.85 μm against MCF-7 and IC50=21.22 ± 5.99 μm against SKBr-3 for compound 33. Compound 34 exhibited a IC50 value of 18.33 ± 4.67 μm against MCF-7 and IC50=8.17 ± 0.11 μm against SKBr-3 [68].
Figure 10: Chemical structures of anticancer drugs constructed via triazole linkage.
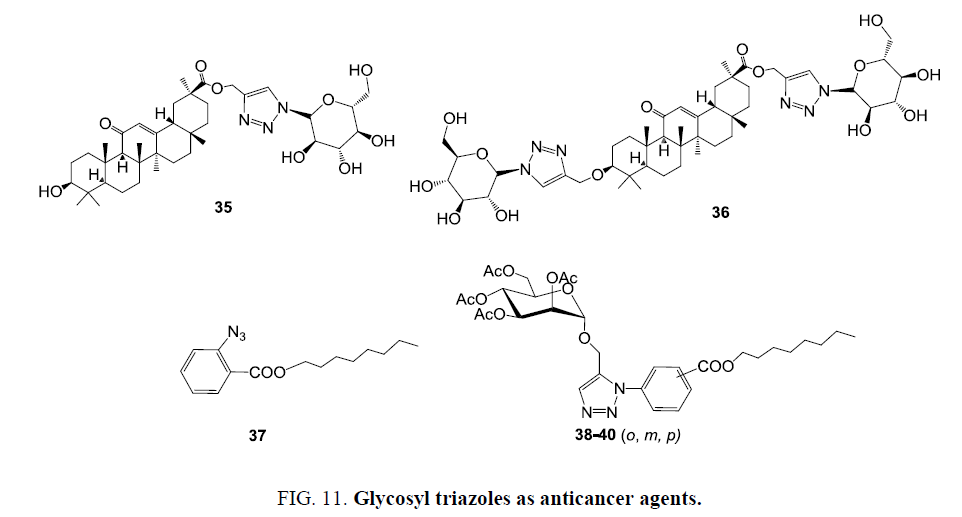
Parida et al. [69] employed click reaction for the synthesis of a series of glycosyl triazole linked 18β-glycyrrhetinic acid (GA) derivatives. Per-O-acetylated glycosyl azide derivatives treated with propargyl ester of 18β-glycyrrhetinic acid (GA) followed the concept of ‘click chemistry afforded glycosyl triazole derivatives of 18β-glycyrrhetinic’. De-O-acetylated triazolyl molecules with free hydroxyl groups in the carbohydrate moieties were evaluated for their anticancer potential against human cervical cancer cells (HeLa) and normal kidney epithelial (NKE) cells. Compound 35 and compound 36 (Figure 11) showed promising anticancer activities for HeLa cells exhibiting values (IC50-13.76 ± 5.30) and (IC50-21.50 ± 2.24) respectively.
Hradilova et al. [70] performed Cu(I) catalyzed Huisgen cycloaddition reaction and obtained new conjugates from terminal alkyne containing D-mannose derivatives and o-, m-, and p-substituted azido benzoic acid esters. All developed molecules were evaluated for their in vitro cytotoxic activity against seven cancer cell lines with/without multidrug resistance phenotype as well as non-tumor MRC-5 and BJ fibroblasts. The conjugated systems were found with increased activity, such as in the case of octyl ester in ortho position of aromatic unit the azido and glycosylated unit displayed IC50 93.2 μm of 37 and 23.8 μm of 38 for CEM lymphoblasts. Surprisingly compound 38 was more active than deacetylated derivative. Similarly, compounds 39 and 40 (Figure 11) having octyl ester in meta and para positions were obtained as most potent molecules and showed significant decrease of IC50 values.
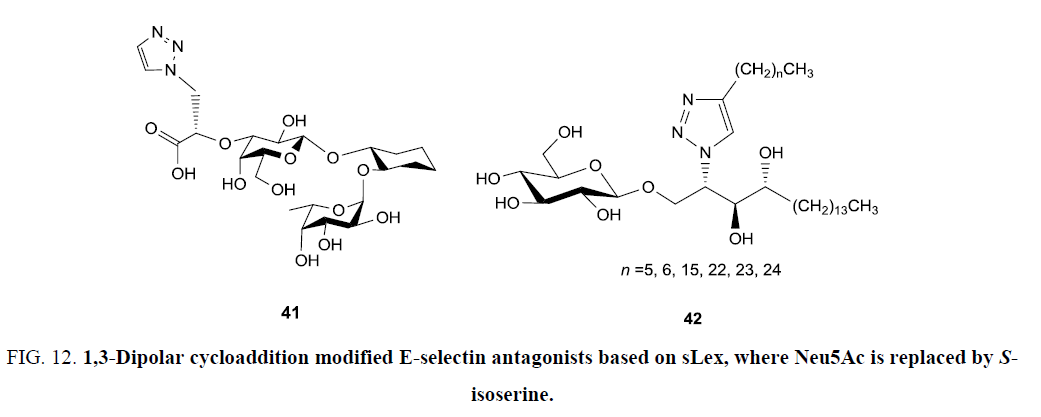
A protective attempt of body against pathogens and commences with interaction of leukocyte of blood vessel with sialyl Lewis x (sLex) residing on physiological selectin ligands is termed as inflammation, which belongs to a class of cell adhesion molecules. However, prolonged inflammatory responses may lead to diseases like hay fever, atherosclerosis, and rheumatoid arthritis etc. Blocking selectin-leukocyte interaction via selectin-antagonists is a promising approach to overcome inflammatory diseases. Recently, Titz et al. [71] have explored click approach for developing a library of selectin antagonists based on sLex structure, where Neu5Ac has been replaced by (S)-isoserine. A series of 7 different alkynes have been inserted over sLex based triazole but, only the unsubstituted triazole (41) (Figure 12) showed slightly improved affinities for E-selectin, whereas other substituted compounds showed affinity similar to sLex.
