Research
, Volume: 19( 1) DOI: 10.4172/tsnp.2023.19(1).001The Chemical Constituents of Soursop Fruit Peels (Annona muricata L.) and their Biological Activities
- *Correspondence:
- Gautam Patil
Department of Chemistry,
Government College Bichhua,
Bichhua, Madhya Pradesh,
India
Tel: 9329762703
E-mail: gautamchem23@gmail.com
Received: October 15, 2022, Manuscript No. TSNP-22-77388; Editor assigned: October 17, 2022, PreQC No. TSNP-22-77388 (PQ); Reviewed: October 31, 2022, QC No. TSNP-22-77388; Revised: January 10, 2023, Manuscript No. TSNP-22-77388 (R); Published: January 17, 2023, DOI: 10.37532/ 0974-7508.23.19.003
Citation: Patil G, Khan S, Pawar D. The Chemical Constituents of Soursop Fruit Peels (Annona muricata L.) and their Biological Activities. Nat Prod Ind J. 2023;19(1):003.
Abstract
In this study, chemical constituents and biological activities of the Annona muricata L. fruit peels were evaluated using Methanol Extract (MEAM) and Hexane (HFAM), Dichloromethane (DFAM), Ethyl Acetate (EFAM) and Butanol (BFAM) fractions. Phytochemical screening (specific chemical reactions), total phenolic and flavonoid contents (spectrophotometric methods) and chemical compounds were assessed (High performance liquid chromatography analysis). The antioxidant activity was determined by 2,2-Diphenyl-1-Picrylhydrazyl (DPPH), Ferric Reducing Antioxidant Power (FRAP), beta-carotene and thiobarbituric acid assays. The inhibitory effect against digestive enzymes (lipase, α-amylase and α-glucosidase) was measured by spectrophotometric assays and toxicity by the brine shrimp lethality bioassay. Flavonoids, tannins, coumarins, terpenes, steroids, saponins and alkaloids were detected. EFAM had the highest values of total phenolic and flavonoids, while a similar compound to annonacin was found in MEAM by HPLC. EFAM was also more active in DPPH and FRAP assays and HFAM was effective in inhibiting the linoleic acid oxidation and the malondialdehyde. MEAM and fractions blocked lipase, α-amylase and α-glucosidase, while HFAM and DFAM were toxic against Artemia salina. The results showed that the A. muricata fruit peels have important biological effects, which can bring great benefits to human and animal health.
Keywords
Annonacin; Antioxidant; Annona muricata, Phenolic compounds; Anti-digestive enzyme; Toxicity
Introduction
Annona muricata is a member of the Annonaceae family and is a fruit tree with a long history of traditional use. A. muricata, also known as soursop, graviola and guanabana, is an evergreen plant that is mostly distributed in tropical and subtropical regions of South and Central America, Africa, Asia and also whole part of the world approximately.
A large number of chemical and biological investigations have been performed in fruits and vegetables, but only a few of them involve waste parts of fruits, as seeds and peels [1,2]. These products are usually thrown in the garbage, but they can be sources of bioactive compounds from extractive, nutritional and biotechnological processes. Among the active constituents, vitamins, minerals, natural pigments and phenolic compounds present in fruits and vegetables are highlighted by their antioxidant activity, since they avoid the oxidation of metabolic reactions, acting both in the initiation stage and in the propagation of the oxidative process. Besides, these constituents can be important in the prevention and treatment of diseases caused by free radical, such as neurodegenerative diseases, diabetes, dyslipidemia, cardiovascular disorders, obesity and cancer among others (Figures 1-4) [3-5].
The fruits are used as natural medicine and consist of a white edible pulp containing protein, carbohydrate and vitamins B and C, as well as esters of aliphatic acids and mono and sesquiterpenes. Alkaloids (annonaine, nornuciferine and asimilobine for example), annonaceous acetogenin epomusenin-A, epomusenin-B and epomurinin-B, among others) and phenolic compounds (5-caffeoylquinic acid, dihydro-kaempferol-hexoside and caffeic acid derivative, for example) were also identified in the fruits, which have been related to several biological properties [6,7]. From a pharmacological point of view, anti-inflammatory and anti-nociceptive, antitumor, anti-arthritic, antibacterial, anticonvulsant, antidiabetic and hypolipidemic and antihypertensive activities, as well as relevant antioxidant action have been reported for A. muricata using extracts from leaves [8].
Considering that the fruit peels are commonly discarded and may represent a strategy for the search for active compounds and nutrients and the oxidative mechanisms are associated with metabolic processes and toxicity, this study investigated the chemical potential and the biological properties of the A. muricata fruit peels [9-11].
Materials and Methods
Plant material
Annona muricata L. (Annonaceae) has been cultivated in Southeast Asia like Indonesia and Vietnam country.
