Original Article
, Volume: 13( 2)New Biotechnological Methods for Producing Therapeutic Compounds (Usnic, Stictic and Norstictic Acids) by Cell Immobilization of the Lichen Cladonia substellata Vainio
- *Correspondence:
- Pereira EC , Department of Geographical Sciences, University of Pernambuco, Federal University of Pernambuco, Av. Prof. Moraes Rego, 1235, CEP 50.740-530, Recife, PE, Brazil, Tel: 55(81)21268275; E-mail: verticillaris@gmail.com.br
Received: October 20, 2017; Accepted: March 23, 2017; Published: March 27, 2017
Citation: Martins MCB, Lima MJG, Santiago R, et al. New Biotechnological Methods for Producing Therapeutic Compounds (Usnic,Stictic and Norstictic Acids) by Cell Immobilization of the Lichen Cladonia substellata Vainio. Biotechnol Ind J. 2017;13(2):133.
Abstract
The aim of this paper is related to the modifications of the most usual method for producing USN and others bioactive compounds by the lichen Cladonia substellata to rationalize ecologically the provision of those at large scale. Different cell immobilization systems were assayed, using NaOAc as biosynthetic precursor at different concentrations. The introduced new systems to the most usual, consisting of the static incubation of immobilizates, were bioreactors in rotary movement and in continuous flow of precursor. They clearly enhance the production of USN. In addition, the kind of immobilization system can vary the content of produced metabolites. The occurrence of reduced USN and the occurrence of both NTI and STI acids in the cell washes were correlated. In this way, the modification of immobilization technique leads to highest production of these metabolites, and to a better use of the precursor, but it does not show any significant influence on the production of accessory and/or degraded compounds of the chemical species. On the basis of our results, USN and other compounds from C. substellata were produced in substantial amounts by kaolinite-immobilized continuous flow system and, in a general way, at a concentration of 10.0 mM, using NaOAc as precursor.
Keywords
Cladoniaceae; Lichen substances; Bioreactor; Kaolinite; Bioproduction
Abbreviations: NaOAc: Sodium Acetate; NTSI: Norstictic Acid; STI: Stictic Acid; USN(R): Usnic Acid (reduced)
Introduction
Lichens or lichenized fungi are symbiotic organisms consisting of, at least, one fungi and one photosynthetic partner (alga or Cyanobacterium). This association also contains a set of unique chemical compounds that are a source of metabolites with biological activity for human use. Lichens synthesize, mostly by a fungal metabolism, various bioactive secondary compounds, that sometimes constitute even more than 30% of the dry mass of thallus [1]. They are found as crystal deposits on the surface of hyphae. Several studies have revealed that these slow-growing organisms produce a diverse array of secondary metabolites with different biological activities [2-8]. USN is a dibenzofuran derivative uniquely found in lichens, displaying a wide range of pharmacological activities including antibiotic, antiviral, antiprotozoal, anti-inflammatory, analgesic, antiproliferative, antioxidant, anticancer activities [8-11]. The use of lichens in medicine is based on the fact that they contain these unique and varied biologically active substances.
For centuries, lichens have been commercially explored as essence fixers, with emphasis to USN and derivatives as therapeutic substances [6,11-18]. In the cosmetic industry, several phenolic compounds from lichens, such as atranorin, have been used for avoiding the maturing skin [19], or USN, used for protecting it from large sunlight exposure [20].
Since the knowledge of the application of lichen substances like USN in both pharmaceutical and cosmetic industries, large amounts of biomass are collected from the environment for extraction, purification and commercial use of these biologically-active compounds. This fact constitutes a problem, due to low growth of lichens that causes difficulties for their reposition in the ecosystem. In this way, the large scale use of lichens implies in an environmental question [21]. In view of the enormous potential of lichens, several techniques have been developed to solve this problem, as symbionts or tissues culture and cell immobilization [22]. Thus, the use of bioreactors using very small amounts of lichen biomass seems to be as yet the most viable alternative to rapid and efficient production of bioactive compounds.
The technique of cell immobilization focuses the continuous production of lichen metabolites, using few amount of thallus, as well as the use of biosynthetic precursors since the entrapped cells in inert matrixes are able to synthesize these compounds for a large time period. In this context, Pereira et al. [23] induced the production of ribitol by cells of Cladonia verticillaris entrapped in alginate spheres, but the highest concentration of the precursor, sodium bis-carbonate, provided a cationic change between the Ca+, used to harden the surface of the spheres that contain the isolated cells, and Na+.
In this way, Pereira et al. [24] tried a new entrapment matrix, kaolinite, for avoiding the interaction described for ribitol production. In this case, the authors used NaOAc for USN bioproduction by immobilized cells of C. substellata. The authors found that the increase of precursor concentration induced the cells to produce USN in a reduced form. Since the immobilization system was performed into a glass column, where lichen cells were mixed to kaolinite and NaOAc (precursor), the cells released to the medium the phenolics produced during the experiment. Thus, it is probable that the USN synthesized by C. substellata cells should be used in any metabolic process, besides its storage in the immobilization medium [24].
