Original Article
, Volume: 16( 15)Estimation of Metformin Hydrochloride in Bulk and Granules by Ion-Pair Liquid Chromatography
- *Correspondence:
- Pritam S. Jain , Department of Pharmaceutics and Quality Assurance, R. C. Patel Institute of Pharmaceutical Education and Research, Shirpur-425405, Maharashtra, India, Tel: 912563255189, Fax: 912563251808; E-mail: pritash79@yahoo.com
Received: November 09, 2016; Accepted: December 14, 2016; Published: December 18, 2016
Citation: Chalikwar SS, Shah DR, Jain PS. Estimation of Metformin Hydrochloride in Bulk and Granules by Ion-Pair Liquid Chromatography. Anal Chem Ind J. 2016;16(15):115.
Abstract
A simple, precise, accurate, cost effective and alternative isocratic high performance liquid chromatography method was developed for the estimation of metformin hydrochloride in bulk and in granule formulation. Chromatographic separation was achieved with Eclipse XDB C18 column (150.00 mm × 4.60 mm) utilizing a mobile phase of sodium chloride, sodium pentane sulfonate along with triethylamine (50.00% W/W). Orthophosphoric acid was added to adjust the mobile phase pH to 3.8. It was filtered through 0.45 μ membrane filter and detection was carried out at 233 nm using PDA detector. Validation was performed as per ICH guidelines to demonstrate system suitability, specificity, linearity, accuracy, precision, limit of detection, limit of quantitation, robustness, ruggedness. The linear response (r2 = 0.999) was observed in the range of 25-75.00 μg mL-1 with good recoveries (99.79% to 101.75%). Validation acceptance criteria were met in all cases. Thus, the method can be used in pharmaceuticals for routine quality control assay of metformin hydrochloride.
Keywords
Metformin hydrochloride; HPLC; Validation; Ion-pair reagent
Introduction
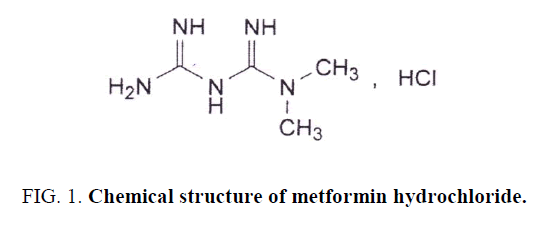
Metformin hydrochloride (MET), a hypoglycemic agent is chemically 1, 1-dimethylbiguanide hydrochloride Figure. 1 [1]. It is the drug of choice for type 2 diabetes mellitus. It improves glucose tolerance in patients with type 2 diabetes, lowering both basal and postprandial plasma glucose. It decreases hepatic glucose production, decreases intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization [2,3].
Figure 1: Chemical structure of metformin hydrochloride.
Various pharmacopoeias like Indian Pharmacopoeia [1], British Pharmacopoeia [4], European Pharmacopoeia [5] and United States Pharmacopeia [6] describe high performance liquid chromatography (HPLC) method for estimation of MET. The detail literature survey revealed estimation of MET by different methods like reverse and normal phase HPLC, [7,8] fluorescence detection [9] capillary electrophoresis (CE) [10,11] UV spectrophotometric method [12,13,14]. Few sophisticated techniques that are reported for determination of MET includes LC-MS/MS [15], supercritical fluid chromatography/tandem mass spectrometry method [16] potentiometry, spectrofluorimetry [17,18] multivariate technique [19] High performance thin layer chromatography [20], Ultra high pressure liquid chromatography [21] Ion pair HPLC in human plasma [22].