The imported a voucher specimen (GCBCHEM 06072022), identified by Dr. Verma N was deposited in the Herbarium of the botanical laboratory. Mature fruits, suitable for consumption, were collected (January to February 2021).
Reagents and chemicals
Aluminum chloride, calcium chloride, potassium chloride, magnesium chloride, potassium bromide, sodium sulfate, sodium bicarbonate, dimethylsulfoxide, potassium ferrocyanide, ferric chloride, sodium carbonate, Folin-Ciocalteu reagent, trichloroacetic acid, sodium chloride, dichloromethane, hexane, butanol, methanol, ethanol, pyridine, 2,2-Diphenyl-1-Picrylhydrazyl (DPPH), linoleic acid, β-carotene, tween 40, Butylated Hydroxytoluene (BHT), gallic acid, rutin, caffeic acid, 5-caffeoylquinic acid, arcabose, orlistat, pancreatic lipase, pancreatic α-amylase α-glycosidase (Sigma Chemical Co, St. Louis,MI, USA) and ascorbic acid [12-15].
Extract preparation
Dried and powdered peels (80 g) were extracted in methanol by static maceration for 3 weeks. The Methanol Extract (MEAM) was filtered and evaporated under a rotary vacuum evaporator (Rotavapor RII, Büchi, Flawil, Switzerland) at controlled temperature (50°C ± 1°C) and yielded 12.78 g. ME (9.45 g) was suspended in water: Methanol (9:1) followed by liquid/liquid partition to obtain the Hexane (HFAM), Dichloromethane (DFAM), Ethyl Acetate (EFAM) and Butanol (BFAM) fractions, which yielded 2.36 g, 1.07 g, 1.28 g and 2.12 g, respectively [16,17].
Phytochemical screening
Chemical constituents (tannins, flavonoids, terpenes and phytosterols, saponins, coumarins, anthraquinones and alkaloids) were investigated with specific reagents.
Total phenolic content determination
As recommended by Sousa, et al., the total phenolic content was determined by Folin-Ciocalteu method using Gallic Acid (GA) as standard. The Folin-Ciocalteu reagent oxidized phenolic compounds present in the samples whose reaction was neutralized with sodium carbonate. After 60 min, in triplicate, the absorbance was measured at 765 nm. The results were expressed as gram of gallic acid equivalent (g GAE/100 g) [18-20].
Total flavonoidal content determination
MEAM, EFAM and BFAM were dissolved in methanol (2 mg/mL) and filtered (0.45 μm filter) and HPLC analysis was performed using an Agilent technologies 1200 series composed of a quaternary pump with a PDA detector and an automatic injector. The column employed was a reversed phase C18 silica column Zorbax SB-18 (4.6 mm × 150 mm; 5 μm of particle size). The mobile phase was composed of ultrapure water (solvent A) and methanol (solvent B). After injection (20 μL), the extract was eluted in a gradient in which the concentration of eluent B was increased from 5% to 75% in 5 min, followed by a 15 minutes gradient increase from 75% to 100% [21-23]. The final gradient condition was maintained for an additional 15 min. The elution flow was 0.8 mL/min and the column temperature was kept at 25°C. Detection was performed at 230 nm. Markers, as quercetin, rutin, gallic acid, caffeic acid and 5-caffeoylquinic acid, were used to identify compounds present in MEAM [24].
High Performance Liquid Chromatography (HPLC) analysis
The total flavonoid content was evaluated spectro photometrically using rutin as standard (from 2 μg/mL to 30 μg/mL). After reaction in media containing acetic acid, pyridine: Ethanol (2:8), 8% aluminum chloride and distilled water at room temperature for 30 min, the absorbance was measured at 420 nm. The results, in triplicate, were expressed as gram of rutin equivalent [25].
DPPH radical sequestration method
The antioxidant activity was determined following the DPPH method described by Mensor, et al. In reaction medium, antioxidant compounds present in the samples neutralized the DPPH (0.03 mM in methanol) after 60 min of incubation. In triplicate, the absorbance was recorded at 518 nm (spectrophotometer Shimadzu, UV-1800). The percentage of Antioxidant Activity (%AA) and the half maximal Inhibitory Concentration (IC50) were determined. Rutin was used as standard.
Ferric reducing antioxidant power assay
The Ferric Reducing Antioxidant Power (FRAP) assay was done according to Oyaizu. Samples and ascorbic acid were added to the medium containing phosphate buffer pH 6.6 and potassium ferrocyanide. After incubation, in triplicate, this mixture reacted with trichloroacetic acid. The supernatant was removed after centrifugation and mixed with distilled water and ferric chloride. The absorbance was recorded at 700 nm in a spectrophotometer (Shimadzu, UV-1800). Absorbance 0.5 was considered as half-maximal Inhibitory Concentration (IC50). Ascorbic acid was used as positive control [26,27].