Based on the efficiency of immobilization system by fix, or traditional method [24], the objective of this work was to introduce modifications in this technique for a high performance in the production of metabolites from C. substellata using new design of bioreactors
Material and Methods
Lichen material
Cladonia substellata Vainio was collected in the Mamanguape district (Paraíba, Northeastern of Brazil-06°50’S e 35°07’W). Taxonomic identification of the lichen was accomplished using standard methods, chemical and morphological thallus characters, by one of the authors (E.C. Pereira). Dried thallus was deposited in the Herbarium of the Universidade Federal de Pernambuco (Brazil), register n°34.402.
Preparation of organic extracts, and isolation and purification of USN
C. substellata (45 g) was macerated and submitted to 4 successive extractions with diethyl ether, chloroform or acetone (P.A 99.5% PA/ACS 1.000 ml-Vetec Sigma-Aldrich®) in a Soxhlet apparatus at 40°C for 16 h. After this period, the extracts were filtered through filter paper (Whatman No. 9) and then evaporated in a rotaevaporator (Flash Evaporator-115V. Buchler Instruments. Fort Lee. NJ, USA) coupled to a water bath (CT 246 CIENTEC) at 40°C until dryness.
For the separation, isolation and purification of USN, the ether extract was fractionated in a column of silica gel (70 mesh to 230 mesh) eluted in the chloroform: hexane (80:20 v/v) solvent system [25]. For the characterization of the product, ether extract and the purified crystals of USN were analyzed by thin layer (TLC) and high performance liquid (HPLC) chromatographies.
Preparation of immobilized systems
Procedures and material used: C. substellata thallus samples (10 g in dry weight) were gently disrupted in a mortar with 50 ml double distilled water, filtered through 4 layered gauze and the filtrate centrifuged for 30 min at 3.200 ×g. The pellet was mixed with fully hydrated kaolinite (30 g in dry weight), previously rinsed with deionized water and dried in an oven (SOC. Fabbe, Sao Paulo/Brazil) at 40°C. NaOAc solutions were prepared at 0.1; 1.0 and 10 mM, pH 8.5. For bioreactors assembly, were used the pellet (about 1 g dry weight) mixed with kaolinite (30 g in dry weight) for each concentration of NaOAc under white light (125 μmol m-2 s-1) at room temperature (25°C ± 3°C).
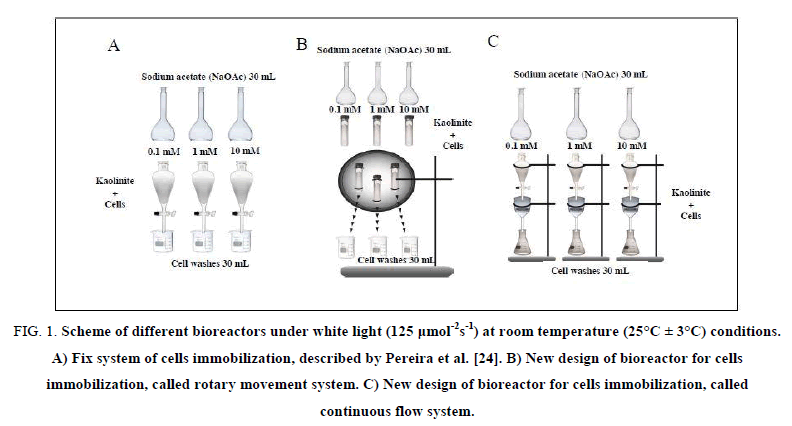
Bioreactor in fix system: The mixture lichen/kaolinite was loaded onto 3 glass columns (125 ml) and 30 ml of NaOAc (0.1; 1.0 and/or 10 mM) were added. The columns were maintained at rest under white light at room temperature (25°C ± 3°C) during the whole experiment period according to Pereira et al. [24] (Figure. 1A).
Figure 1: Scheme of different bioreactors under white light (125 μmol-2s-1) at room temperature (25°C ± 3°C) conditions. A) Fix system of cells immobilization, described by Pereira et al. [24]. B) New design of bioreactor for cells immobilization, called rotary movement system. C) New design of bioreactor for cells immobilization, called continuous flow system.
Bioreactor in rotary movement system: The mixture lichen/kaolinite was placed in 3 glass tubes with capacity of 30 ml and 30 ml of NaOAc (0.1; 1.0 and/or 10 mM) were added. The tubes were fixed in a plate coupled to a motor model MT8-3 7421 (220 V to 240 V) for maintaining the rotational movement at a constant speed (5 rpm) under white light (125 μmol m-2. s-1) at 25°C ± 3°C throughout the experiment (Figure. 1B).
Bioreactor in continuous flow system: The mixture lichen/kaolinite was placed in funnels G4 (7 cm diam. 125 g) and 30 ml of NaOAc (0.1; 1.0 and/or 10 mM) were initially added and precursor was continuously dropped in the funnel with simultaneous recovering of cells washes (30 ml), characterizing a continuous flow of the precursor. The system was maintained under white light (125 μmol m-2. s-1) at room temperature (25°C ± 3°C) throughout the experiment (Figure. 1C).