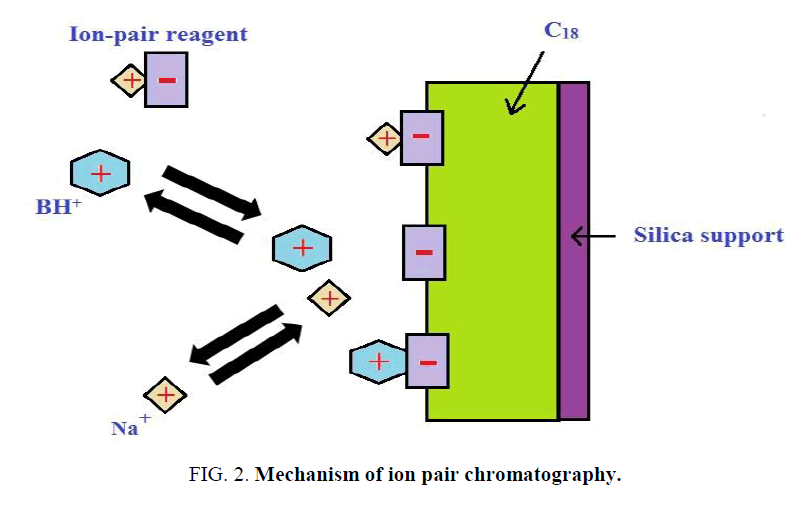
The objective of the present investigation was to establish and validate the fast and sensitive ion pair HPLC method for MET. Apart from its simplicity and sensitivity, the present method highlights on economic one since mobile phase used is complete devoid of organic solvent Figure. 2.
Ion pair reagent is attracted to the stationary phase because of its hydrophobic alkyl group and charged carried by the reagent {C5- SO3- (Sodium pentane sulfonate)} thereby attached to the stationary phase.
Materials and Methods
Chemicals and reagents
Metformin hydrochloride was obtained as gift sample from Shreeji Pharma International, Vadodara (India). MET granules were purchased from market and used as sample under test. Pentane sulphonic acid sodium salt (Sigma), triethylamine (Aldrich Chemical Company, Inc.), sodium chloride and ortho phosphoric acid (OPA) used for preparation of mobile phase. The mobile phase was filtered through a 0.45 μm membrane filter (Gottingen, Germany).
Equipment and chromatographic conditions
The Agilent HPLC system consisted of a degasser G1379B, a bin pump G1312A, an automatic injector G1329A, a thermostatic column oven G1316A and a multi wavelength detector G1365B, all 1200 Series from Agilent Technologies, which were controlled by an Chem-station software. All chromatographic experiments were performed in the isocratic mode. The mobile phase was consisting of 1.2 gm of NaCl and 0.87 gm of sodium pentane sulfonate in 900 mL of water, 3.00 mL 50% of triethylamine was added to it and it was diluted to 1000 mL with water. Orthophosphoric acid was added to adjust the pH to 3.8. The injection volume was 10.00 μL and detection was at 233 nm (LC Solution). The mobile phase flow rate was set at 1.2 mL min-1 and constant column temperature was 25°C.
Preparation of solution
The stock solution was prepared by accurately weighing 50 mg of MET and transferring into 50 mL volumetric flask, 25 mL of water was added as diluent, sonicated for 5 min and volume was made up to the mark. The final concentration of 50 μg mL-1 was achieved by diluting 5 mL of stock solution to 100 mL with diluent.
Assay of formulation
Accurately weighed 20 pouches of granules and the average weight were calculated. Weight equivalent to 500 mg was transferred to 1000 mL of volumetric flask; 500 mL of diluent was added, sonicated for 30 min and volume was made up to the mark. The final concentration of 50 μg mL-1 was achieved by diluting 5 mL of above solution to 50 mL with diluent and filtered through 0.45 μm Millipore PVDF filter filtered through 0.45 μm Millipore PVDF filter.
Validation of the proposed method
The proposed method was validated following the guidelines of International Conference on Harmonization (ICH) [23]. The validation parameters like; specificity, linearity, accuracy, precision, limits of detection and limit of quantification, robustness, ruggedness and stability were determined.
Suitability test: System suitability was determined from six replicate injections of the standard solution before sample analysis
Specificity: To study specificity, drug solution of 50 μg mL-1 was injected into the column, under optimized chromatographic conditions.
Linearity: Linearity was studied over the concentration range of 25-75 μg mL-1. Standard calibration curve was plotted with five calibrators over the said concentration with triplicate determinations at each level. The data of peak area vs. drug concentration were treated by linear least square regression analysis.