β-carotene/linoleic acid assay
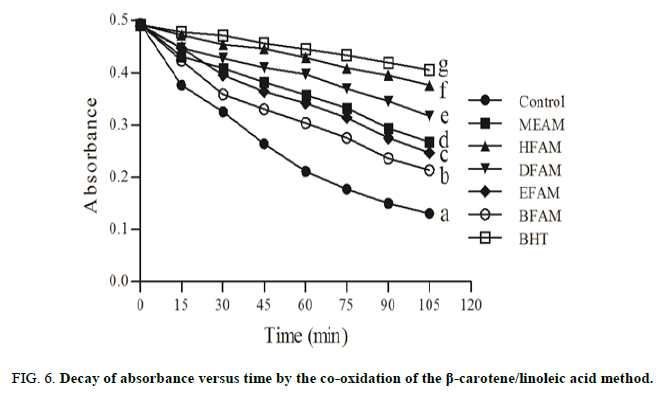
After removal of the solvent, distilled water was added to form an emulsion. Due to the presence of lipophilic compounds in the samples, the linoleic acid and β-carotene system was used to determine the lipid peroxidation inhibition. In this assay, in triplicate, β-carotene (in chloroform), linoleic acid and tween 40 were mixed into a rotaevaporation flask. The assay was performed in a microplate reader (Thermo plate, TP-Reader) and the absorbance was recorded at 492 nm every 15 minutes during 105 minutes. The graph of decay (absorbance x time) and the Inhibition of Lipid Peroxidation (ILP) were determined. Butylated Hydroxyl Toluene (BHT) was used as standard [28-30].
Thiobarbituric acid method
As described by Souza, et al., the anti-lipase activity was evaluated using orlistat as control positive. Solution of porcine pancreatic lipase (Sigma, 10 mg/mL) in Tris-HCl buffer (0.05 mol/L, pH 8.0) containing CaCl2 (0.010 mol/L) and NaCl (0.025 mol/L) was prepared, while the ρ-nitrophenolpalmitate (substrate) was dissolved in Triton-X 100 (0.5%, p/v) and the samples and orlistat (Sigma) were prepared at increasing concentrations (10 μg/mL-1,000 μg/mL). Microplates containing of enzyme solution (100 μL), substrate (50 μL) and sample/orlistat (50 μL) were incubated were incubated in a water bath (37°C) for 10 minutes, 20 minutes, 30 minutes and 40 minutes [31]. The reaction was stopped with an ice bath and absorbances were measured in a micro plate reader (Thermoplate, TP-Reader) at 405 nm.
Pancreatic lipase assay
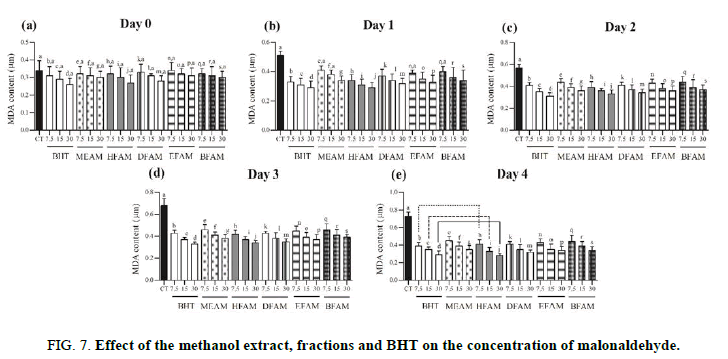
As recommended by Zeb and Ullah, the thiobarbituric acid method was used in this test. From 25 g of low fat ground beef, a homogenate was prepared with distilled water and 7.5 mg, 15 mg or 30 mg of each sample with heating. The homogenate was transferred to the test tubes containing BHT, phosphoric acid and thiobarbituric acid. After cooling in an ice bath, butanol was added to each tube and centrifuged. The formation of the chromogenic complex was measured at 535 nm in a spectrophotometer (Shimadzu, UV-1800) [32,33]. The concentration of the thiobarbituric-malonaldehyde acid complex was calculated from the standard Malonaldehyde (MDA) curve.
Pancreatic α-amylase assay
Pancreatic α-amylase inhibitory activity was performed according to the methodology proposed by Freitas, et al., with some modifications. Porcine pancreatic α-amylase (Sigma, 1 mg/mL) in TRIS-HCl buffer containing CaCl2 was prepared while starch was made. Samples and acarbose were solubilized in DMSO. Microplates containing α-amylase (50 μL), samples or acarbose (50 μL) and substrate (50 μL) were preincubated in a water bath at 37°C for 10 minutes. After this time, the substrate (100 μL) was added to the medium and the reaction was incubated in a water bath at 37°C for 10 minutes, 20 minutes, 30 minutes and 40 minutes. The reaction was stopped with an ice bath and absorbances were measured in a microplate reader (Thermoplate, TP-Reader) at 405 nm.
