Review
, Volume: 13( 6)Degradation of Chicken Feathers: A Review
- *Correspondence:
- Quezada-Cruz M, Laboratorio de Biotecnología Ambiental, Universidad Tecnológica de Tecámac. Km. 37.5 Carretera Federal México – Pachuca. Col. Sierra Hermosa. CP 55740. Tecámac, Edo. de México, México, Tel: +52 55 5938 8400; E-mail: mabelqz@yahoo.com.mx
Received: November 08, 2017; Accepted: November 21, 2017; Published: November 24, 2017
Citation: Pahua-Ramos ME, Hernández-Melchor DJ, Camacho-Pérez B, et al. Degradation of Chicken Feathers: A Review. Biotechnol Ind J. 2017;13(6):153
Abstract
The amount of chicken feathers generated as waste from the poultry industry in the last decade has become of technological interest for many researchers regarding both its treatment and application. At a worldwide level, billions of tons of feathers are produced due to the consumption of poultry and, consequently, leading to problems as they are affecting public health and the environment. Biodegradation processes of chicken feathers with the greatest international impact use pure strains, which have been isolated from wastes of poultry farms. Among the strains with the best degradation efficiency (100% in 1 to 7 days) are: Bacillus sp., Kocuria sp., Pseudomonas sp. and Fervidobacterium sp. At present, microbial consortia or modified strains are also used to increase the efficiency and profitability of biodegradation. Physicochemical methods include alkaline hydrolysis or alkaline-acid hydrolysis, mixture of oleic substances like soy and glycerol, microwaving, pyrolysis and carbonization. After treatment of poultry waste through different methods, relevant by-products like enzymes, amino acids, anti-oxidant, biofuels, biofertilizers, biopolymers, micro-and macro-particles, flame retardant bases, dielectric materials, super-condensers, among others, are obtained. Hence, the objective of this review is to analyze the different biological and physicochemical methods used for the degradation of chicken feathers emphasizing procurement of byproducts and applications in the biotechnological realm.
Keywords
Biodegradation; Physicochemical degradation; Chicken feathers; Keratinolytic; Bypro ducts
Introduction
Production of chicken meat brings about the generation of wastes, among them stand out the feathers. According to some authors [1-3] billions of tons of feathers are generated worldwide becoming a problem as they are wastes that affect public health and the environment [4]. These feathers are mainly produced in chicken processing plants and poultry farms and represent a large source of contamination, because between 5% and 10% of the total chicken weight corresponds to feathers [5]. In Mexico, it has been estimated that the total consumption of chicken will be of 3274 million tons for the year 2018 [6], which means that around 163.7 to 327.4 million tons of feathers will be produced.
Some studies on alternatives to diminish or eliminate the contamination problem propose to incorporate the feathers as feed supplement because 95% of its dry weight corresponds to protein (90% keratin) [7]. To increase their solubility, it has been suggested to subject the feathers to a process with high pressure and temperature, but this has disadvantages, such as high costs and destruction of some thermolabile amino acids, like histidine, methionine and tryptophan [8].
Kowalska and Bohacz [7] mention that there is a radical change regarding the use of feathers due to the aforementioned problems, hence, new cheaper technologies that generate useful byproducts have been developed in recent years. The use of microorganisms or enzymes produced by them to accomplish fermentation processes is one of the most promising technologies. For these processes, metabolism of microorganisms is necessary, such as of bacteria like Bacillus sp., Chryseobacterium sp., Kocuria sp., Fervidobacterium sp., Brevibacillus sp., Xanthomonas sp., Pseudomonas sp., Leuconostoc sp., Scopulariopsis sp., Stenotrophomonas sp. is necessary [1, 9-26] of actinomycetes like Actinomadura sp. [27]; of fungi like Coprinopsis sp., Aspergillus niger [28,29]; of bacterial consortia [30]; and of actinomycetes consortium [31]. In addition, some authors have performed genetic modifications of strains of Escherichia coli, Brevibacillus sp. and Bacillus subtilis, aimed at improving the degradation of chicken feathers in a short time and the bioprocess at different scales (laboratory and industrial level) [3,32,33].
In addition to obtaining by-products of high added value from the biological degradation of chicken feathers, the feathers have been used as adsorbents in the process of removing metals from wastewater [34] and recently its use has been highlighted in the generation of biothermoplastics and nanomaterials [35-37], so that detailed study of its structure results from biotechnological relevance for its application in various industries.