Figure 12: 1,3-Dipolar cycloaddition modified E-selectin antagonists based on sLex, where Neu5Ac is replaced by Sisoserine.
α-GalCer is most potent agonistic antigen of the T cell receptor of natural killer T cells. Lee et al. [72] have synthesized a series of 1,2,3-triazole-containing α-GalCer analogues (42) (Figure 12) by varying length of lipid chain attached. It has been observed that replacement of amide group with isosteric triazole enhances the IL-4 versus IFN-γ bias of released cytokines. Also, the analogues carrying long chained triazoles exhibited stimulatory effect on cytokine production comparable to α-GalCer and showed a stronger Th2 cytokine response [72]. Likewise, Slamova et al. [73] have reported the synthesis β-2-acetamido-2- deoxygluco GlcNAc-triazole (Figure 12) by click reaction under microwave condition, which was found to be hydrolyzed by Talaromyces flavus CCF 2686 β-N-acetylhexosaminidase and was furthermore established to act as a strong ligand of rat and human natural killer cell activating receptors (Figure 12) [74].
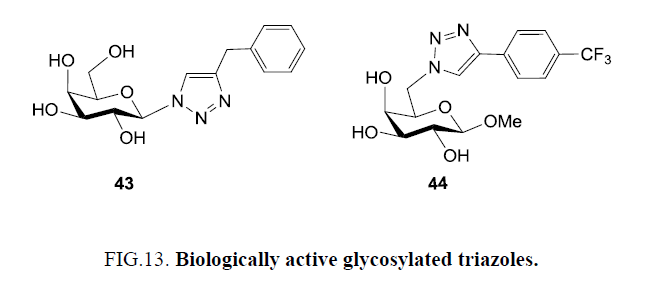
Trypanosoma cruzi ‘a parasite’ sheds a developmentally regulated enzyme called trans sialidase. This involves in the interactions between host cells and parasite which is responsible for cellular invasion processes and infections and is a potential therapeutic target for Chagas’ disease. Carvalho et al. [17] explored Cu(I)-catalyzed azide-alkyne 1,3-dipolar cycloaddition for designing a small library of 1,4-disubstituted 1,2,3-triazole galactose derivatives, and identified them as potent inhibitor for Trypanosoma cruzi trans-sialidase (TcTS) enzyme. Galactose scaffolded with azide at either C-6 or C-1 positions were reacted with a panel of 23 different terminal alkynes employing click catalytic system under microwave irradiation, which afforded regioselectively pure disubstituted triazoles). Further, evaluation of all the synthesized sugar-triazoles for their inhibitory activity against TcTS suggested them as moderate to weak inhibitors of the enzyme at 0.5 mm to 1.0 mm range, and compound 43 exhibited maximum inhibitor activity (with 37% inhibition). Whereas assessment of the same library for in vitro trypanocidal activity against Trypomastigote forms of T. cruzi Y strain, using lead drug benznidazole as control, verified compound 44 as the most powerful inhibitor with optimum activity in 100s of μm range (Figure 13) [74].
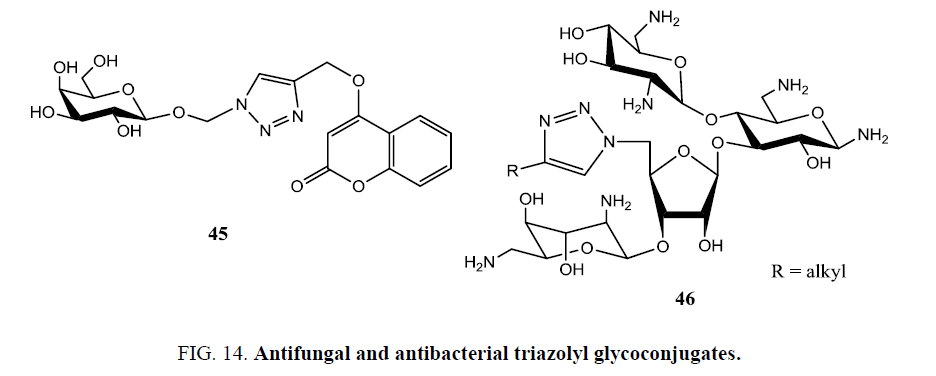
The incidence of life-threatening fungal infections has increased in the last two decades due to greater use of immunosuppressive drugs, prolonged use of broad spectrum antibiotics, widespread use of indwelling catheters, and in cancer and AIDS. The presently marketed antifungal and antibacterial drugs are either highly toxic or becoming ineffective due to the appearance of resistant strains. This necessitates continuing research into new classes of antimicrobial agents. 1,2,3-triazole-containing molecules is one of these classes. A series of sugar modules carrying alkyne/azide functionality were synthesized by glycosidase-mediated glycosylation of alkynyl alcohols and azide-containing alcohols with unprotected sugars in one step. And their subsequent click with a variety of alkyne/azide counterparts resulted in triazole-containing glycosides (45) (Figure 14) and their subsequent submission to bioassays showed that this procedure is a feasible approach to the development of antifungal drugs [75].