α-Glucosidase assay
α-Glucosidase inhibitory activity was determined according to the methodology proposed by Chelladurai and Chinnachamy, with some modifications. Solutions of α-glycosidase and ρ-nitrophenyl-α-D-glycopyranoside substrate were solubilized in citrate phosphate buffer, while samples and acarbose (Sigma) were prepared at increasing concentrations and solubilized in DMSO. Microplates containing α-glucosidase, samples or acarbose and substrate were incubated in a water bath at 37°C for 10 minutes, 20 minutes, 30 minutes and 40 minutes. The reaction was stopped with an ice bath and absorbances were measured in a microplate reader at 405 nm [34].
Determination of Inhibition percentage (I%) and half-maximal Inhibitory Concentration (IC50)
Absorbance values obtained from digestive enzyme assays were used to calculate the Inhibition percentage (I%) by linear regression using the least square method according to the equation 1 below.
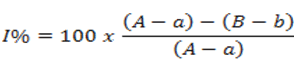
Where, A=Angle coefficient value of enzyme only reading (enzyme+substrate); a: Value of the angular coefficient of the reading without enzyme and without sample (substrate); B: Angular coefficient value of enzyme plus inhibitor reading (enzyme+substrate+inhibitor); b=Angle coefficient value of reading without enzyme and inhibitor.
The half-maximal Inhibitory Concentration (IC50) was determined by linear least square regression using samples and Inhibition percentage (I%) [35].
Brine shrimp lethality bioassay
The brine shrimp lethality bioassay was performed according to Meyer, et al. In this assay, five concentrations of MEAM, HFAM, DFAM, EFAM and BFAM and thymol (positive control) were prepared in artificial seawater and transferred to the test tubes. Then, ten shrimps (Artemia salina Leach) were placed in each tube (n=4). After 24 hours of exposure, the surviving larvae were counted and the 50% Lethal Concentration (LC50) was determined by the probit method. This assay has been used to assess the toxicity of acetogenins present in plants of the Annonaceae family.
Statistical analysis
The results were expressed as mean ± Standard Error Mean (SEM). Analysis of Variance (ANOVA) followed by Tukey’s HSD (Honest Significant Difference) test was applied to measure the degree of significance for p<0.05. Graph pad prism 5.0 Software was used in this analysis.
Results and Discussion
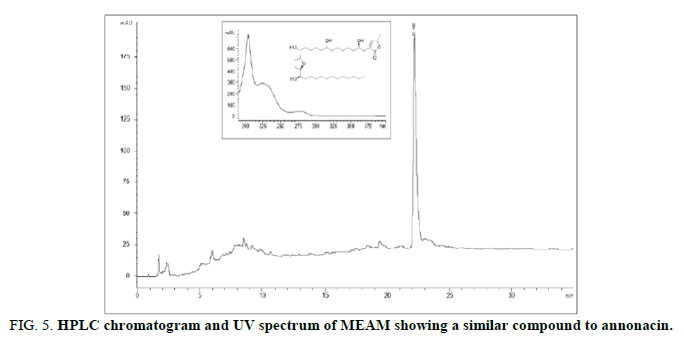
According to the polarity of the solvent, these chemical classes were also detected in HFAM (terpenes and steroids), DFAM (terpenes, steroids and alkaloids), EFAM (tannins, flavonoids, coumarins and alkaloids) and BFAM (tannins, flavonoids, coumarins, saponins and alkaloids). Tannins, flavonoids, coumarins, terpenoids and steroids, saponins and alkaloids were revealed in MEAM. Considering the quantification of constituents, total phenolic varied from 0.40 ± 0.03 g/100 g to 8.45 ± 0.05 g/100 g and flavonoid ranged from 1.35 ± 0.07 g/100 g to 2.74 ± 0.06 g/100 g (Table 1). EFAM exhibited the highest phenolic and total flavonoid contents. In addition, after HPLC analysis, the markers were not identified. However, considering the retention time and UV spectrum, a compound with similar characteristics to annonacin, an acetogenin from Annonaceae, was detected (Figure 5). Due to the lack of standard compound, it was not possible to confirm the authenticity of this substance [36].
| Tested product | Total phenolic (g GAE/100 g) | Total flavonoid (g RUE/100 g) |
|---|---|---|
| MEAM | 6.57 ± 0.16a | 1.35 ± 0.07a |
| HFAM | 0.40 ± 0.03b | - |
| DFAM | 1.38 ± 0.02c | - |
| EFAM | 8.45 ± 0.05d | 2.74 ± 0.06b |
| BFAM | 4.54 ± 0.06e | 1.75 ± 0.05c |
| Note: Mean ± SEM (n=3). Different letters, there was significant difference between the means (P<0.05) after ANOVA-Tukey's test. (-) Not detected or not quantified. | ||
TABLE 1. Total phenolic and flavonoid contents of A. muricata.