Extraction and analysis of phenolic acids from immobilized samples
The washes of each immobilization system at 24, 48, 72, 96 and 120 h; 1 and 2 months of experiment were extracted with organic solvents. The same volume (30 ml) of fresh NaOAc solution was replaced in each bioreactor after obtaining the cell washes. For separation of phenolic fraction from the cell washes, samples were placed in a separation funnel and treated with 2 solvent systems: 30 ml of diethyl ether/ethyl acetate (60:40, v/v) for the first extraction and 30 ml of chloroform/acetonitrile (65:35, v/v) for a second extraction. Organic phases were mixed when indicated. Then, the phenolic extracts were analyzed by spectrophotometry at 254 nm and 366 nm, thin layer chromatography (TLC) and high performance liquid chromatography (HPLC).
Quantification of lichen phenolics
The phenolic content of the organic extract was estimated by using a quantitative UV spectrophotometric method. Absorbance was measured with a spectrophotometer (BIOCHROM, Libra S 22) at 254 nm and 366 with a quartz cell (1 cm path length). A calibration curve was plotted using USN (Merck®) as a standard phenolic compound (0-5 mg ml-1). The results were expressed as mg of USN equivalent mg-1 dry weight.
Thin layer chromatography (TLC)
The organic extracts of natural lichen thallus, and extracts from immobilization (0.1 mg ml-1), after evaporation, the USN purified and USN standard (Merck®) were dissolved in diethyl ether/ethyl acetate and/or chloroform/acetonitrile and diethyl ether (0.5 ml), respectively, being applied on silica gel Merck F254+366 plates and developed in A solvent system (toluene/dioxane/acetic acid, 180:45:5, v/v/v). The spots were visualized under short and long UV wavelengths, 254 nm and 322 nm. After this procedure, the plates were sprayed with H2SO4 solution (10%) and heated at 50°C for 30 min, for spots color reaction [26].
High performance liquid chromatography (HPLC)
The same material analyzed by TLC was submitted to HPLC assays as described Legaz and Vicente, in a Hitachi Chromatograph (655 A-11, Tokyo, Japan) coupled to a CG437-B UV detector set at 254 nm. For the separation, a C-18 reverse phase column MicroPack MCH-18 de 300 mm × 4 mm, Berlin, Germany (Merck® KGaA, Darmstadt, Germany) was used. Organic extracts from cell immobilization (1.0 mg ml-1) and Merck standard USN (0.1 mg ml-1) were developed in an isocratic mode using as mobile phase methanol/deionized water/acetic acid, 80:19.5:0.5 (v/v). Other analytical parameters were: volume of injection 20 ?l, attenuation 0.16, pressure 87 atm, flow rate1.0 ml min-1 at room temperature (28°C ± 3°C). The results were analyzed by comparing the retention time of substance in the column, and peak area, taking the standard (Merck®) USN as reference. Organic phases were evaporated to dryness; residues were re-dissolved in 1 ml of diethyl ether and used for HPLC analysis.
Image analysis
To study the influence of technique modification on the cells bioproduction cell washes of immobilization systems were crystallized and observed using a stereomicroscope.
Statistical analysis
Analytical measurements were done in triplicate from biological samples. Statistical analysis of the differences between the mean values measured from different bioreactor systems conditions were performed using the multiple ANOVA test followed by post hoc analysis with Tukey's honestly significant difference test. Differences were considered to be significant at p<0.05.
Results and Discussion
The lichen symbiosis state makes possible the production of several families of organic compounds, secondary metabolites developing varied biological roles. Between these compounds, the most well-known are those phenols from the acetate-polymalonate pathway [27], which are secreted outside the fungal hyphae and crystallized on the cortex to form a protective screen against UV radiation [28]. Legaz et al. [29] described that C. verticillaris develops higher enzymatic activities in the light than in the dark. This could be in agreement with the requirement of the photochemical activity of the chlorobionts to maintain the pool of acetyl-CoA. Thus, the use of NaOAc as precursor induced the cells on producing lichen metabolites, under light conditions, allowing the algal cells to photosynthesize.
