Original Article
, Volume: 13( 6)Green Applications of Nanoparticles Derived from Spent Tea Leave as Solar Photocatalyst and Water Purifier
- *Correspondence:
- Savita Belwal, Department of Chemistry , Anurag Group of Institutions, Hyderabad, India, Tel: 09704080295, E-mail: saovita29@gmail.com
Received: October 30, 2017; Accepted: November 08, 2017; Published: November 10, 2017
Citation: Savita B, Ganesh M, Shubham G, et al. Green Applications of Nanoparticles Derived from Spent Tea Leave as Solar Photocatalyst and Water Purifier. Biotechnol Ind J. 2017;13(6):151
Abstract
Newly synthesized micro and Nanoparticles are evaluated for possible application in DSSC (dye sensitized solar cell) and a low-cost adsorbent. Naturally occurring anthocyanin dyes, extracted from four fruits/juices were used as sensitizers for DSSCs (dye sensitized solar cells). The DSSCs fabricated with anthocyanin extracts of beet root (Beta vulgaris) with carbon Nanoparticles of spent tea leaves has shown the highest power conversion efficiencies (η) of 0.75% discussed in experimental part. Nanoparticles are also used to treat polluted water of different lakes of Hyderabad. The waste water samples were characterized physico-chemically before and after treatment using activated micro and nanoparticles as adsorbent according to standard procedure. Results are compared based on statistical technique and the magnitude of the difference among the samples is explicated. The results obtained from the various pollution indicators show an appreciable improvement to the quality of the water. The synthesized Nanoparticles were characterized by XRD (X-ray powder diffraction), FTIR (Fourier transforms infrared spectroscopy), SEM (Scanning electron microscopy), UV–visible spectroscopy.
Keywords
Adsorbent activated charcoal; Dye-sensitized solar cell; Physico-chemical analysis; Pollutant; SEM; TiO2; T-test
Introduction
This manuscript deals with the development of Nanoparticles from tea wastes. Tea wastes symbolize an unused resource and create increasing disposal problems. Once the tea is prepared, even at the domestic level, tea leaves are then redundant and considered as throw away waste [1]. For these reasons, policies are being made and research is going on to evaluate their possible use as an energy source or in other value-added applications [2]. Tea is assumed the extensively consumed beverage in the world, as its annual production and consumption are over 3, 000, 000 tons of tea leaves [3]. Instead of discarding the waste it has been developed as Nanoparticles using few techniques. Tea leaves act as natural adsorbents and has the capacity to adsorb a number of heavy metal ions [4,5]. So, Nanoparticles of tea waste were being used as an adsorbent in waste water treatment and various parameters were also tested [6]. Advancement in science and engineering specifically in the field of nanoscale suggest that various current problems concerning water quality could be resolved or greatly diminished by using bioactive nanoparticles, nanostructured catalytic membranes, Nano-adsorbent, nanocatalysts and magnetic nanoparticles [7]. It is due to the diffusion process especially at elevated temperatures [8] and the high surface area to volume ratio of nanoparticles offers a tremendous dynamic force for diffusion.
Recent developments show various applications of nanoparticulate which includes open wound and burn treatment and it has been established that 20 ppm silver colloidal suspension (~30 nm diameter) in purified water has a 100% cure rate for malaria [9]. Anatase nanoparticle form of titanium dioxide (TiO2) is known as the cytotoxic agent and potent against antibacterial activity with extraordinary photocatalytic action and it can be severely toxic in the respiratory system [10,11]. Nanocapsules and nanodevices may present new possibilities for drug delivery, gene therapy, medical diagnostics, antimicrobial activity etc. Because of their small size which is adequate to confine their electrons and produce quantum effects, nanoparticles acquire unexpected optical properties [12]. Yellow gold and gray Si nanoparticles are red in colour whereas gold nanoparticles emerge deep-red to black in solution [13]. Absorption power for solar radiation is much higher in materials composed of nanoparticles than the thin films of material. In both solar PV and solar thermal applications, controlling the size, shape and material of the particles, it is possible to control solar absorption [14-16]. Adsorption of heavy metal ions onto activated carbon is an efficient and well-established method for their removal from contaminated waters, but high costs limit its widespread use. Thus, in recent years much research has been undertaken to develop comparably effective but less expensive adsorbents [17]. An economical adsorbent is defined as one which is abundant in nature, or is a by-product or a waste from industry and requires little processing [18]. A major technological challenge for the human race in the 21st century is the transition from fossil-fuel-based energy economy to renewable (sustainable) energy one. Current solar power technology has little chance to compete with fossil fuels or large electric grids. Cost is a crucial factor in the success of any solar technology. Utilizing nanotechnology in an inexpensive solar cell would help to preserve the environment. Nanomaterials reveal good result than other techniques because of its high surface area (surface/volume ratio). It is suggested that these may be used in future at large scale applications. Nanotechnology has already shown huge breakthroughs in the solar field. Nanotechnology might be able to increase the efficiency of solar cells, but the most promising application of nanotechnology is the reduction of manufacturing cost. The synthetic dyes used in DSSC pose a serious threat to the environment and health due to their non-biodegradable and carcinogenic nature. Replacement of synthetic dyes with eco-friendly, bio-degradable and inexpensive natural dyes extract is the better way considering the impact on environment, health and economy [19].