Using DPPH assay, the IC50 values of the samples were statistically different (P<0.05) and ranged from 20.03 ± 0.08 to 204.50 ± 1.12 μg/mL, while FRAP varied from 16.35 ± 0.04 to 249.70 ± 2.54 μg/mL (Table 2). According to the Table 1, EFAM (20.03 ± 0.08 and 16.35 ± 0.04 μg/mL) was more active in both methods, respectively, when compared to the other fractions and extract (Figures 6 and 7).
| Tested product | IC50 (µg/mL) | ILP (%) | |
|---|---|---|---|
| DPPH | FRAP | ||
| MEAM | 67.57 ± 0.58a | 81.75 ± 0.78a | 37.70 ± 1.10a |
| HFAM | 204.5 ± 1.12b | 249.7 ± 2.54b | 68.26 ± 0.41b |
| DFAM | 82.26 ± 0.13c | 171.50 ± 0.84c | 51.83 ± 0.48c |
| EFAM | 20.03 ± 0.08d | 16.35 ± 0.04d | 33.30 ± 0.60d |
| BFAM | 31.61 ± 0.46e | 25.96 ± 0.11e | 23.02 ± 1.29e |
| Rutin | 10.58 ± 0.14f | - | - |
| Ascorbic acid | - | 7.45 ± 0.10f | - |
| BHT | - | - | 75.82 ± 0.92f |
| Note: Mean ± S.E.M. (n=3). Different letters, there was significant difference between the means (P<0.05) after ANOVA-Tukey's test. (-) Not detected or not quantified. | |||
TABLE 2. Antioxidant activity of A. muricata.
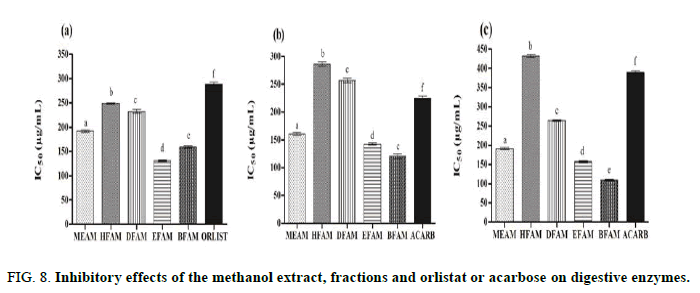
The IC50 values of MEAM, HFAM, DFAM, EFAM and BFAM on pancreatic lipase were 192.13 μg/mL ± 2.31 μg/mL, 248.67 μg/mL ± 1.28 μg/mL, 232.51 μg/mL ± 3.50 μg/mL, 131.14 μg/mL ± 1.70 μg/mL and 159.32 μg/mL ± 1.70 μg/mL, respectively (Figure 8). In this figure, these values were significantly different (P<0.001) when compared to orlistate (289.07 μg/mL ± 3.65 μg/mL) and these extracts were more potent in inhibiting pancreatic lipase.
Acarbose, a synthetic pancreatic α-amylase inhibitor, produced IC50 of 225.14 ± 4.11 μg/mL, being significantly (P<0.001) less potent than MEAM (160.60 ± 2.29), EFAM (142.42 ± 1.95) and BFAM (120.43 ± 3.88). HFAM (286.02 ± 4.08) and DFAM (257.1 ± 3.63) also inhibited pancreatic α-amylase, but were less active than positive control [37,38].
Regarding α-glucosidase inhibition, acarbose produced IC50 of 389.3 μg/mL ± 4.01 μg/mL, while MEAM (191.1 μg/mL ± 2.67 μg/mL), DFAM (264.4 μg/mL ± 2.51 μg/mL), EFAM (157.3 μg/mL ± 2.64 μg/mL) and BFAM (109.3 μg/mL ± 1.76 μg/mL) were more active. Although HFAM (432.02 μg/mL ± 3.39 μg/mL) inhibited α-glucosidase, it was significantly (P<0.001) less potent than acarbose and other extracts.
FIG 8: Inhibitory effects of the methanol extract, fractions and orlistat or acarbose on digestive enzymes.
After the Artemia salina toxicity test, MEAM, HFAM and DFAM were cytotoxic producing LC50 values lower than 1,000 μg/mL (Table 3). According to this table, HFAM (LC50=184.30 μg/mL) and DFAM (LC50=149.53 μg/mL) fractions were more active than thymol (LC50=433.23 μg/mL), used as reference substance.
| Tested product | Concentrations (mg/mL) | LC50 (mg/mL) | Confidence interval (95%) |
|---|---|---|---|
| MEAM | 10, 50, 100, 500 and 1,000 | 385.24 | 264.68-560.74 |
| HFAM | 10, 50, 100, 500 and 1,000 | 184.3 | 129.45-262.40 |
| DFAM | 10, 50, 100, 500 and 1,000 | 149.53 | 103.48-216.07 |
| EFAM | 10, 50, 100, 500 and 1,000 | >1000 | - |
| BFAM | 10, 50, 100, 500 and 1,000 | >1000 | - |
| Thymol | 10, 50, 100, 500 and 1,000 | 433.23 | 305.88-613.59 |
| Note: (-) Not detected or not quantified. | |||
TABLE 3. Toxicity of the methanol extract, fractions and thymol on the Artemia salina.