Experimental
Structure of the feather
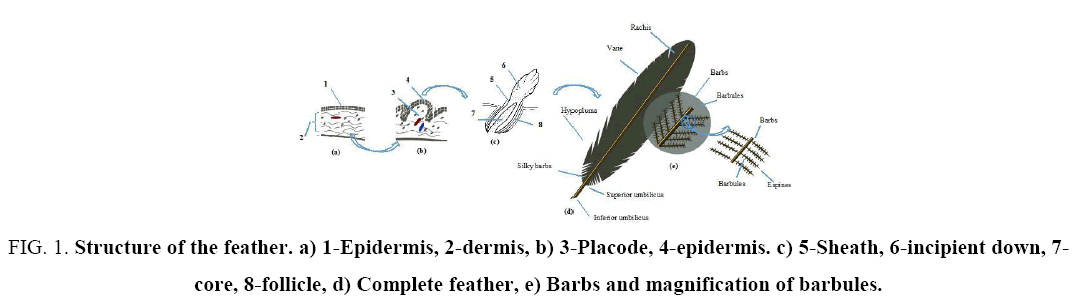
The process of feather development starts with the formation of a conjunctive papilla (Figure 1a), as a first placode, with the participation of the dermis and epidermis (Figure 1b). Later on, it differentiates from the epidermal layer to give rise to the sheath that contains the radii of the down (Figure 1c). The last stage of the development is characterized by rupture of the sheath that already envelops the incipient down. In the basal strata is the core. The whole placode becomes immersed in the follicle (Figure 1d) [38].
Figure 1: Structure of the feather. a) 1-Epidermis, 2-dermis, b) 3-Placode, 4-epidermis. c) 5-Sheath, 6-incipient down, 7-core, 8-follicle, d) Complete feather, e) Barbs and magnification of barbules.
Once the development is complete, the feather is composed of three different units, the central shaft of the feather called rachis, to which the secondary structure (the barbs) attaches. The tertiary structure of the feathers corresponds to the barbules, which are associated to the barbs (Figure 1e).
The axis is formed by a calamus and a rachis, despite being a very light structure; it provides the needed rigidity to keep it firm. The rachis is filled with foam (medulla) and its cross-section has a quasi-rectangular shape that tapers toward the feather's tip. The rachis consists of alternating oppositely oriented fibre layers. The medulla core, made from keratin, shows two levels of porosity, which minimizes feather weight, especially because it occupies a large part of the rachis volume. Besides the rachis is filled with dead substances, pigments and proteins that remained there, resulting from the development of the feather. The lower part of the rachis is wider and hollow, usually nude, it is called the calamus or quill and it is the part by which the feather is inserted into the skin. The calamus has in its lower part an orifice, named inferior umbilicus, through which the feather is fed during its growth [39]. On the upper part of calamus, the rachis starts to flatten and there is, just where the calamus ends, another orifice called the superior umbilicus, through which the laminar body of the feather emerged as it started to grow. In the lateral margins of the rachis grows the vane, a structure like a lamina divided in two opposite parts, it is the visible body and the largest area of the feather, formed by a complicated network of interwoven hooks that are the barbicels that connect barbules to each other, interlocking neighboring barbs, providing the texture of a very light tissue able to support a heavy load per area unit, the principle that allows birds to fly.
The rachis runs along the whole feather and could be of 7 inches in length. Barbs have lengths ranging from 1 cm to 4.5 cm depending on their location along the rachis. Barbs in the base of the rachis are longer than those on the tip. The tertiary structures, the barbules, have lengths of approximately 0.3 mm-0.5 mm and have hook structures in their tips [40].
Physicochemical characteristics of the feather
According to Costa et al. [20] the feather has a high percentage of volatile solids of 99 ± 1.4 (Table 1), which represents most of the possible degradable matter as it contains 92.0 ± 0.48% of crude protein, of which 82.8 ± 0.51% corresponds to keratin [30] constituted by different amino acids from which nitrogen can be obtained; nitrogen is an essential nutrient for the development of microorganisms capable of degrading chicken feathers. Some other constituents of the feather are fat with 2.79 ± 0.032% [30] and ashes with 0.69 ± 0.08% [1]. Chemical demand of oxygen ranges from 1200 to 1408 g/kg [20,30].
| Variable/Fraction | Costa et al. [20] | Xia et al. [30] |
|---|---|---|
| Total solids (%) | 100 ± 0.5 | NR* |
| Volatile solids (%) | 99± 1.4 | NR* |
| Dry matter (%) | NR* | 94.7 ±0.44 |
| Chemical oxygen demand | 1408 ±59 (g/kg) | 1200 ±0.002 (g/kg) |
| N-Kjeldahl | 137 ±9 (g N/kgwaste) | NR* |
| Crude protein (%) | NR* | 92.0 ± 0.48 |
| Organic matter (%) | NR* | 99.2±0.69 |
| Fat (%) | NR* | 2.79± 0.032 |
| Keratine (%) | NR* | 82.8 ±0.51 |
| *NR: Not reported | ||
Table 1:Physicochemical characteristics of chicken feathers.