As mentioned before the percentage of availability of freshwater is so less but the demand for the water is more, so this condition leads to scarcity of water. There are many factors responsible for the scarcity of water like overpopulation, pollution, rapid urbanization, climate change, depletion of aquifers etc. All these factors they lead to water pollution. An estimated 580 people in India die of water pollution related illness every day [20]. Due to unplanned nitrogen and phosphorous compounds, animal wastes, toxic chemicals, insecticides management, tremendous development of industry and agriculture and disposal of untreated public sewage water, agricultural runoff and other human and animal wastes in to river, lakes, reservoirs and other water bodies are continuously deteriorating their water quality and biotic resources [21]. Natural phenomena such as volcanoes, algae blooms, storms and earthquakes also cause major changes in water quality and the ecological status of water. Polluted water may contain suspended solids, dissolved inorganic, pesticides, medical waste, toxic heavy metals and biological pollutants such as pathogenic bacteria, fungi, protozoa, viruses, parasitic worms, etc [22,23]. Major sources of water pollution are from municipal water, industrial water, agricultural water, sewage water etc. Contamination of water is becoming a serious problem at a global level as fresh water resources are getting deteriorated day-by-day at an alarming rate [24-26]. These waste materials near factories are subjected to reaction with percolating rain water and reach aquifer system and hence degrade water quality [27]. These waste materials also consist of heavy metals which are toxic in nature, they enter the food chain through dietary intake or drinking water [28]. Eutrophication is another major factor to be considered when we talk about lakes. This condition reduces dissolved oxygen levels and results in mortality of benthic communities [29]. Thus, water for different purposes has its own requirements in terms of composition and purity as well as the presence of different components in water has to be analyzed on a regular basis to confirm its suitability [30]. For the treatment of water, many techniques have been introduced in the recent times. Adsorption is an effective purification and separation technique used in the industry especially in wastewater treatments. [31]. Chemical precipitation, reverse osmosis and filtration techniques cannot be used for trace analysis, while some of the methods are not economically feasible for large-scale industrial use due to high cost [32]. Among all the techniques Nano being the most advanced and effective method used widely. There are many types of research on waste water treatment and DSSC using nanotechnology.
Materials and Methods
Present paper consists of the following major steps:
1. Preparation of Nanoparticles from spent Tea and their characterization.
2. Use of nanoparticles adsorbents for treatment of polluted water samples and as photo catalyst.
Preparation of Nanoparticles from spent tea
The dried spent tea is crushed in a china dish with the help of a mortar and then it is transferred to a crucible which can withstand high temperatures up to 1000°C. The tea waste is then burnt in a muffle furnace at 650°C to 700°C and it was allowed to cool down for an hour in the furnace itself and the burnt material was removed. This tea waste gets activated due to its exposure to high temperature for 30 min. After this sonication is done for 20 min, where particles are uniformly spread with the help of UV rays. The activated tea waste is then agitated twice with the magnetic stirrer for 20 min.
Preparation of fruit extracts for DSSC
Dyes were extracted from beet root, pomegranate, mango and guava by a modified procedure similar to the procedure reported earlier [33]. The skin and pulp were ground by hand for two hour using mortar and pestle and then sonicated in 75% ethanol acidified with 10 M HCl for one hour. The mixture was then centrifuged at 1500 rpm for 10 min and decanted. Large particles were removed from the solution using a stainless-steel mesh and the solution was filtered using a filter paper.
Fabrication of DSSCs
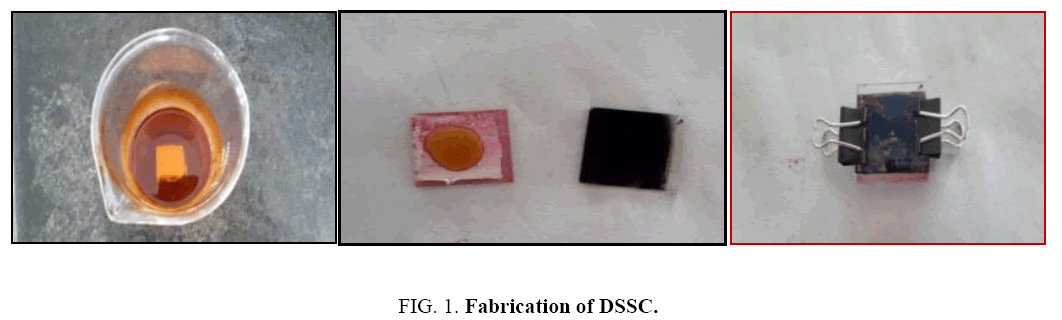
Dye sensitized solar cells (DSSC’s) were fabricated using the indium titanium oxide (ITO) glasses as shown in Figure 1. The photo anode is TiO2 powder, was then calcined in a muffle furnace at 673 K to produce anatase TiO2 nanoparticles [34]. TiO2 slurry was then obtained by slowly adding pH 3 acetic acid solution. The slurry was ultrasonicated for 30 min and was placed on a magnetic stirrer at 1200 rpm for 30 min. Ultrasonication and stirring were repeated 4 times to get the consistent viscous slurry of TiO2. The counter electrode was the nano-tea particles. The plates were fabricated manually instead of the doctor blade process. On one plate of ITO the TiO2 was plated with and then it was heated directly on the flame for about 10 min, till the TiO2 becomes brown in colour and comes back to white. This plate was dipped in the respective dye solutions for 12 h for complete dye loading. The other plate was decorated with tea Nanoparticles for increasing the conductivity of the cell. These tea plates were then sandwiched together as shown in the pictures below. Cells were tested under 350 W/m2 illuminations using artificial sunlight.