These compounds may be separated with solvents of increasing polarities and have been detected by specific reagents. For example, the chemical structure of cholesterol is identified by the Libermann-Burchard reaction, while the color reaction with 3,5-diinitrobenzoic acid depends upon the presence of an β-unsaturated lactones, as occurs in annonaceous acetogenins. Positive reactions to alkaloids confirm the presence of these compounds in A. muricata, as well as phenolic compounds. In particular, the extraction in ethyl acetate allows the removal of free flavonoids, tannins and xanthones, while the butanol extracts glycosylated flavonoids and tannins. In this study, these solvents extracted 12.99% and 4.49% of total phenols and flavonoids, respectively, confirming previously described data. In addition, annonacin, an acetogenins found in fruits of A. muricata, has been related to the toxic effects on the nervous system and this may be a limitation on the use of A. muricata fruit peels for food. However, the fractionation process performed in this study may be an alternative for the consumption of part of the fruit peels as food and herbal medicines, especially EFAM and BFAM that are rich in phenolic compounds.
Although the antioxidant effect has been studied in different parts of soursop, this property has not been explored in A. muricata fruit peels. MEAM, EFAM and BFAM exhibited significant IC50 values in DPPH and FRAP assays, which can be explained by the high content of phenolic and flavonic substances. These compounds have ability to donate hydrogen or electrons and prevent the oxidation in biological media, which can bring great benefits to human health.
Lipid peroxidation is related to oxidative degradation of lipids in which free radicals capture electrons in cell membranes and initiates a chain reaction mechanism. Based on this phenomenon, the linoleic acid forms the peroxyl radical that reacts with beta-carotene resulting in the loss of coloration. Our results showed that HFAM and DFAM were more effective than MEAM, EFAM and BFAM, since they present decay closer to BTH. Probably, this effect might be due to the presence of nonpolar compounds that interact with the lipid emulsion by inhibiting the peroxyl radical. In addition to these data, thiobarbituric acid assay showed that all the samples were active in reducing the formation of free radicals from the day 2, which corroborated the results showed in Table 1 and Figure 2. These findings are relevant, since the lipid peroxidation assays mimic cellular alterations related to different pathophysiological mechanisms. On the other hand, the adipose tissue secretes adipokines that generate Reactive Oxygen Species (ROS) and leads the Oxidative Stress (OS). In cases of obesity, several mechanisms, as mitochondrial and peroxisomal oxidation of fatty acids, produce OS and are capable of generating ROS, which promote cardiovascular diseases, among others. Natural products that inhibit the pancreatic lipase constitute an important therapeutic alternative in the treatment of obesity and oxidative stress. MEAM, HFAM, DFAM, EFAM and BFAM were able to inhibit the pancreatic lipase, especially HFAM and DFAM that were more potent in inhibiting this enzyme. This effect may be related to the presence of different compounds, mainly phenolics, and confirm the antioxidant effect. In addition, docking studies have revealed that the possible binding sites of polyphenolic compounds with pancreatic lipase were located close to the enzyme active site, Serine153 (Ser153), Aspartic acid176 (Asp176) and Histidine263 (His263).
Pancreatic α-Amylase and α-glucosidase are the main enzymes involved in carbohydrate digestion, such as dietary starch, releasing oligosaccharides that are later degraded to glucose to be absorbed. Inhibition of these enzymes is considered a promising strategy to lower serum glucose levels and manage the symptoms of diabetes related diseases. Our results indicated that methanol extract and fractions of A. muricata fruit peels are efficient inhibitors of pancreatic α-amylase and α-glucosidase activity. As for pancreatic lipase, EFAM and BFAM were more effective in inhibiting both enzymes. As shown in Table 1, these fractions are rich in phenolic compounds that may be related to inhibition of these digestive enzymes. Currently, one of the therapeutic approaches for the treatment of type 2 diabetes is the reduction of postprandial hyperglycemia by preventing glucose absorption by inhibiting α-amylase and α-glucosidase in the digestive tract. Among the inhibitors of these enzymes that slow carbohydrate digestion, causing a reduction in the rate of glucose absorption and thereby attenuating the postprandial increase in plasma glucose, acarbose, miglitol and voglibose are clinically used. Another important aspect is that inhibitors help in the management of obesity, which may also contribute to the treatment of metabolic syndrome.