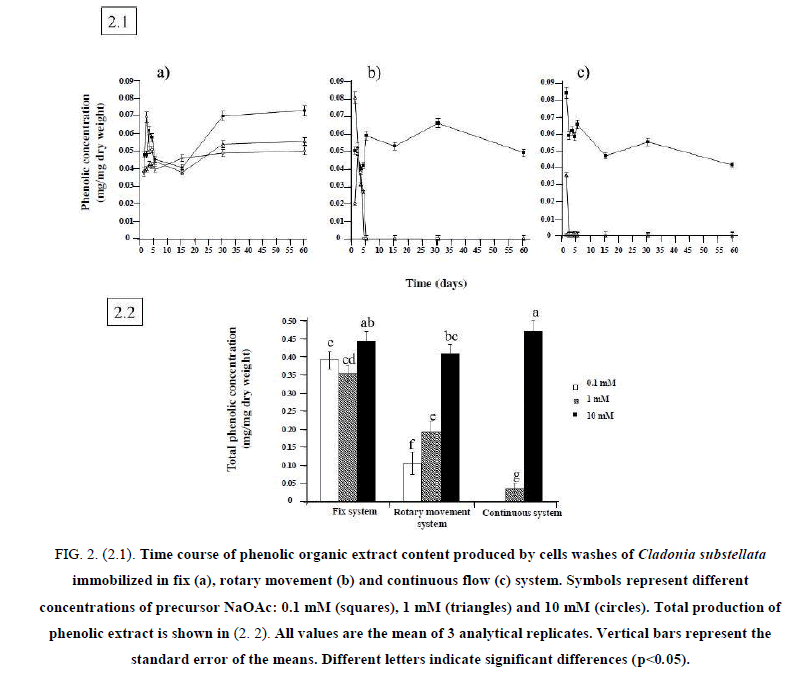
To establish the optimal conditions for bioreactors, 3 different concentrations of NaOAc were assayed. The time-course of phenols production and secretion changed for the different immobilization systems. Cells washes of C. substellata immobilized in fix system (Figure. 2.1.a) showed a high production of phenolics during the first 5 days of incubation, decreasing until 30 days, and increasing the production after that, mainly at 10 mM NaOAc.Figure. 2.1.b shows the time-course of phenolic acid concentration produced in rotary movement system. The same production peak was observed, but it not increased by increasing NaOAc from 0.1 mM to 1.0 mM. High levels of phenolic acids were obtained at 10 mM NaOAc, being this concentration the most efficient for bioproduction. The same behavior was observed for cells washes of C. substellata immobilized in continuous flow system at 10 mM of NaOAc, which maintained its continuous production throughout the experiment. No significant production was observed for 0.1 mM and 1.0 mM NaOAc (Figure. 2.1.c). These results can be explained by the short time of contact between the cells and their bioproduced metabolites. This problem is mentioned for fix immobilization system, when cells of C. substellata could use the produced substances for any metabolic process [23].
Figure 2: (2.1). Time course of phenolic organic extract content produced by cells washes of Cladonia substellata immobilized in fix (a), rotary movement (b) and continuous flow (c) system. Symbols represent different concentrations of precursor NaOAc: 0.1 mM (squares), 1 mM (triangles) and 10 mM (circles). Total production of phenolic extract is shown in (2. 2). All values are the mean of 3 analytical replicates. Vertical bars represent the standard error of the means. Different letters indicate significant differences (p<0.05).
Based on these results, it is possible to say that immobilized cells of C. substellata are capable of producing phenolic compounds. However, different design of bioreactors (Fix, Rotary movement and Continuous Flow Systems) influenced on bioproduction by immobilized cells. Kaolinite immobilized cells loaded on continuous flow system showed better production of phenolic compounds at 10 mM NaOAc as precursor.
Total production of phenolics by C. substellata cells is shown in 2.2. The fix system showed a considerable production at 0.1 mM NaOAc that decreased at 1.0 mM, but the highest production was achieved at 10 mM NaOAc. In the rotary movement and continuous flow systems, we observed no production of phenolic acids at 0.1 mM of precursor, low at 1.0 mM and a remarkable increase at 10 mM. Significant differences occurred in the production of phenolic acids between system immobilization type and precursor concentration. Thus, the concentration of 10 mM of NaOAc was the most efficient for cells bioproduction, and the continuous flow, the better employed system. We can find a number of reports for the antioxidant activity of lichen extracts with high content of phenolic compounds, because of their ability to scavenge free radicals such as singlet oxygen, superoxide, and hydroxyl radicals [7,30,31]. Thus, immobilization in continuous flow system at 10 mM of precursor seems to be the best bioreactor to produce phenolic compounds with antioxidant activity.
The influence of the precursor concentration on the content of produced metabolites can vary from one species to another. While C. substellata increases its production as a function of high concentrations of NaOAc [23], C. clathrata cells showed to be more efficient at 0.1mM NaOAc [32] while C. corallifera did not enhanced its production, for mid and highest precursor concentrations [33]. In this paper, it may observe that the kind of immobilization system can vary the content of produced metabolites.
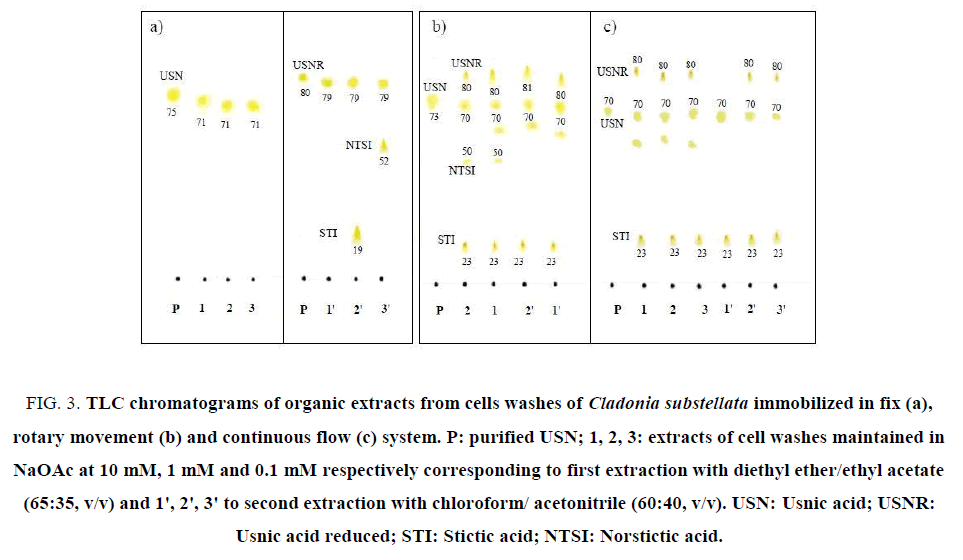
The chemical composition of phenolics obtained in cells washes of immobilized C. substellata was determined using TLC and HPLC-UV analysis. The major phenolic compounds in these extracts were: USN, its reduced form, NSTI acid STI (Figure. 3-5). All of them are bioactive compounds [4,5,11,34,35]. USN is produced by several species of lichens and it is a common metabolite in lichen genus Cladonia. Since its first isolation in 1844, USN has become the most extensively studied lichen metabolite and one of the few that is commercially available. The depsidones NSTI and STI are a wide spread secondary metabolites produced by lichen-forming fungi. Like other lichen substances, most of them are deposited as crystals in the apoplast of lichens, whereas only a small proportion is soluble in water. For this reason, they can usually be isolated from lichen by means of organic diluents.