Treatment of water sample with nanoparticles
The polluted water of four lakes of Hyderabad is tested with waste Nanoparticles and the values before and after treatment were noted down and compared with the help of T-test. T-test’s statistical significance indicates whether there is a difference between two groups or not and averages reflect a “real” difference in the population from which the groups were sampled.
Analytical procedure
The parameters, their units and methods of analysis are summarized in Table 1. Dissolved oxygen is determined by adding MnSO4, alkaline iodide azide, conc. H2SO4 to sample and is titrated against standard hypo solution using starch as an indicator. All water samples were analyzed for different physico-chemical parameters within 48 h, COD [35] determined on the same sampling day. pH of each water sample was measured by a digital pH meter. Total hardness [36] was measured by EDTA complexometric titration. Total alkalinity [37] is determined by acid titration using methyl-orange and phenolphthalein indicators. Acidity is determined by base titration using phenolphthalein as an end point. Chlorides in water are found by Mohr’s method. Total residual chlorine [38] is found by using KI and glacial acetic acid. COD was found by using potassium dichromate and is titrated against hypo using starch indicator for the end point.
| Variables | Abbreviations | Units | Analytical methods |
|---|---|---|---|
| pH | pH | pH unit | pH-meter |
| Total hardness | T-Hard | mg/L | Titrimetric |
| Dissolved oxygen | DO | mg/L | Prob method |
| Chemical oxygen demand | COD | mg/L | Dichromate method |
| Chloride | Cl | mg/L | Titrimetric |
| Total alkalinity | T-Alk | mg/L | Titrimetric |
| Acidity | - | pH unit | Titrimetric |
| Residual Chlorine | Cl2 | mg/L | Titrimetric |
Table 1: Water quality parameters with their units and analytical methods used.
Results and Discussion
Characterization of carbon Nanoparticles
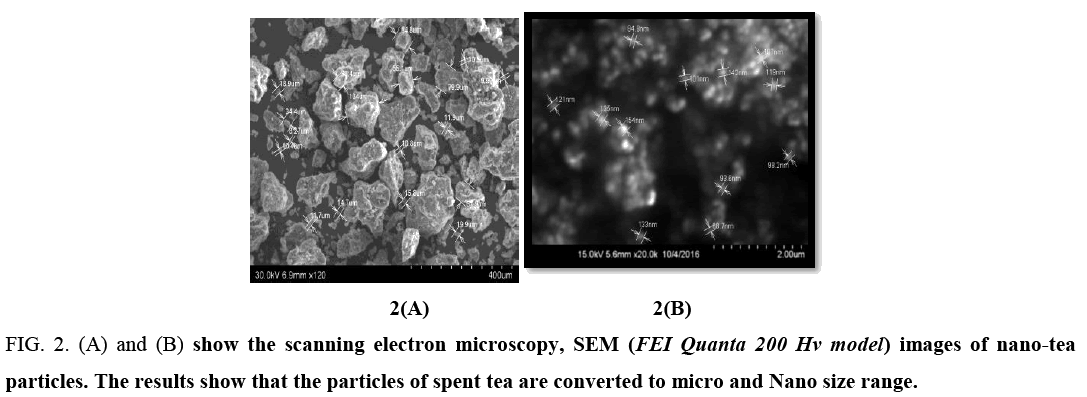
SEM (Scanning electron microscopy): The particles of spent tea are converted to micro and Nano size range.
Figure 2: (A) and (B) show the scanning electron microscopy, SEM (FEI Quanta 200 Hv model) images of nano-tea particles. The results show that the particles of spent tea are converted to micro and Nano size range.
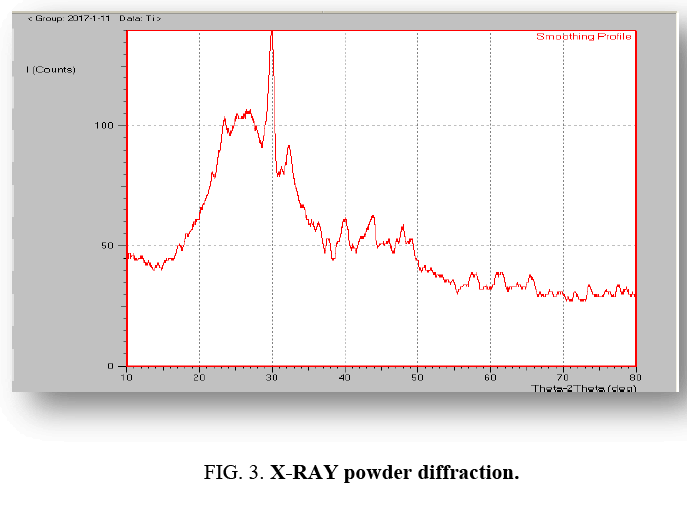
X-RAY powder diffraction: Powder X-ray diffraction patterns of the prepared sample were taken by a two-circle (2q-q). X-ray powder diffractometer (X'Pert PRO XRD PW 3040 system, model) using Copper kα1 of wavelength 1.54056´10-10 m and kα2 of wavelength 1.544426´10-10 m. The scan was taken between 2θ of 10° and 2θ of 45° at increments of 0.04° with a count time of 4 seconds for each step. The XRD spectrum of carbon nanoparticles in Figure 3 shows that there are two Bragg diffraction peaks at near 2θ=23.68° and 44.01°. In the present study, the peaks at near 2θ=23.68° and 44.01° were correspond to the presence of large amounts of amorphous carbon nano-materials in association with hexagonal graphite lattice.