To assess the toxicity of MEAM and fractions, we opted for the brine shrimp (Artemia salina Leach) method that is considered a simple bioassay for natural product research, which determines LC50 values. In addition, brine shrimp could be a valuable tool in the search for compounds that are protective against damage by superoxide or other active oxygen species. MEAM, HFAM and DFAM were toxic against A. salina and were more active than thymol (positive control). Among the compounds that can be found in A. muricata, acetogenins, as annonacin, have been studied for their cytotoxic potential. Several annonaceous acetogenin (annonacin, annomuricin A, annomuricin B, annomuricin C, annohexocin, muricatocin C, corossolone, among others) of A. muricata exhibited toxicity against brine shrimp. However, as chemical markers of Annonaceae species, the alkaloids have also been considered in toxicity studies. Although the toxicity of MEAM and fractions can be an impediment to the use of fruit peels as food and medicine, our findings may be relevant for safety studies, since the EFAM and BFAM fractions were not toxic on A. salina in the tested concentrations.
Our results are in accordance with Chel-Guerrero, et al., which showed a valuable source of bioactive compounds from the tropical fruit peels using Annona squamosa, A. reticulata, Chrysophyllum cainito and Melicoccus bijugatus. Thus, fruit peels of A. muricata can be used for the development of new therapeutic products.
Conclusion
The study show that the peels of A. muricata fruit peels are a source of chemical compounds (phenolic) and other chemical classes, which they may be, related to their antioxidant, anti-lipase, anti-amylase, anti-glucosidase and toxic properties. In addition, the peels could play a crucial role in the prevention and treatment of oxidative and metabolic disorders.
References
- Mann FM. Identification and analysis of bioactive components of fruit and vegetable products. J Chem Educ. 2015;92 (5):892-895.
- Amusa NA, Ashaye OA, Oladapo MO, et al. Pre-harvest deterioration of Soursop (Annona muricata) at Ibadan Southwestern Nigeria and its effect on nutrient composition. Afr J Biotechnol. 2003;2(1):23-25.
- Jirovetz L, Buchbauer G, Ngassoum MB. Essential oil compounds of the Annona muricatafresh fruit pulp from Cameroon. J Agric Food Chem. 1998;46(9):3719-3720.
- Hasrat JA, Pieters L, de Backer JP, et al. screening of medicinal plants from Suriname for 5-HT1A ligands: Bioactive isoquinoline alkaloids from the fruit of Annona muricata. Phytomedicine. 1997;4(2):133-140.
[Crossref] [Google Scholar] [PubMed]
- Sun S, Liu J, Kadouh H, et al. Three new anti-proliferative annonaceous acetogenins with mono-tetrahydrofuran ring from graviola fruit (Annona muricata). Bioorg Med Chem Lett. 2014;24(12):2773-2776.
[Crossref] [Google Scholar] [PubMed]
- Jimenez VM, Gruschwitz M, Schweiggert RM, et al. Identification of phenolic compounds in soursop (Annona muricata) pulp by high performance liquid chromatography with diode array and electrospray ionization mass spectrometric detection. Food Res Int. 2014;65:42-46.
- George VC, Kumar DN, Suresh PK. Antioxidant DNA protective efficacy and HPLC analysis of Annona muricata(soursop) extracts. J Food Sci Technol. 2015;52(4):2328-2335.
[Crossref] [Google Scholar] [PubMed]
- Olakunle S, Onyechi O, James O. Toxicity, anti-lipid peroxidation, in vitro and in vivo evaluation of antioxidant activity of Annona muricataethanol stem bark extract. Am J Life Sci. 2014;2(5):271-277.
- Wang S, Zhu F. Dietary antioxidant synergy in chemical and biological systems. Crit Rev Food Sci Nutr. 2017;57(11):2343-2357.
[Crossref] [Google Scholar] [PubMed]
- Septembre-Malaterre A, Remize F, Poucheret P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Res Int. 2018;104:86-99.
[Crossref] [Google Scholar] [PubMed]
- Sharma N. Free radicals, antioxidants and disease. Biol Med. 2014;6(3):1-6.
- Moghadamtousi SZ, Fadaeinasab M, Nikzad S, et al. Annona muricata (Annonaceae): A review of its traditional uses, isolated acetogenins and biological activities. Int J Mol Sci. 2015;16(7):15625-15658.
[Crossref] [Google Scholar] [PubMed]
- Muiara AM, Bruna CS, Rodrigo LF, et al. Pharmacological potential of Palicourea rigida kunth: A possible participation of flavonoid compounds. J Med Plants Res. 2017;11(10):194-206.
- Chelladurai GR, Chinnachamy C. Alpha amylase and alpha glucosidase inhibitory effects of aqueous stem extract of Salacia oblonga and its GC-MS analysis. Braz J Pharm Sci. 2018;54(1):1-10.
- Meyer BN, Ferrigni NR, Putnam JE, et al. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982;45(5):31-34.
[Crossref] [Google Scholar] [PubMed]
- Xiong Q, Wilson WK, Pang J. The Liebermann-Burchard reaction: Sulfonation, desaturation and rearrangement of cholesterol in acid. Lipids. 2007;42(1):87-96.