Zhang et al. [75] developed a facile synthetic strategy for the preparation of numerous neomycin B derivatives 46 (Figure 14) by modifications at the 5´´ position. The structure-activity relationship (SAR) against aminoglycoside resistant bacteria equipped with various aminoglycoside-modifying enzymes (AMEs) resulted into the identification of numerous derivatives with an increased antibacterial activity compared to neomycin. Moreover, antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin resistant Enterococci (VRE), the prominent activity was observed in some compounds [76].
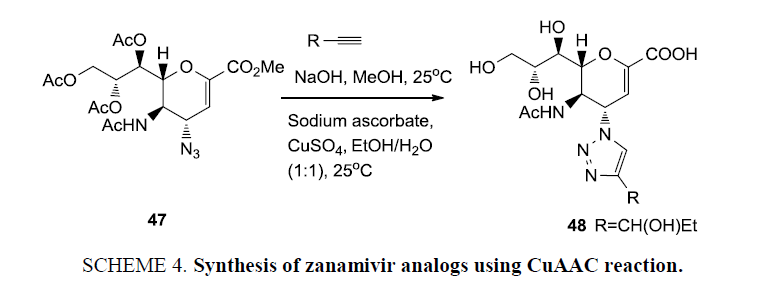
The coupling of two or more molecular entities with distinct properties to form novel conjugates with combined properties of parent components, is a fast-growing technology in recent years. By employing click reaction, Li et al. [76] synthesized analogues of drug zanamivir (Scheme 4) which is an effective drug for combating avian influenza (H5N1) because of its inhibitory activity against the enzyme neuraminidase. This enzyme stimulates cleavage of sialic acid from glycoproteins and glycolipids of the host cells, which is a key step for replication of influenza virus. These neuraminidase inhibitors cure influenza directly by blocking the synthesis of neuraminidase enzyme synthesis. A library of sixteen C-4 triazole substituted zanamivir analogues have been prepared utilizing a common precursor (47), and were subsequently screened. Compound 48 substantially showed maximum anti-AIV activity (IC50=6.4 μm) comparable to zanamivir (IC50=2.8 μm) [77].
Weiwer et al. [77] developed an attractive library of non-hydrolysable 1,2,3-triazole derivatives of sialic acid using CuAAC reaction of methyl (5-acetamido-3,5-dideoxy-2-azido-D-glycero-α-D-galactonon-2-ulopyranoside)onate (49) with different terminal alkyne functionalized molecules. All the synthesized 1,2,3-triazole containing sialic acid derivatives were screened for neuraminidase inhibitory activity using a 96-well plate fluorescence assay; for the SAR study, to develop a novel class of neuraminidase inhibitors for the treatment of influenza. 5-acetamido-3,5-dideoxy-2-(4-octyl-1H-1,2,3-triazol-1-yl)-D-glycero α-D-galacto-non-2-ulopyranosidic acid (50) has been recognized as potential lead compound (IC50=28 μm), comparable to the known sialidase inhibitor (Neu5Ac2en) [78]. Similarly, some interesting multivalent derivatives, including sialic acid disaccharide mimic 52 (IC50=17 μm) and dendrimer 53 (IC50=20 μm) were also investigated along with compound 51. Therefore, aldehyde of sialic acid derivatives can be useful for the conjugation with protein surface by reductive amination process, for example KLH-carrier protein for the production of antibodies (Figure 15).
Figure 15: Synthesis of library of triazole linked sialic acid using CuAAC reaction./p>
Designing and synthesis of glycosylated porphyrins has gained immense popularity owing to their application in photodynamic therapy (PDT), which is a noninvasive approach of cancer treatment. Porphyrin upon exposure to a particular wavelength of light generates lethal singlet oxygen that kills tumor cell whereas, sugars installed over this hydrophobic core provides water solubility and increased tumor cell specificity. Therefore, the whole glycoconjugated porphyrin system can acts as a promising PDT sensitizer. In this context, recently Kushwaha et al. [78] reported a click chemistry inspired synthesis of a series of porphyrin-cored glycodendrimers containing 8, 12, 16, and 24 peripheral β-D-glucopyranose units. Same group [79] established the synthesis of glycoconjugated triazolyl azido-alcohols via one-pot click reaction of glycosyl alkynes with epichlorohydrin in aqueous medium. The resulting triazolyl azido-alcohols were further utilized for the synthesis of bis-triazolyl ethisterone glycoconjugates using CuAAC reaction. These triazole-linked ethisterone glycoconjugates would be crucial in androgen receptor pharmacology and chemical biology [80]. Mishra et al. [81] reported a concise one-pot protocol for the synthesis of novel glycosyl-β-azidoester from glycosyl olefinic ester utilizing diazotriansfer reaction which was further utilized via click chemistry and a diverse range of triazolyl glycoconjugates of potential chemotherapeutic values has been achieved in high yields. Mishra et al. [82] reported the click inspired synthesis of morpholine-fuzed triazole, which is biologically relevant heterocyclic moiety known to possess excellent medicinal properties. Further chemistry was extended for the synthesis of triazole-linked O-benzylquercetin glycoconjugates, where developed glycoconjugates were evaluated for anti-leishmanial activity against the promastigotes and amastigotes of Leishmania donovani and shown promising activities [83]. Furthermore, 7-O-propargyl noscapine, obtained via two successive steps eg. O-demethylation of noscapine, and subsequent propargylation has been utilized to click with of glycoconjugated triazolyl azido-alcohols and afforded regioselective triazole-linked 7-Onoscapine glycoconjugates as promising anticancer agent [84].