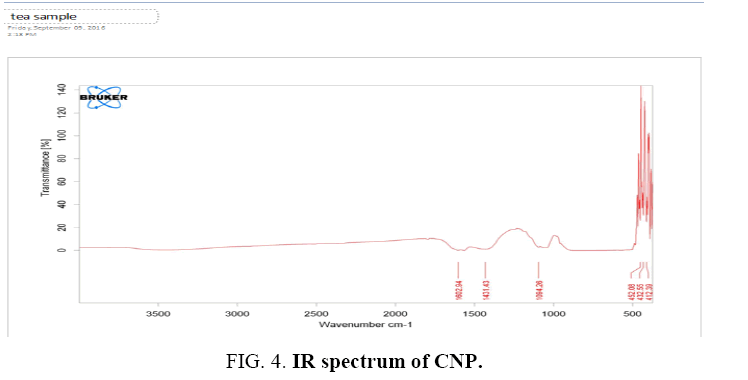
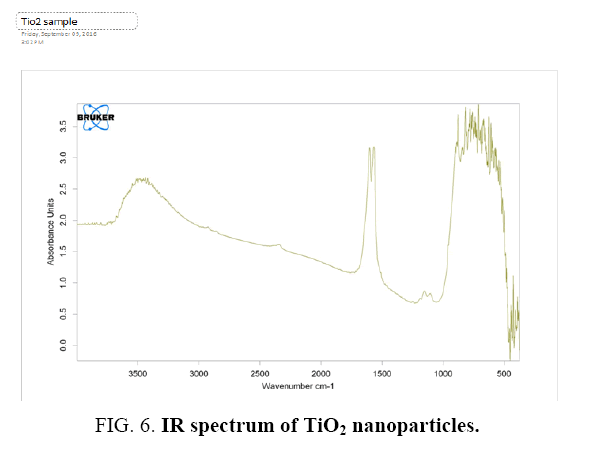
IR Study: Fourier transforms infrared spectroscopy (FTIR) spectroscopic study of the sample was performed by Shimadzu, Japan model, using KBr as a reference. 0.5 g dried carbon sample was mixed with KBr (sample/KBr ratio was 1/100) and were pressed into the transparent thin pellet (Figure 4).
A FTIR spectrum of carbon materials was obtained in the range on 4000 cm-1 to 400 cm-1.
The FTIR spectrum of prepared dried carbon Nanoparticles indicates the presences of some functional groups of hydrocarbon and oxygen. In the spectrum, peak at 3436 cm-1 (m) is for O-H stretch, peak at 2927 cm-1 is for C-H stretch in the alkane or in aromatic compounds. A very weak peak at 1630 is for C=C aromatic stretch. Peaks at 1465 cm-1, 1383 cm-1 and 1327 cm-1 are for the C-H bend in CH3 and the peak at 1118 is for C-O stretch.
Characterization of TiO2 Nanoparticles and extracted dyes
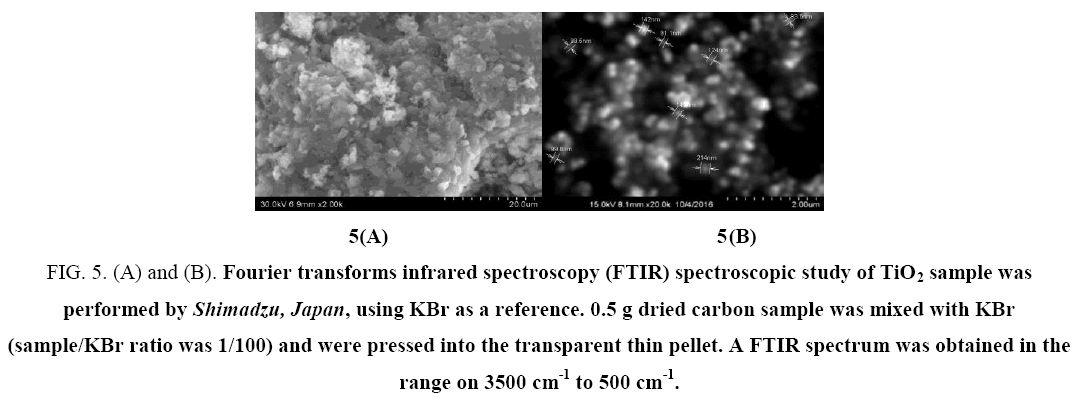
The presence of holes and extensive bridging as depicted in Figure 5 (A and B) of SEM (FEI Quanta 200 Hv model) of TiO2 is an indicator of highly conducting and crystalline TiO2 layers reveals extensive adsorption of the anthocyanin dye on the TiO2 nanoparticles. Highly porous layers with large surface areas are readily available to dyes for rapid adsorption, thus enhancing the short circuit current density. As a result, materials show enhanced electrochemical performance compared with their bulk counterparts [39] (Figure 5 & Figure 6).
Figure 5: (A) and (B). Fourier transforms infrared spectroscopy (FTIR) spectroscopic study of TiO2 sample was performed by Shimadzu, Japan, using KBr as a reference. 0.5 g dried carbon sample was mixed with KBr (sample/KBr ratio was 1/100) and were pressed into the transparent thin pellet. A FTIR spectrum was obtained in the range on 3500 cm-1 to 500 cm-1.