Figure 3: TLC chromatograms of organic extracts from cells washes of Cladonia substellata immobilized in fix (a), rotary movement (b) and continuous flow (c) system. P: purified USN; 1, 2, 3: extracts of cell washes maintained in NaOAc at 10 mM, 1 mM and 0.1 mM respectively corresponding to first extraction with diethyl ether/ethyl acetate (65:35, v/v) and 1', 2', 3' to second extraction with chloroform/ acetonitrile (60:40, v/v). USN: Usnic acid; USNR: Usnic acid reduced; STI: Stictic acid; NTSI: Norstictic acid
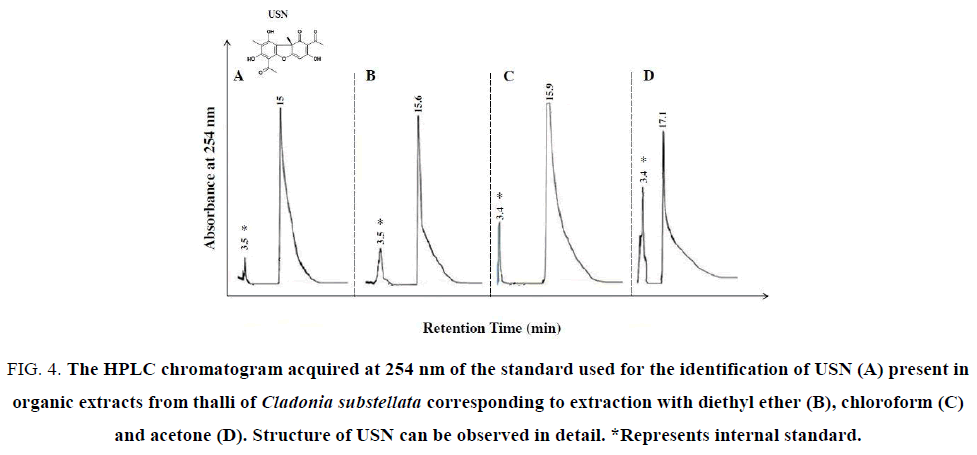
Figure 4: The HPLC chromatogram acquired at 254 nm of the standard used for the identification of USN (A) present in organic extracts from thalli of Cladonia substellata corresponding to extraction with diethyl ether (B), chloroform (C) and acetone (D). Structure of USN can be observed in detail. *Represents internal standard.
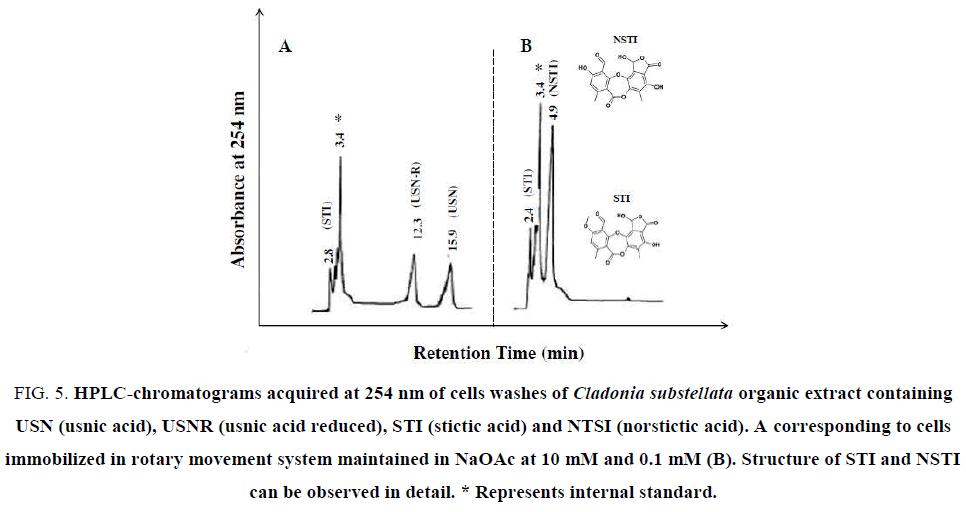
Figure 5: HPLC-chromatograms acquired at 254 nm of cells washes of Cladonia substellata organic extract containing USN (usnic acid), USNR (usnic acid reduced), STI (stictic acid) and NTSI (norstictic acid). A corresponding to cells immobilized in rotary movement system maintained in NaOAc at 10 mM and 0.1 mM (B). Structure of STI and NSTI can be observed in detail. * Represents internal standard.