Accuracy: Accuracy was determined by applying the method in triplicate samples of mixture of placebo to which known amount of standard drug added at three different levels of analyzed sample solution (50%, 100% and 150%). The % recovery and % RSD at each level was calculated.
Precision: The precision studies were performed as intra-day, interday and repeatability. Intra-day was performed by analyzing, the three-different concentration of drug for three times in the same day. Inter-day precision was performed by analyzing three different concentration of the drug for three consecutive days. Repeatability was performed by analyzing same concentration of drugs for six times.
Limit of detection (LOD) and limit of quantification (LOQ): Sensitivity of the proposed method was estimated in terms of LOD and LOQ. These were determined by using standard deviation of the response and slope.
Robustness: Robustness of the method was performed by deliberately changing the critical chromatographic parameters such as the pH and flow rate of the mobile phase, column temperature and the injection volume
Ruggedness: Ruggedness of the proposed method is determined by analysis of aliquots from homogenous slot by two different analysts using similar operational and environmental conditions.
Results and Discussion
Optimization of chromatographic conditions
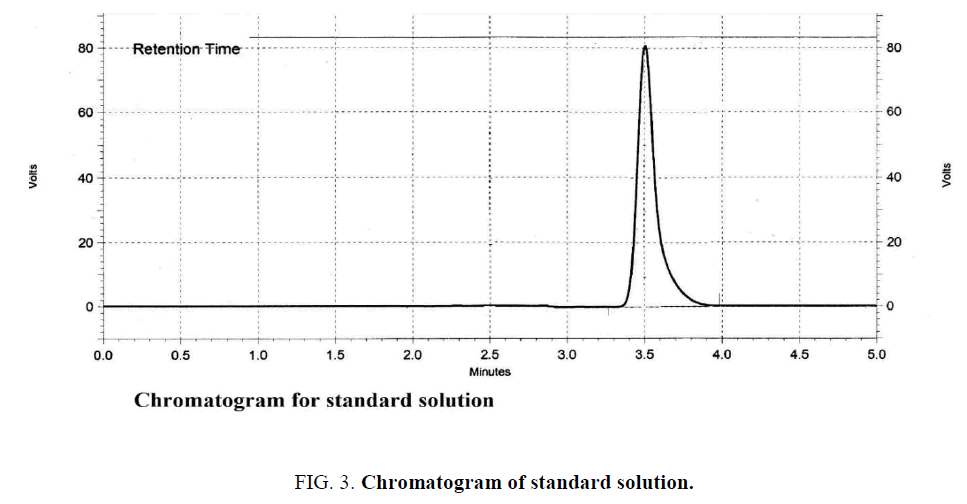
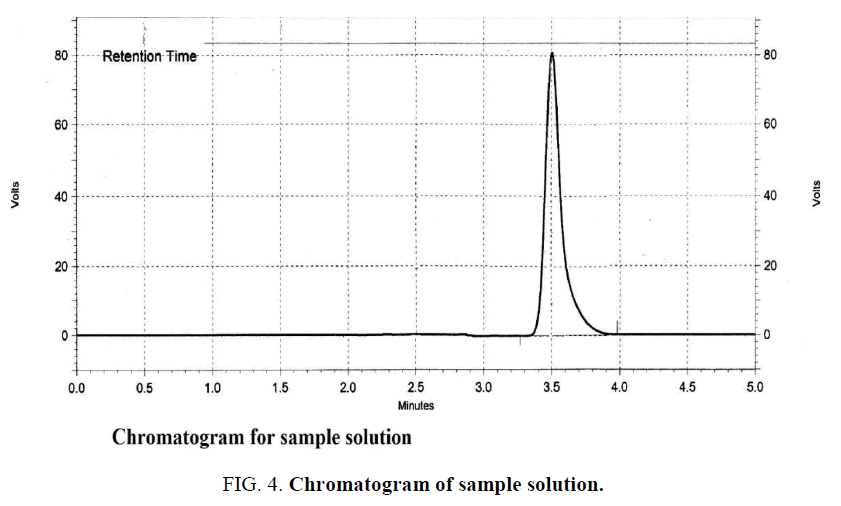
The chromatographic conditions were optimized based on selection of column, optimization of mobile phase, wave length detection, and column temperature and flow rate. The conditions were found to be best suited for analysis as good resolution and sharp peak were observed. The mobile phase found to be the best suited since it devoid of organic solvents. The retention time was obtained at 3.5 min for both standard Figure. 3 and sample Figure. 4.