Carbonic anhydrase (CA) enzymes, particularly membrane-bound isozymes CA IX and CA XII, support a pH-regulating system that enables hypoxic tumor cell survival and proliferation. Carbonic anhydrase IX and XII are concerned as potential targets for the advancement of new hypoxic cancer therapies. To date, there are limited numbers of molecules which have been characterized in CA-relevant cell and animal model systems.
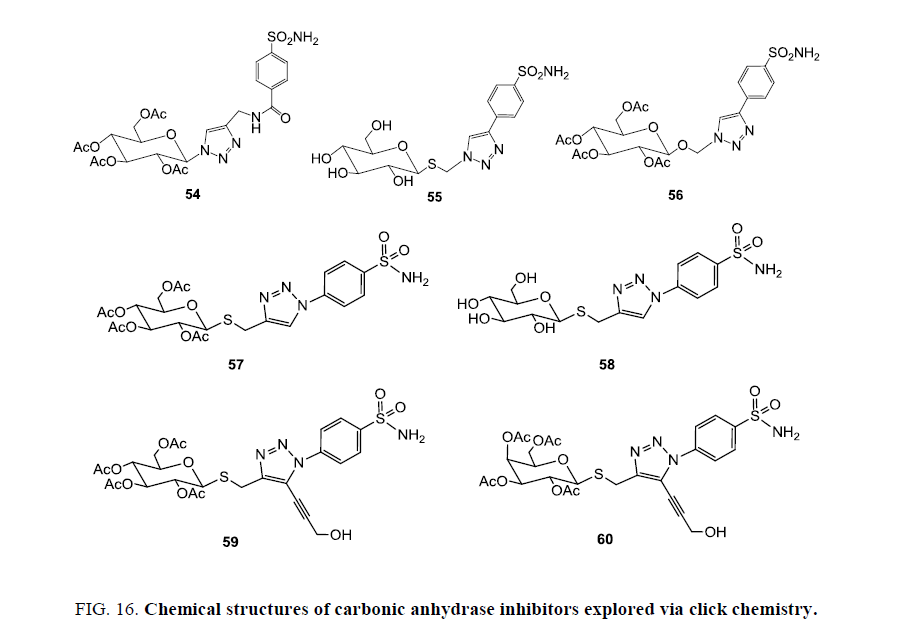
Novel glycoconjugate benzene sulfonamides were synthesized using click approach that contains diverse carbohydrate-triazole. Developed compounds were assessed for their ability to inhibit three human carbonic anhydrase (CA) isozymes in vitro: cytosolic hCA-I, hCA-II, hCA-XII, hCA-XI, and transmembrane, tumor-associated hCA-IX with Ki=4.3 nm against alpha hCA-XII 54, Ki=7.0 nm against alpha hCA-I 55, Ki=5.3 nm against alpha hCA-IIC 56 and Ki=8.6 nm against alpha hCA-XI 56 (Figure 16). Stereochemical diversity in carbohydrates part of the developed molecules interrogated the CA active site topology, to originate various inhibitors that were potent and selective toward hCA-IX. This was an important outcome in the quest for potential cancer therapy applications based on carbonic anhydrase inhibition [85-87].
Morris et al. [88] designed and developed new carbohydrate-based small molecule as CA inhibitors. Some of the synthesized molecules inhibit CA IX and XII within a narrow range of low nanomolar Ki values (5.3 nm to 11.2 nm). They evaluated also CA inhibitors in cell-based models first time that emulate the protective role of CA IX in an acidic tumour microenvironment. Out of various prepared molecules, two compounds 56 and 57 block CA IX-induced survival and have potential for development as in vivo cancer cell selective inhibitors. Compound 56 and a galactose derivative were in trisubstituted analogues 58 and 59, both with a short hydroxyl bearing group at the 5-position on the triazole, confirmed some noteworthy SAR regarding isozyme selectivity for the cancer associated CAs but compound 59 displayed much lower inhibition for both cytosolic CAs as compared to all other compounds (Figure 16) [88].