The spectrum of TiO2 nanoparticles obtained by the standard procedure is shown in Figure. It clearly shows the TiO2 absorption band near 530 cm–1. The peaks at 3450 cm-1 and 1650 cm–1 indicate the presence of –OH and C=O residues, probably due to atmospheric moisture and CO2 respectively. Some very small peaks can be seen which could be due to the traces of the contaminants present in the sample.
UV-Visible
Beet root pomegranate
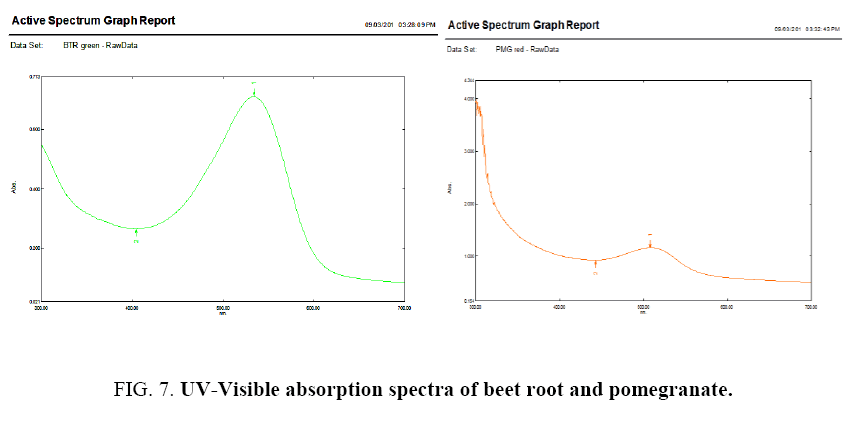
Figure 7 shows the UV-Visible (UV Double Beam Spectrophotometer, Shimadzu, Japan) absorption spectra of different sensitizers used in this study. A band due to the >C=N chromophore in the spectrum of the compound at 365 nm shifts to a higher wavelength. Such a shift in n-π * transition band is probably due to the donation of a lone pair of electrons by the nitrogen. Further, two bands at 260 nm and 305 nm are due to π-π * transitions, these are assigned to the benzenoid ring and (>C=N) band of the azomethine group respectively. The K band π-π * showed a red shift due to the increase in conjugation and the B-band undergoes a hypsochromic shift. The peak or the hump is observed at 545 nm explains the presence of betanin moiety, "omega-5" moiety [40,41].
These Nanoparticles were used in the fabrication of dye sensitized solar cells which are more efficient than that of the existing ones and could be the near future to produce electricity. Table 2 depicts the I-V characteristics of the DSSCs made from extracts of fruit juices. Highest power conversion efficiency (η) of 0.75% and 0.54% were obtained for beet root and pomegranate with Voc of 0.677 V and 0.611 V and Fill Factor (FF) of 0.803 and 0.718 respectively. The corresponding values are given in Table [1].
| S.No | Dye | Voc (V) | Jsc (mA/cm2) | FF | η(%) |
|---|---|---|---|---|---|
| 1 | Beet Root (Beta Vulgaris) | 0.677 | 0.438 | 0.803 | 0.75 |
| 2 | Pomegranate (Punicagranatum) | 0.611 | 0.3966 | 0.718 | 0.54 |
| 3 | Mango (Mangiferaindica) | 0.555 | 0.561 | 0.521 | 0.52 |
| 4 | Guava (Psidiumguajava) | 0.668 | 0.314 | 0.38 | 0.25 |