Validation of proposed method
The proposed method was validated by validating various parameters. The method was found to be system suitable as % RSD was 0.78%, tailing factor was 1.51 and column efficiency (N) was above 3500 plates per column. All these values were within the acceptance range. The specificity of the method was checked for the interference against blank.
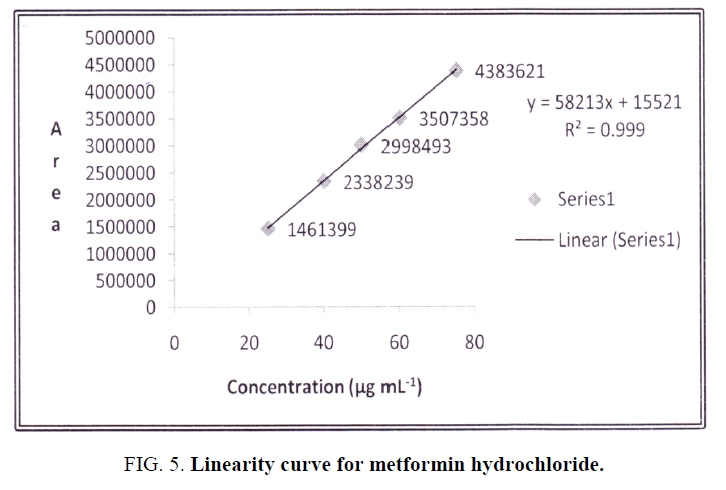
Separation of the drug indicated no interference of blank and placebo. Standard calibration curve was plotted for linearity over the concentration range of 25-75 μg mL-1. The r2 value of 0.999 confirmed good linearity over selected concentration range as shown in Figure. 5. The percentage recovery was found to be in range of 99.79-101.75%. The detailed results of linearity, accuracy and precision are tabulated in (Table 1). The values obtained in the respective limits are indicative of accurate and precise method.
Figure 5: Linearity curve for metformin hydrochloride.
| Linearitya | |||||||
| Parameters | Values | ||||||
| λ max | 233 nm | ||||||
| Concentration range (m mL-1) | 25-75 | ||||||
| Correlation coefficient | 0.999 | ||||||
| Regression equation | y= 58213x+15521 | ||||||
| Accuracy | |||||||
| Concentration (%) | Placebo (mg) | Sample wt. taken (mg) | %Recovery (Mean ±SD)b | % RSD | |||
| 50 | 68.0 | 250.0 | 99.79 ±0.40 | 0.003 | |||
| 100 | 68.0 | 500.0 | 101.75 ±0.01 | 0.010 | |||
| 150 | 68.0 | 750.0 | 100.72 ±0.53 | 0.014 | |||
| Precision | |||||||
| Type of precision | Mean ±SDa | % RSD | |||||
| Intra-day Precision | 2948508 ± 1039.48 | 0.03 | |||||
| Inter-day Precision | 2948634 ± 643.25 | 0.02 | |||||
| Repeatability | 2966157 ± 18571.66 | 0.63 | |||||
a: mean of three determinations.
b: Percent recovery according to three replication of same concentration of sample.
Table 1: Optical characteristic of linearity, accuracy and precision for proposed method.
The proposed method was found to be sensitive from LOD and LOQ values. The values obtained were found to be 1.5 μg mL-1 and 4.5 μg mL-1 for LOD and LOQ respectively. All the values for peak areas were within the acceptance limits as per ICH guidelines for robustness. Also in case of ruggedness, % RSD of less than 2% indicated the method was rugged. The results of robustness and ruggedness studies are summarized in (Table 2). Stock solution of sample and standard were found to be sufficiently stable for the specified period.