Through TLC assays it was possible to observe that the samples of C. substellata collected in Brazil produced high content of USN (Figure. 3A- 3C). In the first extraction with diethyl ether/ethyl acetate, of the cells loaded with 0.1 mM, 1.0 mM and 10 mM NaOAC maintained in fix system, USN was only detected (Figure. 3A). After a second extraction with chloroform/ acetonitrile, it was observed the USNR production for all NaOAc concentrations, and 2 extra spots, probably STI and NSTI acids. STI spot was detected in cell washes maintained on NaOAc at 1.0 mM and NSTI spot at 0.1 mM (Figure. 3A). TLC of organic extracts in rotary movement and continuous flow systems, showed enhanced spots of USN and USNR, besides 2 extra spots, probably the STI and NSTI acids (Figure. 3B and 3C) for all NaOAc concentrations. The last phenolic only was extracted with ether/ethyl acetate, according to the eluotropic capacity of the solvents systems used for extracting. It is probably that NTSI acid was extracted with diethyl ether/ethyl acetate, whereas STI acid with both solvents systems. The cells immobilized in rotary movement system showed an extra spot, near USN, that can be other refereed compound to this species (Figure. 3B ). In particular, the disruption of the strong intramolecular hydrogen bonds of USN increases the overall hydro-solubility of the molecule, which can generate more polar active compounds with small Rf values. Derivatives of USN should be considered as prospective compounds for their further optimization as substances with biological activity [36,37].
For detecting occurrence of these phenolic acids in the washing solutions, HPLC assays were achieved. USN was detected in HPLC analysis from thallus extracts (Figure. 4). The Figure. 4A exhibits a peak of standard USN, registered at 15.7 min. The crude extracts showed the same peak at 15.6 min, and other at 15.9 min and 17.1 min, respectively for diethyl ether, chloroform and acetone extracts (Figure. 4B-4D), where chloroform showed higher extractive capacity of USN in relation to other solvents. In the same figure, an additional peak at 3.5 min corresponds to the excess of diethyl ether used for dissolution of standard USN and crude extracts. This compound was used as internal standard. This peak was enhanced when the extract polarity increased, probably due to higher amounts of accessory compounds. This lichen species is described as rich in USN, but also contains these mentioned phenols as well as cryptostictic acid as accessory compound [38], also found in TLC analysis (Figure. 3).
Some characteristics of each compound, including their retention time value and the range of concentration (%) are shown in Table 1. HPLC analysis revealed that USN, USNR, STI and NSTI were the main compounds produced during cell immobilization of lichen C. substellata (Figure. 5). Retention time values of these compounds varied from 2.7 ± 0.5 to 15.9 ± 0.9 min (Table 1), whereas the concentration range of these 4 metabolites was similar. NTSI showed a higher range (about 50%), whereas the amount of USN, USNR and STI was slightly lower (about 30%).
| Compound | Substance class | Retention time (min)(tR ± SD) | Range concentration (%) |
|---|---|---|---|
| STI | Depsidone | 2.7 ± 0.5 | 7.4-28.2 |
| NTSI | Depsidone | 4.9 ± 0.7 | 0.5-46.8 |
| USNR | Dibenzofurane | 12.3 ± 0.7 | 0.5-30.9 |
| USN | Dibenzofurane | 15.9 ± 0.9 | 0.2-27.9 |
Table 1: Some characteristics of each compound, including their retention time value and the range of concentration (%).
It is possible that the lichen secondary metabolism was modified to produce accessory compounds (STI and NSTI) when cells were immobilized, because STI and NSTI (Figure. 5) were not detected in the organic extracts of the thallus (Figure. 4). The production of some different compounds by thallus and immobilized cells could depend on the way of attachment between the symbionts. It is probably that lichens collected in humid areas show apressorial contact, while species from semi-arid region of Brazilian Northeast prefer the haustorial contact [39].
In relation to bioproduction efficiency, differences between the content of compounds depending on the type of cells immobilization system, since rotary movement and flow systems showed higher values were observed. However, it is necessary to understand that samples analyzed by HPLC were mixtures of the organic extracts and peaks resolution of some samples were not always satisfactory. So, the purpose of the HPLC analysis was to confirm the presence of bioactive compounds detected by TLC to know their range of total bioproduction.
The importance of lichen compounds as anticancer agents has been confirmed in recent years. STI can be considered as a promising lead compound for the design of novel human colon adenocarcinoma drugs [17], also induces neuroprotection through their antioxidant ability. This suggests the possibility that they could be used in those neurodegenerative disorders associated to oxidative damage such as Alzheimer’s and Parkinson’s disease [40].