Table 2: (1-4) Characteristics of dye based solar cells at 350W/m2 illumination water samples with CNP and results are shown below.
• HS: Hussian Sagar BT: Before treatment
• SG: Safilguda AF: After treatment
• SN: Sarrornagar
• UP: Uppal
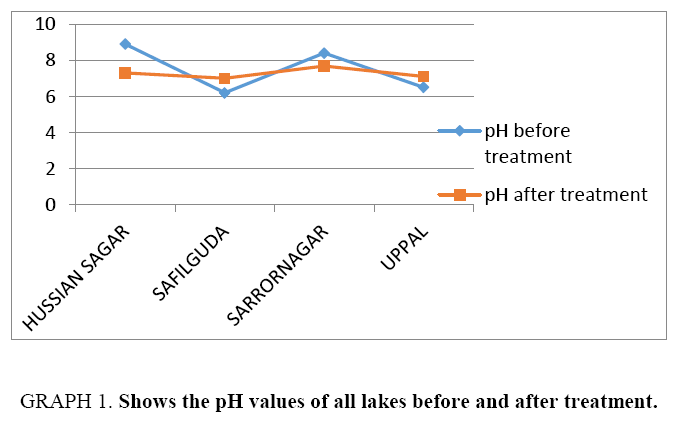
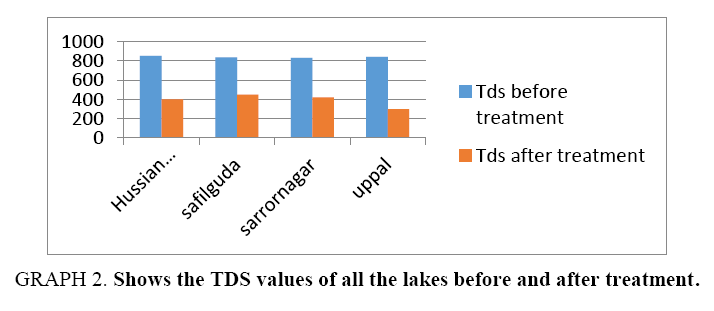
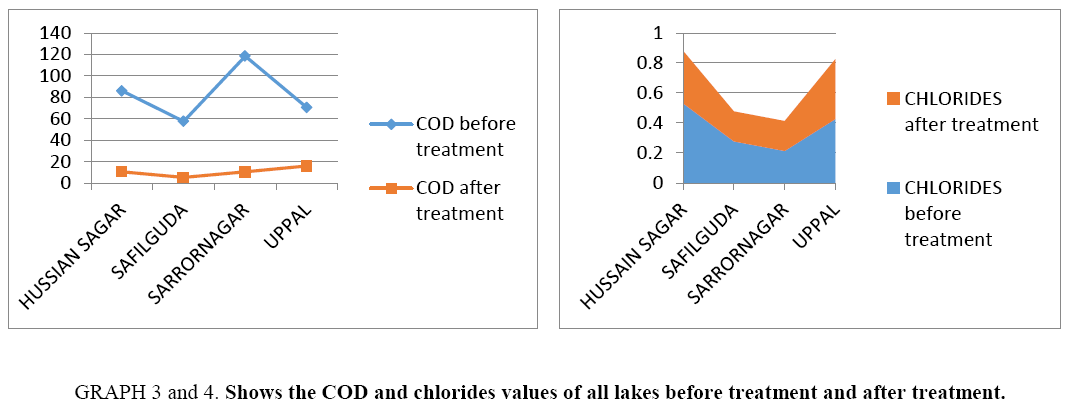
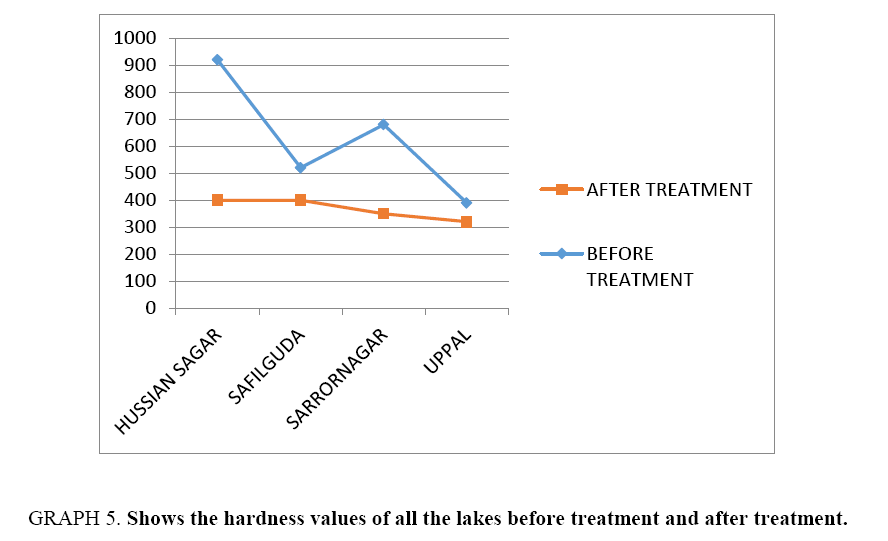
Results of Table 3 are summarized and depicted with the help of graphs (Graph 1-5).
Graph 3 and 4: Shows the COD and chlorides values of all lakes before treatment and after treatment.
| Parameters | HS | SG | SN | UP | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BT | AT | BT | AT | BT | AT | BT | AT | ||||
| pH | 8.9 | 7.3 | 7.2 | 7.00 | 8.40 | 7.69 | 7.5 | 8.1 | |||
| Alkalinity | p-end | 100 | 500 | 50 | 150 | 100 | 200 | 0 | 150 | ||
| m-end | 450 | 300 | 370 | 320 | 250 | 300 | 500 | 400 | |||
| Acidity | - | - | - | - | |||||||
| Chlorides | 0.53 | 0.35 | 0.2769 | 0.20 | 0.213 | 0.20 | 0.426 | 0.42 | |||
| Residual chlorine | - | - | - | - | |||||||
| COD | 86.4 | 10.66 | 57.6 | 5.33 | 118.4 | 10.5 | 70.4 | 16 | |||
| Hardness | Temphard | 540 | 100 | 180 | 170 | 440 | 120 | 190 | 150 | ||
| Per.hard | 380 | 300 | 340 | 230 | 250 | 230 | 200 | 170 | |||
| Total hard | 920 | 400 | 520 | 400 | 680 | 350 | 390 | 320 | |||
| DO | 11.2 | 7 | 10.5 | 6.5 | 8 | 9 | 17.6 | 7.5 | |||
| TDS | 850 | 400 | 834 | 450 | 830 | 420 | 840 | 300 | |||
| Sulphates | Wavelength | 448 | 208.4 | 424.5 | 213 | 425 | 222.8 | 432 | 213.8 | ||
| Absorbance | 0.006 | 2.231 | 0.151 | 1.94 | 0.152 | 0.103 | 0.009 | 1.98 | |||
| E. coli | Prsent | Nil | Present | Nil | Present | Nil | Present | Nil | |||
Table 3: Shows result of 11 parameters of all the lake samples.
CNP is very effective for water treatment in reducing COD, BOD and chlorides, hardness and other parameters. This technique is a bit cheaper in comparison with other waste water treatment techniques, so can be used effectively in order to treat highly polluted water.