[Crossref] [Google Scholar] [PubMed]
- Sousa CM, Silva HR, Ayres MC, et al. Fenois totais e atividade antioxidante de cinco plantas medicinais. Quim Nova. 2007;30(2):351-355.
- Peixoto Sobrinho TJ, Silva CH, Nascimento JE, et al. Validacao de metodologia espectrofotometrica para quantificacao dos flavonoides de Bauhinia cheilantha (Bongard) Steudel. Rev Bras Cienc Farm. 2008;44(4):683-689.
- Mensor LL, Menezes FS, Reis AS, et al. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res. 2001;15(2):127-130.
[Crossref] [Google Scholar] [PubMed]
- Oyaizu M. Studies on product of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. Japanese J Nutr Diet. 1986;44(6):307-315.
- Koleva II, Van Beek TA, Linssen JP, et al. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem Anal. 2002;13(1):8-17.
[Crossref] [Google Scholar] [PubMed]
- Zeb A, Ullah F. A simple spectrophotometric method for the determination of thiobarbituric acid reactive substances in fried fast foods. J Anal Methods Chem. 2016;2016:9412767.
[Crossref] [Google Scholar] [PubMed]
- Souza SP, Pereira LL, Souza AA. Inhibition of pancreatic lipase by extracts of Baccharis trimera: Evaluation of antinutrients and effect on glycosidases. Braz J Pharmacogn. 2011;21(3):450-455.
- Oliveira RJ, Mendonça RJ, Candido PA, et al. Identification of bioactive compounds and analysis of inhibitory potential of the digestive enzymes from Syzygium sp. extracts. J Chem. 2019;(47):1-10.
- Yashin A, Yashin Y, Xia X, et al. Antioxidant activity of spices and their impact on human health: A review. Antioxidants. 2017;6(3):1-18.
[Crossref] [Google Scholar] [PubMed]
- Yin H, Xu L, Porter NA. Free radical lipid peroxidation: Mechanisms and analysis. Chem Rev. 2011;111(10):5944-5972.
[Crossref] [Google Scholar] [PubMed]
- Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur J Med Chem. 2015;97(31):55-74.
[Crossref] [Google Scholar] [PubMed]
- Marseglia L, Manti S, Nicotera A, et al. Oxidative stress in obesity: A critical component in human diseases. Int J Mol Sci. 2015;16(1):378-400.
[Crossref] [Google Scholar] [PubMed]
- Milagro FI, Boque N, Campion J, et al. Natural inhibitors of pancreatic lipase as new players in obesity treatment. Planta Med. 2011;77(8):773-785.
[Crossref] [Google Scholar] [PubMed]
- T Buchholz T, Melzig MF. Polyphenolic compounds as pancreatic lipase inhibitors. Planta Med. 2015;81(10):771-783.
[Crossref] [Google Scholar] [PubMed]
- Martinez-Gonzalez AI, Alvarez-Parrilla E, Diaz-Sanchez AG, et al. In vitro inhibition of pancreatic lipase by polyphenols: A kinetic, fluorescence spectroscopy and molecular docking study. Food Technol Biotechnol. 2017;55(4):519-530.
[Crossref] [Google Scholar] [PubMed]
- Abbas Q, Hassan M, Raza H, et al. In vitro, in vivo and in silico anti-hyperglycemic inhibition by sinigrin. Asian Pac J Trop Med. 2017;10(4):372-379.
[Crossref] [Google Scholar] [PubMed]
- Jiang P, Xiong J, Wang F, et al. α-Amylase and α-glucosidase inhibitory activities of phenolic extracts from Eucalyptus grandis × E. urophylla bark. J Chem. 2017;1-7.
- Kalita D, Holm DG, LaBarbera DV, et al. Inhibition of α-glucosidase, α-amylase and aldose reductase by potato polyphenolic compounds. PLoS One. 2018;13(1): 0191025.
[Crossref] [Google Scholar] [PubMed]
- Mohamed EA, Siddiqui MJ, Ang LF, et al. Potent α-glucosidase and α-amylase inhibitory activities of standardized 50% ethanolic extracts and sinensetin from Orthosiphon stamineus Benth as anti-diabetic mechanism. BMC Complement Altern Med. 2012;12:176.
[Crossref] [Google Scholar] [PubMed]
- Jayaraj S, Suresh S, Kadeppagari RK. Amylase inhibitors and their biomedical applications. Starch. 2013;65(7-8):535-542.
- Aguilar-Salinas CA, Viveros-Ruiz T. Recent advances in managing/understanding the metabolic syndrome. Res. 2019;8:1-9.
[Crossref] [Google Scholar] [PubMed]
- Matthews RS. Artemia salina as a test organism for measuring superoxide mediated toxicity. Free Rad Biol Med. 1995;18(5):919-922.
[Crossref] [Google Scholar] [PubMed]