| Robustness Parameters | Percent recoverya, b± SD |
| Optimal conditions | 100.64 ± 0.63 |
| Variation of the mobile phase flow rate (mL min-1) | |
| 1.0 | 99.58 ± 1.09 |
| 1.4 | 99.48 ± 1.37 |
| Variation of column temperature (°C) | |
| 20 | 100.69 ± 1.07 |
| 30 | 101.27 ± 0.62 |
| Variation of mobile phase (pH) | |
| 3.6 | 100.24 ± 0.38 |
| 4.0 | 100.39 ± 0.70 |
| Variation of the injection volume (μg mL-1) | |
| 8 | 99.63 ± 0.70 |
| 12 | 100.42 ± 1.54 |
| Ruggedness parameters | |
| Analyst | Mean ± SD% RSD |
| I | 101.14 ± 0.1285 0.13 |
| II | 101.165 ± 0.1259 0.12 |
a: Percent recovery according to the calibration curve obtained under optimal conditions (mean of six injections ± standard deviation);
b: each sample contained 50 μg mL-1 (100% concentration level)
Table 2: Results of robustness and ruggedness studies.
Conclusion
In the present study, an attempt had made to develop and validate a simple, efficient, and sensitive ion pair HPLC method. The proposed method was found to be accurate, precise, and linear across the analytical range. The method was found to be suitable for the determination of drug content in said formulation. The method may be used to assess the quality of commercially available drug products of metformin hydrochloride. The most significant advantage of proposed method is that mobile phase does not contain organic phase and no pretreatment of sample is required. The obtained results indicated that the drug can be separated completely and there was no interference of excipients present in dosage form. Thus, it will serve as an alternative to routine existing analytical methods in pharmaceutical industry.
Acknowledgment
Authors are thankful to Shreeji Pharma International, Vadodara (India) for providing gift sample of Metformin hydrochloride.
References
- Ashraf G. Heterologous expression of stress-responsive DUF538 domain containing protein and its morpho-biochemical consequences. Plant J. 2011;30(5):351-8.
- Takahashi S, Yoshikawa M, Kamada A, et al. The photoconvertible water-soluble chlorophyll-binding protein of Chenopodium album is a member of DUF538, a superfamily that distributes in Embryophyta. J Plant Physiol. 2013;170(17):1549-52.
- Ashraf G, Kohnehrouz SB.Identification ofDUF538cDNA clone from Celosia cristata expressed sequences of nonstressed and stressed leaves. RussJPlant Physiol. 2010;57(2):247-52.
- Nakagami H, Sugiyama N, Mochida K, et al. Large-scale comparative phosphoproteomics identifies conserved phosphorylation sites in plants. Plant Physiol. 2010;153(3):1161-74.
- Ashraf G, Kohnehrouz SB. ProtJ.2013;32:163.
- Ashraf G.Prediction of tertiary structure homology between bactericidal/permeability increasing protein of innate immune system and hydrolase enzymes. IntJBiosci. 2014;5(2):1-6.
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seiolman JG, Smith JA, et al. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 1991.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402-8.
- Rabbani MA, Maruyama M, Abe H, et al. Monitoring expression profiles of rice genes under cold, drought, and high-salinity stresses and abscisic acid application using cDNA microarray and RNA gel-blot analyses. Plant physiol. 2003;133(4):1755-67.
- Shinozaki K, Yamaguchi SK, Seki M, et al. Regulatory network of gene expression in the drought and cold stress responses.CurrOpinPlant Biol. 2003;6(5):410-7.
- Shinozaki K, Yamaguchi SK. Gene networks involved in drought stress response and tolerance. JExpBot. 2007;58(2):221-7.
- Yamaguchi SK, Shinozaki K. Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci. 2005;10(2):88-94.
- Bartels D, Sunkars R. Drought and Salt Tolerance in Plants. CritRevPlant Sci. 2005;24(1):23-58.
- Seki M, Narusaka M, Abe H, et al. Monitoring the expression pattern of 1300 Arabidopsis genes under drought and cold stresses by using a full-length cDNA microarray. Plant Cell. 2001;13(1):61-72.
- Schimid M, Davison TS, Hens SR, et al. A gene expression map of Arabidopsis thaliana development. NatGenet. 2005;37(5):501-6.