T-Test
The results for the water samples from 6 lakes before and after treatment with Nanoparticles are further examined by the statistical method i.e. T-Test, which can be used to determine if two sets of data are significantly different from each other. Table 4 given below shows the correlation between the factors i.e. BEFORE TREATMENT AND AFTER TREATMENT. The table shows the positive changes in the values.
| N | Correlation | Sig. | ||
|---|---|---|---|---|
| Pair 1 | BTHS and ATHS | 10 | .639 | .047 |
| Pair 2 | BTSG and ATSG | 10 | .981 | .000 |
| Pair 3 | BTSN and ATSN | 10 | .874 | .001 |
| Pair 4 | BTUppaland ATUppal | 10 | .822 | .004 |
| Where, BTHS:Before treatment HussainSagar ValueATHS:After treatment HussainSagar Value BTSG: Before Treatment Safilguda; ATSG: After Treatment Safilguda BTSN: Before Treatment Saroornagar; ATSN: After Treatment Saroornagar; BT Uppal: Before Treatment Uppal AT Uppal: After TreatmentUppal | ||||
Table 4: The correlation between the factors.
| Paired samples test | Paired differences | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Std. deviation | Std. Error Mean | 95% Confidence interval of the difference | ||||||
| Lower | Upper | T | df | Sig. (2-tailed) | |||||
| Pair 1 | BTHS-ATHS | 127.17200 | 270.17065 | 85.43546 | -66.09644 | 320.44044 | 1.489 | 9 | .171 |
| Pair 2 | BTSG-ATSG | 42.95469 | 79.75918 | 25.22207 | -14.10159 | 100.01097 | 1.703 | 9 | .123 |
| Pair 3 | BTSN-ATSN | 85.76230 | 154.62938 | 48.89810 | -24.85289 | 196.37749 | 1.754 | 9 | .113 |
| Pair 4 | BTUppal-ATUppal | 69.39060 | 178.39132 | 56.41229 | -58.22286 | 197.00406 | 1.230 | 9 | .250 |
Table 5: Result of T-test.
Conclusion
Water pollution in India is serious than originally thought it was. Water pollution in India has a wide range of reasons, ranging from pesticide runoff due to its usage in agriculture, heavy metal contamination by irresponsible factories and mines, tradition occupations, religious practices, sewage contamination attributed to the shortage of waste water treatment plants.
In this study, Nanoparticles are derived and they are used for water treatment. The values of before and after treatment are noted down and compared. A change in COD, hardness, DO and TDS were seen in water samples after treatment. Acidity and residual chlorine were absent in both the cases. The Hardness value changed hugely and chemical oxygen demand (COD) was reduced for all the lake waters.
The main cause of pollution in these lakes is due to the discharge of industrial effluents and sewage water into the lakes from different ‘nalas’. Every lake has an inlet of sewage or industrial water into the lake and dumping of garbage piles also added to the coliform woes of the lake.
To determine the similarities and variations in the parameter of the lakes, T-test was used in our manuscript. For this analysis, the parameters of different lake samples were taken and average change between the samples was found out. Consequently, the search for persistently but effective, efficient and economic methods has been increased in recent times.
Acknowledgments
The authors are thankful to Dr. P. Sivakrishna, Director, Synteny Lifesciences Pvt, Hyderabad, for providing the facilities to characterize the samples.
References
- Tiwari DK, Behari J, Sen P. Application of nanoparticles in waste water treatment. World Applied Science Journal. 2008;3(3):417-33.
- Mamadou SD, Savage N. Nanoparticles and water quality. Journal of Nanoparticles Research. 2005;7(4):325-30.
- Hewakuruppu YL, Dombrovsky LA, Chen C, et al. Plasmonic pump-probe method to study semi-transparent nanofluids. Appl Opt. 2013;52(24):6041-50.
- Muhammad A, Khan MA, Farooq U, et al. Carbonized green tea dredge, a potential adsorbent for removal of remazolbrillian yellow dye. J Mater Environ Sci. 2012;3(1):149-56.
- Jeyaseelan C, Gupta A. Green tea leaves as a natural adsorbent for the removal of Cr(VI) from aqueous solutions. Air, Soil and Water Research. 2016;9:913-19.
- Taylor RA, Todd O,Yasitha H, et al. Feasibility of nanofluid-based optical filters. Appl Opt. 2013;52(7):1413-22.
- Taylor RA, Phelan PE, Otanicar TP, et al. Nanofluid optical property characterization towards efficient direct absorption solar collectors. Nano Scale Research Letters. 2011;6(1):225-32.
- Mamadou SD, Savage N. Nanoparticles and water quality. J Nanopart Res. 2005;7(4):325-330.
- Shivaji S, Madhu S, Singh S. Extracellular synthesis of antibacterial silver nanoparticles using psychrophilic bacteria. Process Biochemistry. 2011;46(9):1800-7.
- Ishibashi KI. Generation and deactivation processes of super oxide formed on TiO2 film illuminated by very weak UV light in air or water. J PhysChem B. 2000;104:4934-8.
- Babel S, Kurniawan TA. Low-cost adsorbents for heavy metals uptake from contaminated water. Journal of Hazardous Materials. 2003;B97:219-43.
- Aksu Z, Isoglu I A. Removal of copper(II) ions from aqueous solution by biosorption onto agricultural waste sugar beet pulp. Process Biochemistry. 2005;40(9):3031-44.