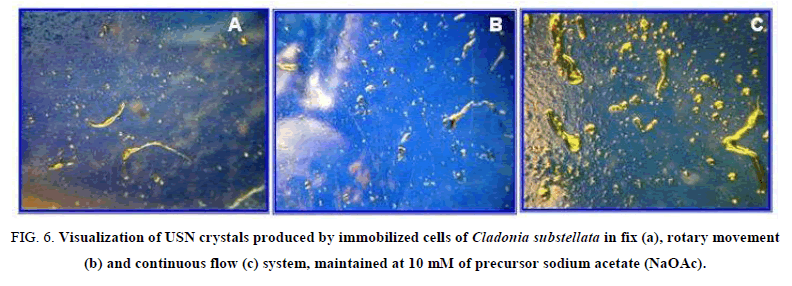
The enhance of USN production by our 3 systems was also ratified through crystallization of obtained USN from cell washes (Figure. 6), where the continuous flow immobilization system is clearly the best for USN production, at 10 mM NaOAC (Figure. 6C). The crystals from cell washes of immobilization systems, maintained in NaOAc at 10 mM, exhibited different weights. From the fix system, the crystallized USN showed a yield of 3 mg, 5 mg in rotary movement, and 7 mg in continuous flow, that ratifies the influence of technique modification on the bioproduction efficiency.
Figure 6: Visualization of USN crystals produced by immobilized cells of Cladonia substellata in fix (a), rotary movement (b) and continuous flow (c) system, maintained at 10 mM of precursor sodium acetate (NaOAc).
The polyketide biosynthetic pathway appears to be responsible (in whole or in part) for USN, STI and NTSI acids. However, their biotechnological production requires a process economically sustainable. The previous purification of the adequate enzyme and the chemical preparation of their substrates are very expensive to be used at an industrial scale. There is a great interest to find new systems that could produce them, hence the use of immobilized cells basically supplemented with acetate is a process sufficiently rapid, accurate and reproducible to produce this lichen compounds at a semi-industrial level.
In this way, the modification of the immobilization technique promotes the highest production of the cells and a better use of the precursor NaOAc, but it does not influence on the production of compounds of the species. The production of those metabolites from cell immobilizates, without use of large thalli amounts, is a promissory technique for bioconservation and sustainable use of natural resources, with emphasis to lichens, whose growth is very slow and their reposition to ecosystem very difficult [21].
Conclusion
It is possible to say that USN of C. substellata was well produced by continuous flow system and, in a general way, at 10 mM of NAOAc as precursor. Furthermore, other active compounds, such as STI and NTSI acids, were produced under the experimental conditions tested. New immobilization systems for production bioactive compounds were validated.
Acknowledgements
One of the authors (E. C. Pereira) thanks The National Council for Scientific and Technological Development (CNPq) for individual grant and financial project support.
References
- Rankovic B, Kosanic M. Antimicrobial activities of different extracts of Lecanora atra,Lecanora muralis, Parmeliasaxatilis, Parmelia sulcata andParmeliopsis ambigua. Pakistan J Bot. 2012;44:429-33.
- Molnár K, Farkas E, Naturforsch ZC. Studies in natural products chemistry: Bioactive Natural Products. 2010;65:157-73.
- Shukla V, Joshi JP, Rawat MSM. Lichens as a potential natural source of bioactive compounds: A review. Phytochem Rev. 2010;9:303-14.
- Johnson CJ, Bennett JP, Biro SM, et al. Degradation of the disease-associated prion protein by a serine protease from Lichens. PLoS One. 2011;6(5):e19836.
- Manojlovic NT, Rankovic B, Kosanic M, et la. Chemical composition of three Parmelia lichens and antioxidant, antimicrobial and cytotoxic activities of some their major metabolites. Phytomedicine. 2012;19:1166-72.
- Rankovic B, Kosanic M, Stanojkovic T, et al. Biological activities of Toninia candida and Usnea barbatatogether with their norstictic acid and usnic acid constituents.Int J Mol Sci. 2012;13:14707-22.
- Kosanic M, Manojlovic N, Jankovic S, et al. Evernia prunastri and Pseudoevernia furfuraceae lichens and their major metabolites as antioxidant, antimicrobial and anticancer agents. FoodChem Toxicol. 2013;53:112-8.
- White PAA, Oliveira RCM, Oliveira AP, et al. Antioxidant activity and mechanisms of action of natural compounds isolated from lichens: A systematic review.Molecules. 2014;19:14496-527.
- Müller K. Pharmaceutically relevant metabolites from lichens. Appl Microbiol Biotechnol. 2001;56:9-16.
- Bucar F, Schneider I, Ogmundsdóttir H, et al. Anti-proliferative lichen compounds with inhibitory activity on 12(S)-HETE production in human platelets. Phytomedicine. 2004;11:602-6.
- Kosanic M, Rankovic B, Stanojkovic T, et al. Biological activities and chemical composition of lichens from Serbia. Excli J.2014;13:1226-38.
- Maia MBS, Silva NH, Silva EF, et al. ActaFarm Bonaer. 2002;21:259-64.
- Piovano MJA, Garbarino FA, Giannini ER, et al. Evaluation of antifungal and antibacterial activities of aromatic metabolites from lichens. BolSocChilQuim. 2002;47:235-40.
- Vijayakumar CS, Viswanathan S, Reddy Kannappa M, et al. Anti-inflammatory activity of (+)-usnic acid. Fitoterapia. 2002;71:564-66.
- Santos NP, Nascimento SC, Wanderley MSO, et al. Nanoencapsulation of usnic acid: An attempt to improve anti-tumour activity and reduce hepatotoxicity.Eur J Pharm Biopharm. 2006;64:154-60.