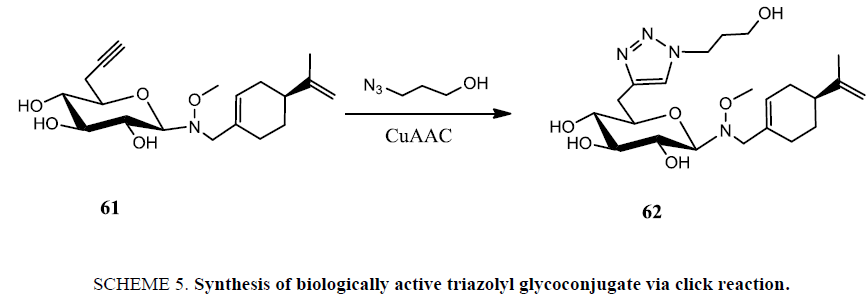
Recently, Nandurkar et al. [89] prepared a series of perillyl alcohol (POH) neoglycosides, and evaluated their antiproliferative activity against A549 and PC3 cell lines. Authors observed a sugar dependent in vitro antiproliferative activity and the inhibition of S6 ribosomal protein phosphorylation as a putative mechanism of action. In order to assess the compatibility of developed neoglycosides with Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition and the potential impact of sugar triazole substitution upon POH bioactivity, the 6’-azido perillyl glucoside was converted to the corresponding 6’-triazole perillyl glucoside via CuAAC in 82% yield. However, C6’-triazole substitution was detrimental to activity based upon the comparative activities of 61 (C6’-azido) and 62 (Scheme 5).
Conclusion and Future Perspective
Carbohydrates constitute a major class biomolecules lying inside or on the surface of cell and govern several pathologically and physiologically vital biological events such as cellular recognition, adhesion, migration, invasion, communication, bacterial/viral infection, tumor metastasis, post-translational modifications of protein etc. Therefore, it is challenging to craft glycoconjugates, which can fulfil the objective of understanding role of carbohydrates in these pivotal interactions and also regulate these processes majorly by acting as inhibitors of those enzymatic transformations. Several 1,2,3-triazole core containing drug like molecules with good pharmacokinetic properties have been synthesized, however, there is lot more to explore about this vast pool of triazolyl glycoconjugates. The triazole forming click reaction promisingly facilitates creation lead and its optimization due to its modular nature, reliance on extremely short sequences of near-perfect reactions. Therefore, it’s time to recognize the real potential of versatile click reaction and design highly active, selective, pharmacologically important triazolyl scaffolds, which can contribute to major advances in pharmaceutical sciences.
Acknowledgements
The author thanks Department of Chemistry, H. D. Jain College, Veer Kunwar Singh University, Arrah for the departmental and infrastructural facilities. Author gratefully acknowledges the help and consistent encouragement from Dr. R. T. Singh (Ex. Dean, Faculty of Science, VKSU, Arrah) during the preparation of manuscript.
References
- Tiwari VK, Mishra RC, Sharma A, et al. Carbohydrate based potential chemotherapeutic agents: recent developments and their scope in future drug discovery: Mini review. Med Chem. 2012;12(14):1497-1519.
- Cao H, Jeol H, Chen X. Carbohydrate-containing natural products in medicinal chemistry. In: Tiwari VK, Mishra BB, editors.Opportunity, Challenges and Scope of Natural Products in Medicinal Chemistry, Kerala: Research Signpost Publications, India; 2011. p. 411.
- Varki A. Biological roles of oligosaccharides: All of the theories are correct. Glycobiology. 1993;3(2):97-130.
- Oliveira RN, Sinou D, Srivastava, RMJ. Efficient synthesis of some unsaturated [1,2,3]?triazole?linked glycoconjugates. Carbohydr Chem. 2006;25:407-25.
- Bertozzi CR, Kiessling LL. Chemical glycobiology. Science. 2001;291(5512):2357-64.
- Holgersson J, Gustafsson A, Breimer ME. Characteristics of protein–carbohydrate interactions as a basis for developing novel carbohydrate-based antirejection therapies. ImmunolCell Biol. 2005;83:694-708.
- Lindhorst TK, Dubber M, Krallmann-Wenzel U, et al. Cluster Mannosides as Inhibitors of Type 1 Fimbriae-Mediated Adhesion of Escherichia coli: Pentaerythritol Derivatives as Scaffolds. EurJOrgChem. 2000;11:2027-34.
- Joosten JAF, Loimaranta V, Appeldoorn CCM, et al. Inhibition of Streptococcus suis Adhesion by Dendritic Galabiose Compounds at Low Nanomolar Concentration. JMedChem. 2004;47:6499-508.
- Fan E, Zhang Z, Minke WE, et al. High-Affinity Pentavalent Ligands of Escherichia coli Heat-Labile Enterotoxin by Modular Structure-Based Design. JAmChemSoc. 2000;122:2663-4.
- Vrasidas I, Andre S, Valentini P, et al. Rigidified multivalent lactose molecules and their interactions with mammalian galectins: a route to selective inhibitors. OrgBiomolChem. 2003;1:803-10.
- Wittmann V, Seeberger S. Spatial Screening of Cyclic Neoglycopeptides: Identification of Multivalent Wheat Germ Agglutinin Ligands. AngewChemIntEd. 2004;43:900-3.
- Kale RR, Prasad V, Tiwari VK. Facile synthesis of novel glycosyl carboxamide with sugar in furanose and pyranose form using benzotriazole methodology. LettOrgChem. 2010;7:136-43.
- Anand N, Jaiswal N, Pandey SK, et al. Application of click chemistry towards an efficient synthesis of 1,2,3-1H-triazolyl glycohybrids as enzyme inhibitors. Carbohydr Res. 2011;346(1):16-25.