- Arvanitoyannis I, Varzakas TH. Vegetable waste treatment: Comparison and critical presentation of methodologies. Critical Reviews in Food Science and Nutrition. 2008;48(3):205-47.
- Laufenberg G, Kunz B, Nystroem M. Tranformation of vegetable waste into value added products: (A) The upgrading concept: (B) Practical implementation. BioresourceTechnology.2003;87(2):167-98.
- Wan L, Li D, Zhang D, et al. Conservation and implications of eukaryote transcriptional regulatory regions across multiple species. BMC Genomics.2008;9:623.
- Gao H, Lv S, Dou J, et al. The efficient adsorption removal of Cr(VI) by using Fe3O4 nanoparticles hybridized with carbonaceous materials. RSC Adv. 2015;5:60033-40.
- Reich E, Schibil A, Widmer V, et al. HPTLC methods for identification of green tea and green tea extract. J Liquid Chromatograph Relat Tech. 2006;29(14):2141-51.
- Aksu Z, Isoglu IA. Removal of copper(II) ions from aqueous solution by biosorption onto agricultural waste sugar beet pulp. Process Biochem. 2005;40(9):3031-44.
- Hardin BE, Snaith HJ, McGehee MD. The renaissance of dye-sensitized solar cells. Nat Photon. 2012;6(3):162-9.
- Ichinose N, Ozaki Y, Kashu S. Superfine particle technology. Springer London; 1992.
- Wang ZS, Yan C, Yasufumi D, et al. Molecular design of coumarin dyes for stable and efficient organic dye-sensitized solar cells. The J PhysChem A. 2008;112:17011-17.
- Elmaci A, Topac FO, Ozengin N, et al. Evaluation of physical, chemical and microbiological properties of lake Ulubat,Turkey. J Environ Bio. 2008;29(2):205-10.
- Aggarwal R, Arora S. A study of water quality of Kaushalya River in The SubmountaneousShivalik Region. International Journal of Scientific Technology Research. 2102;1(8):52-68.
- Pardeshi DS, Vaidya S. Physico-chemical assessment of Waldhuni River Ulhasnagar (Thane). A case study. International Journal of Current Research and Academic Review. 2015;3(4):234-48.
- Mahananda MR. Physico-chemical analysis of surface water and ground water of Bargarh District, Orissa, India. International Journal of Research and Review in Applied Sciences. 2010;2(3):284-95.
- Venkatesharaju K, Ravikumar P, Somashekar RK, et al. Physico chemical and bacteriological investigation on the river cauvery of kollegal stretch in Karnataka. Journal of Science Engineering and Technology. 2010;6(1):50-9.
- Shaji C, Nimi H, Bindu L. Water quality assessment of open Wells in and around Chavara industrial area Quilon,Kerala. Journal of Environmental Biology. 2009;30(5):701-4.
- Oketola A, Adekolurejo SM, Osibanjo O. Water quality assessment of River Ogun using multivariate statistical techniques. Journal of Environmental Protection. 2013;4(5):466-79.
- Treseder KK. Nitrogen additions and microbial biomass a meta-analysis of ecosystem studies. Ecological Letter. 2008;11(10):1111-20.
- Sharma V, Walia YK. Water quality assessment using physico-chemical Parameters and heavy metals of GobindSagar Lake, Himachal Pradesh (India). Current World Environment. 2015;10(3):967-74.
- Al-Asheh S, Banat F, Al-Omari R, et al. Predictions of binary sorption isotherms for the sorption of heavy metals by pine bark using single isotherm data. Chemosphere. 2000;41:659-65.
- Sirimanne PM, Isenevirathna MK, Premalal EVA, et al. Tennakore, utilization of pigment extracted from flowers as sensitizer in solid state solar cell. J PhotochemPhotobiol A. 2006;177:324-7.
- Adachi M, Murata Y, Okada I, et al. Formation of titania nanotubes and applications for dye-sensitized solar cells. Journal of The Electrochemical Society. 2003;150:G448-G493.
- Sachidanandamurthy KL, Yajurvedi HN. Monthly variations in water quality parameters (physico-chemical) of a Perennial lake in Mysore city. Indian Hydrobiol. 2004;7:217-28.
- Ong CN, Grandjean AC, Heaney RP. The mineral composition of water and its contribution to calcium and magnesium intake. In: Calcium and magnesium in drinking-water: public health significance. Geneva, World Health Organization. 2009;p:36-58.
- Kim HC, Lee K. Significant contribution of dissolved organic matter to seawater alkalinity. Geophys Res Lett. 2009;36:L20603.
- Das AK. Phytoplankton primary production in some selected reservoirs of Andra Pradesh. Geobios. 2002;29: 52-7.
- Premalala EVA, Dematagea N, Kumarab GRA, et al. Shorter nanotubes and finer nanoparticles of TiO2 for increased performance in dye-sensitized solar cells. ElectrochimicaActa. 2012;63:375-80.
- Gandia-Herrero F, Garcia-Carmona F, Escribano J. Purification and characterization of a latent polyphenol oxidase from beet root (Beta vulgaris L.). Journal of Agricultural and Food Chemistry. 2004;52(3):609-15.
- Gandia-Herrero F, Escribano J, Garcia-Carmona F. Betaxanthins as pigments responsible for visible fluorescence in flowers. Planta. 2005;222(4):586-93.
- Mc Donald JH. Handbook of Biological Statistics, Sparky House Publishing, Baltimore; 2008.