- Bazin MA, Lamer ACLe, Delcros JG, et al. Synthesis and cytotoxic activities of Usnic acid derivatives. Bioor Med Chem. 2008;16:6860-6.
- Pejin B, Iodice C, Bogdanovic G, et al. Stictic acid inhibits cell growth of human colon adenocarcinoma HT-29 cells. Arab J Chem. 2013.
- Su ZQ, Mo ZZ, Liao JB, et al. Usnic acid protects LPS-induced acute lung injury in mice through attenuating inflammatory responses and oxidative stress. Int Immunopharmacol. 2014;22:371-8.
- Proksa B, Adamcova J, Sturdikova M, et al. Metabolites of Pseudevernia furfuracea and their inhibition potential of proteolytic enzymes. Pharmazie. 1994;49:282-3.
- Racan F, Rosan S, Boehm K, et al. Protection against UVB irradiation by natural filters extracted from lichens. J Photochem Photobiol. 2002;68:133-9.
- Vicente C, Solas MT, Pereyra MT, et al. Immobilization of lichen cells and enzymes for bioproduction of lichen metabolites: Technical requirements and optimization of product recovering. In: Daniels JA, Schulz M, Peine J, editors.Flechten Follmann: Contributions to lichenology in honour of Gerhard Follmann. Botanical Institute, University of Cologne, Germany, 1990; p97-110.
- Pereira EC, Andrade LHC, Silva NH, et al. Production of metabolites by immobilized cells of Cladiaaggregata (SW) Nyl. at different status of fertility. In: Calvelo S, Feuerer T, editors.Lichenology in Latin America. Germany: 2002; p185-194.
- Pereira EC, Molina MC, Pedrosa MM, et al. Production of ribitol by alginate-immobilized cells of the lichen cladonia-verticillaris. Chem Ann. 1995;91:253-9.
- Pereira EC, Pereyra T, Matos SC, et al. Bioproduction of usnic acid from acetate by kaolinite immobilized cells of cladonia-substellata vain. Acta Soc Bot Pol. 1995;64:171-4.
- Odabasoglu F, Cakir A, Suleyman H, et al. Gastroprotective and antioxidant effects of usnic acid on indomethacin-induced gastric ulcer in rats. J Ethnopharmacol. 2006;103:59-65.
- Culberson CF. J Chromatogr. 1972;72:113-25.
- Huneck S, Yoshimura I. Identification of lichen substances. 1st Ed. Springer; Verlag, Berlin, 19960, p11-123.
- Nguyen KH, Chollet-Krugler M, Gouaulta N, et al. UV-protectant metabolites from lichens and their symbiotic partners. Nat Prod Rep. 2013;l30:1490-1508.
- Legaz ME, deArmas R, Vicente C. Bioproduction of depsidones for pharmaceutical purposes. In: Rundfeldt C, editors. Drug development: A case study based insight into modern strategies. InTech, Europa. 2011;487-508.
- Praveen-Kumar SV, Prashith-Kekuda TR, Vinayaka KS, et al. Anthelmintic and Antioxidant efficacy of two Macrolichens of Ramalinaceae. Pharmacogn J. 2009;1:238-42.
- Kosanic M, Rankovic B, Stanojkovic T. J Sci Food Agr. 2012;92:1909-16.
- Pereira EC, Vicente C, Legaz ME, et al. Productionof lichen met abolites by immobilized cells of Cladonia clathrate. Phyton.1999;39:79-89.
- Pereira EC, Silva EF, Silva MIL, et al. Production of Cladonia corallifera metabolites by cellular immobilization. Rev UA Ser C Biol. 1999;2:25-41.
- Turgay T, Türk A, Yilmaz M, et al. Evaluation of the antimicrobial activity of the acetone extract of the lichen Ramalina farinacea and its (+)-Usnic Acid, Norstictic Acid, and Protocetraric Acid Constituents. Z Naturforsch. 2004;59:384-8.
- Manojlovic NT, Vasiljevic PJ, Maskovic PZ, et al. Chemical composition, antioxidant, and antimicrobial activities of lichen Umbilicaria cylindrica (L.) Delise (Umbilicariaceae). Evid Based Complement Alternat Med. 2012;8.
- Campanella L, Delfini M, Ercole P, et al. Molecular characterization and action of Usnic acid: A drug that inhibits proliferation of mouse polyomavirus in vitro and whose main target is RNA transcription. Biochimie.2002;84:329-34.
- Sokolov DN, ZarubaevVV, Shtro AA, et al. Anti-viral activity of (-)- and (+)-usnic acids and their derivatives against influenza virus A(H1N1)2009. Bioorg Med Chem Lett. 2012;22:7060-4.
- Ahti T, Stenroos S, Xavier-Filho L. The lichen family Cladoniaceae in Paraiba, Pernambuco and Sergipe, Northeast Brazil. Trop Biol. 1993;7:55-70.
- de Paz G, Raggio J, Gómez-Serranillos MP, et al. HPLC isolation of antioxidant constituents from Xanthoparmelia spp. J Pharm Biomed Anal. 2010;53:165-171.
- Vasconcelos TL, de Oliveira AK, Pereira EC, et al. Catena. 2015;135:70-7.