- Velázquez S, Alvarez R, Pérez C, et al. Regiospecific synthesis and anti-human immuno-deficiency virus activity of novel 5-substituted N-alkylcarbamoyl and N,N-dialkyl carbamoyl 1,2,3-triazole-TSAO analogues anti-human. Antiviral ChemChemother. 1998;9:481-9.
- Wilkinson BL, Long H, Sim E, et al. Synthesis of Arabinoglycosyltriazoles as potential inhibitors of mycobacterial cell wall biosynthesis. BioorgMedChemLett. 2008;18:6265-7.
- Reddy LVR, Mishra NN, Shukla PK, et al. Synthesis and biological evaluation of glycal-derived novel tetrahydrofuran 1,2,3-triazoles by 'click' chemistry. CarbohydrRes. 2010;345:1515-21.
- Carvalho I, Andrade P, Campo VL, et al. 'Click chemistry' synthesis of a library of 1,2,3-triazole-substituted galactose derivatives and their evaluation against Trypanosoma cruzi and its cell surface trans-sialidase. Bioorg Med Chem.2010;18(7):2412-27.
- Singh BK, Yadav AK, Kumar B, et al. Preparation and reactions of sugar azides with alkynes: synthesis of sugar triazoles as antitubercular agents. Carbohydr Res. 2008;343:1153-62.
- Huisgen R. 1,3-dipolar cycloadditions. Past and future. AngewChemInt Ed. 1963;2:565-98.
- Huisgen R. Kinetics and mechanism of 1,3-dipolar cycloadditions. AngewChemInt Ed. 1963;2:633-96.
- Meldal CW, Tornoe C, Meldal M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. JOrgChem. 2002;67(9):3057-64.
- Kolb HC, Finn MG, Sharpless KB. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. AngewChemInt Ed. 2001;40(11):2004-21.
- Rostovtsev VV, Green LG, Fokin VV, et al. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective Ligation of Azides and Terminal Alkynes. AngewChemInt Ed. 2002;41:2596-99.
- Zhang L, Chen X, Xue P, et al. Ruthenium-Catalyzed Cycloaddition of Alkynes and Organic Azides. JAmChemSoc. 2005;127(46):15998-9.
- Rasmussen LK, Boren BC, Fokin VV. Ruthenium-Catalyzed Cycloaddition of Aryl Azides and Alkynes. OrgLett. 2007;9(26):5337-9.
- Dondoni A. Triazole: the keystone in glycosylated molecular architectures constructed by a click reaction. ChemAsian J. 2007;2(6):700-8.
- Hein JE, Fokin VV.Copper-catalyzed azide–alkyne cycloaddition (CuAAC) and beyond: new reactivity of copper(I) acetylides. ChemSocRev. 2010;39:1302-15.
- Dondoni A, Marra A. Calixarene and Calixresorcarene Glycosides: Their Synthesis and Biological Applications. ChemRev. 2010;110(9):4949-77.
- Holub JM, Kirshenbaum K. Tricks with clicks: modification of peptidomimetic oligomers via copper-catalyzed azide-alkyne [3 + 2] cycloaddition. ChemSocRev. 2010;39:1325-37.
- Tron GC, Pirali T, Billington RA, et al. Click chemistry reactions in medicinal chemistry: applications of the 1,3-dipolar cycloaddition between azides and alkynes. Med Res Rev. 2008;28(2):278-308.
- Kappe CO, der Eycken EV. Click chemistry under non-classical reaction conditions. ChemSocRev. 2010;39:1280-90.
- Aragao-Leoneti V, Campo VL, Gomes AS, et al. Application of copper (I)-catalysedazide/alkyne cycloaddition (CuAAC)‘click chemistry’in carbohydrate drug and neoglycopolymer synthesis. Tetrahedron. 2010;66(49):9475-92.
- Tiwari VK, Mishra BB, Mishra KB, et al. Cu-Catalyzed Click Reaction in Carbohydrate Chemistry. Chem Rev. 2016;116:3086-40.
- Kushwaha D, Dwivedi P, Kuanar SK, et al. Click reaction in carbohydrate chemistry: recent developments and future perspective. Curr Org Synth. 2013;9:89-135.
- He XP, Zeng YL, Zang Y, et al. CarbohydrRes. 2016;429:1-22.
- Wilkinson BL, Bornaghi LF, Poulsen SA, et al. Synthetic utility of glycosyltriazoles in carbohydrate chemistry. Tetrahedron. 2006;62(34):8115-25.
- Rodionov VO, Fokin VV, Finn MG. Mechanism of the ligand-free CuI-catalyzed azide-alkyne cycloaddition reaction. AngewChemInt Ed. 2005;44(15):2210-15.
- Bock VD, Hiemstra H, Maarseveen JHV. CuI-Catalyzed Alkyne–Azide “Click” Cycloadditions from a Mechanistic and Synthetic Perspective. Eur J Org Chem. 2006;51-68.
- Straub BF. µ-Acetylide and µ-alkenylidene ligands in “click” triazole syntheses. ChemCommun. 2007;37:3868-3870.
- Himo F, Lovell T, Hilgraf R, et al. Copper(I)-catalyzed synthesis of azoles. DFT study predicts unprecedented reactivity and intermediates. J Am Chem Soc. 2005;127(1):210-16.
- Ahlquist M, Fokin VV. Enhanced Reactivity of Dinuclear Copper(I) Acetylides in Dipolar Cycloadditions. Organometallics. 2007;26(18):4389-4439.
- Zhou Y, Lecourt T, Micouin L. Direct synthesis of 1,4- disubstituted-5-alumino-1,2,3-triazoles: copper-catalyzed cycloaddition of organic azides and mixed aluminiumacetycides. AngewChemInt Ed. 2010;49:2607-10.
- Meldal M, Tornoe CW. Cu-catalyzed azide-alkyne cycloaddition. Chem Rev. 2008;108(8):2952-3015.
- Wong CH. Carbohydrate-based Drug Discovery. New York: Wiley-VCH, USA; 2003.
- He XP, Deng Q, Gao LX, et al. Facile fabrication of promising protein tyrosine phosphatase (PTP) inhibitor entities based on ‘clicked’serine/threonine–monosaccharide hybrids. Bioorg Med Chem. 2011;19(13):3892-900.
- Li C, He XP, Zhang YJ, et al. Click to a focused library of benzyl 6-triazolo(hydroxy)benzoic glucosides: novel construction of PTP1B inhibitors on a sugar scaffold. Eur J Med Chem. 2011;46(9):4212-8.
- Wang J, He X, Gao L, et al. Synthesis of Triazole-Linked Amino Acid-Aryl C-Glycoside Hybrids via Click Chemistry as Novel PTP1B Inhibitors. J Chin Chem. 2011;29(6):1227-32.
- He XP, Li C, Jin XP, et al. Microwave-assisted construction of triazole-linked amino acid–glucoside conjugates as novel PTP1B inhibitors. New J Chem. 2011;35(3):622-631.
- Butters TD, Dwek RA, Platt FM, et al. Therapeutic applications of imino sugars in lysosomal storage disorders. CurrTopMedChem. 2003;3:561-74.
- Platt FM, Neises GR, Reinkensmeier G, et al. Prevention of lysosomal storage in Tay-Sachs mice treated with N-butyldeoxynojirimycin. Science. 1997;276(5311):428-31.
- Greimel P, Spreitz J, Stutz AE. Iminosugars and relatives as antiviral and potential anti-infective agents. Curr Top Med Chem. 2003;3(5):513-23.
- Zitzmann N, Mehta AS, Carrouee S. Imino sugars inhibit the formation and secretion of bovine viral diarrhea virus, a pestivirus model of hepatitis C virus: implications for the development of broad spectrum anti-hepatitis virus agents. Proc Natl Acad Sci USA. 1999;96(21):11878-82.
- Gross PE, Baker MA, Carver JP, et al. Inhibitors of carbohydrate processing: A new class of anticancer agents. Clin Cancer Res. 1995;1(9):935-44.
- Perion R, Ferrieres V, Garcia-Moreno MI, et al. 1,2,3-Triazoles and related glycoconjugates as new glycosidase inhibitors. Tetrahedron 2005;61:9118-28.
- Dedola S, Hughes DL, Field RA, et al. Synthesis of alpha- and beta-D-glucopyranosyltriazoles by CuAAC 'click chemistry: reactant tolerance, reaction rate, product structure and glucosidase inhibitory properties. Carbohydr Res. 2010;345:1123-34.
- Rossi LL, Basu A. Glycosidase inhibition by 1-glycosyl-4-phenyl triazoles. Bioorg Med Chem Lett. 2005;15:3596-99.
- Wu P, Malkoch M, Hunt JN, et al. Multivalent, bifunctional dendrimers prepared by click chemistry. ChemCommun. 2005:5775-7.
- Polakova M, Stanton R, Wilson IBH, et al. 'Click chemistry' synthesis of 1-(α-D-mannopyranosyl)-1,2,3-triazoles for inhibition of α-mannosidases. CarbohydrRes. 2015;406:34-40.
- Zhou Y, Zhao Y, O’Boyle KM, et al. Hybrid angiogenesis inhibitors: synthesis and biological evaluation of bifunctional compounds based on 1-deoxynojirimycin and aryl-1,2,3-triazoles. Bioorg Med Chem Lett. 2008;18(3):954-8.
- Hosoguchi K, Maeda T, Furukawa JI, et al. An efficient approach to the discovery of potent inhibitors against glycosyltransferases. Med Chem. 2010;53(15):5607-19.
- Li T, Guo L, Zhang Y, et al. Design and synthesis of O-GlcNAcase inhibitors via 'click chemistry' and biological evaluations. Carbohydr Res. 2011;346:1083-92.
- Cheng K, Liu J, Liu X, et al. Synthesis of glucoconjugates of oleanolic acid as inhibitors of glycogen phosphorylase. Carbohydr Res. 2009;344(7):841-50.
- Cheng K, Liu J, Sun H, et al. Tethered derivatives of D-glucose and pentacyclic triterpenes for homo/heterobivalent inhibition of glycogen phosphorylase. New J Chem. 2010;34:1450-64.
- Tejler J, Tullberg E, Frejd T, et al. Synthesis of multivalent lactose derivatives by 1,3-dipolar cycloadditions: Selective galectin-1 inhibition. Carbohydr Res 2006;341:1353-62.
- Gao YJ, Eguchi A, Kakehi K. Synthesis and molecular recognition of carbohydrate-centered multivalent glycoclusters by a plant lectin RCA120. Bio Org Med Chem. 2005;13(22):6151-57.
- Giguere D, Patnam R, Bellefleur MA, et al. Carbohydrate triazoles and isoxazoles as inhibitors of galectins-1 and -3. ChemCommun. 2006;14(22):2379-81.
- Cecioni S, Matthews SE, Blanchard H, et al. Synthesis of lactosylatedglycoclusters and inhibition studies with plant and human lectins. CarbohydrRes. 2012;356:132-41.
- Peterson LB, Blagg BSJ. Click chemistry to probe Hsp90: synthesis and evaluation of a series of triazole-containing novobiocin analogs. Bioorg Med Chem Lett. 2010;20:3957-60.
- Parida PK. Sau A, Ghosh T, et al. Synthesis and evaluation of triazole linked glycosylated 18β-glycyrrhetinic acid derivatives as anticancer agents. Bioorg Med Chem Lett. 2014;24:3865-68.
- Hradilova L, Polakova M,Dvorakova B, et al. Synthesis and cytotoxicity of some D-mannose clil conjugates with aminobenzoic acid derivatives. CarbohydrResearch. 2012;361:1-6.
- Titz A, Patton J, Smiesko M, et al. Probing the carbohydrate recognition domain of E-selectin: The importance of the acid orientation in sLe x mimetics. Bioorg Med Chem. 2010;18:19-27.
- Lee T, Cho M, Ko SY. Synthesis and evaluation of 1,2,3-triazole containing analogues of the immunostimulant alpha-GalCer. J Med Chem. 2007;50:585-9.
- Slamova K, Marhol P, Bezouska K, et al. Synthesis and biological activity of glycosyl-1H-1,2,3-triazoles. Bioorg Med Chem Lett. 2010;20:4263-5.
- Lu WY, Sun XW, Zhu C, et al. Expand the application scope of glycosidase using click reaction. Tetrahedron. 2010;66:750-7.
- Zhang J, Chiang FI, Wu L, et al. Surprising alteration of antibacterial activity of 5″-modified neomycin against resistant bacteria. J Med Chem. 2008;51:7563-73.
- Li J, Zheng M, Tang W, et al. Syntheses of triazole-modified zanamivir analogs via click chemistry and anti-AIV activities. Med Chem Lett 2006;16:5009-13.
- Weiwer M, Chen CC, Kemp MM, et al. Synthesis and Biological Evaluation of Non?Hydrolyzable 1, 2, 3?Triazole?Linked Sialic Acid Derivatives as Neuraminidase Inhibitors. Eur J Org Chem. 2009;2611-20.
- Kushwaha D, Tiwari VK. Click chemistry inspired synthesis of glycoporphyrin dendrimers. J Org Chem. 2013;78(16):8184-90.
- Mishra BB, Tiwari VK, Mishra KB. Efficient synthesis of ethisterone glycoconjugate via bis-triazole linkage. CarbohydrRes. 2014;399:02-7.
- Mishra A, Tiwari VK. One-Pot Synthesis of Glycosyl-β-azido Ester via Diazotransfer Reaction Toward Access of Glycosyl-β-triazolyl Ester. J Org Chem. 2015;80(10):4869-81.
- Mishra KB, Tiwari VK. Click Chemistry Inspired Synthesis of Morpholine-Fused Triazoles. J Org Chem. 2014;79(12):5752-62.
- Mishra KB, Shashi S, Tiwari VK, Metal free synthesis of morpholine fused [5, 1-c] triazolyl glycoconjugates via glycosyl azido alcohols. RSC Adv. 2015;5(105):86840-8.
- Dwivedi P, Mishra KB, Mishra BB, et al. Click inspired synthesis of antileishmanial triazolyl O-benzylquercetin glycoconjugates. Glycoconjugate J. 2015;32(4):127-40.
- Mishra KB, Mishra RC, Tiwari VK. First noscapine glycoconjugates inspired by click chemistry. RSC Adv. 2015;5(64):51779-89.
- Singer M, Lopez M, Bornaghi LF, et al. Inhibition of carbonic anhydrase isozymes with benzene sulfonamides incorporating thio, sulfinyl and sulfonyl glycoside moieties. Bioorg Med Chem Lett. 2009;19(8):2273-6.
- Wilkinson BL, Bornaghi LF, Houston TA, et al. Carbonic anhydrase inhibitors: inhibition of isozymes I, II, and IX with triazole-linked O-glycosides of benzene sulfonamides. J Med Chem. 2007;50(7):1651-7.
- Winum JY, Poulsen SA, Supuran CT. Therapeutic applications of glycosidic carbonic anhydrase inhibitors. Med Res Rev. 2009;29(3):419-35.
- Morris JC, Chiche J, Grellier C, et al. Targeting Hypoxic Tumor Cell Viability with Carbohydrate-Based Carbonic Anhydrase IX and XII Inhibitors. J Med Chem. 2011;54(19):6905-18.
- Nandurkar NS, Zhang J, Thorson JS, et al. The Identification of Perillyl Alcohol Glycosides with Improved Antiproliferative Activity. J Med Chem. 2014;57(17):7478-84.