Feathers are composed of keratin. It is a semi-crystalline protein of small molecular weight (~10 kDa) [41], aside of being rich in amino acids like leucine and serine. Stilborn et al. [42] identified the amino acids present in chicken feathers at 112 days of age, the essential amino acids were arginine, cystine, histidine, isoleucine, lysine, methionine, phenylalanine, threonine, tryptophan, tyrosine and valine; the non-essential amino acids were alanine, aspartic acid, glutamic acid, glycine, proline and serine; the amino acids with the highest concentration were leucine (7.75%), glutamic acid (10.34%) and serine (11.44%).
Due to the aforementioned, the alternative of greatest interest for their degradation is the biological one, from submerged cultures and through fermentation in solid state, as this allows obtaining the enzymes capable of degrading keratin (proteases), as well as byproducts of high added value like the previously mentioned amino acids, proteins, enzymes (keratinases), etc. [21,23,24].
Biological Treatment of Chicken Feathers
Microorganisms capable of degrading chicken feathers
The amount of chicken feathers generated as waste in the last decade has become of biotechnological interest for many researchers [26,43,44]. The degradation processes most studied at the international level have used pure strains, most of these strains have been isolated from wastes of the poultry farms [1,13,25,27]. Among the strains that have shown the best efficiency for degradation are Bacillus pumilis A1, Bacillus cereus Wu2, Bacillus megaterium F7-1, Kocuria rosea, Pseudomonas stutzeri, Fervidobacterium islandicum AW-1, Alcaligenes sp., Stenotrophomonas maltophilia and Actinomadura keratinilyca Cpt29, accomplishing a total chicken feathers degradation in a period of 1.5 to 7 days [1,9,11,13,22,24,26,27]. Considering the difficulty to maintain under sterile conditions the strains with complex substrates, like chicken feathers, studies have been performed for their degradation using microbial consortia or mixed cultures. Xia et al. [30] and Tonkova et al. [31] observed that by the degradation of feathers in a mixed culture, the concentration of soluble protein obtained is two to three times higher than in a culture with pure strains. According to these authors, the use of microbial consortia and mixed cultures leads to lower operational costs and allows escalating the process. An example of this is the one reported by Xia et al. [30], who used reactors at a pilot level (42 L), for the degradation of chicken feathers using an anaerobic consortium isolated from manure and wastes from a slaughterhouse. The concentration of feathers represented 37% of the total solids in the reactor; removal of feathers was accomplished in 146 days. Tonkova et al. [31] who worked with a mixed culture of actinomycetes, observed a degradation of up to 91% at 72 h, however, it must be pointed out that the operational conditions and type of consortium were different (Table 2).
| Strain | Pretreatment | Mineral medium | Carbon source | Reactor type//Operation conditions | Fermentation time (d)/Degradation (%) | Enzymatic activity | Reference |
|---|---|---|---|---|---|---|---|
| Bacillus subtilis AMR | -Wash with detergent(linear alkylbenzene sulfonic acid) -Rinse with tap water -Dry overnight at 60°C. -Delipidate by immersion in methanol:chloroform (1:1) solution for 1 hour, -Dry at 60°C -Store at room temperature |
-Yeast extract 5 g/L -Peptone 5 g/L -KCl 20 g/L |
sucrose 0.5 g/L feathers 10 g/L |
-2000 mL Erlenmeyer flask -medium 500 mL -26 ± 2°C -150 rpm -pH 8 |
12 d/95% | Keratinolytic: 360.6 U/mL | [4] |
| B. methylotrophicus |
-Wash under running tap water -Dryat 60°C for48 h -Ground with ultra-?ne friction grinder |
-Na2HPO4 0.376 g/L -NaH2PO4 0.46 g/L |
feathers 10 g/L | -Bottles 100 mL -medium 70mL -30°C -170 rpm -pH: not reported |
2 d/30% | Not reported | [45] |
| Bacillus subtilis | -Wash with tap water 3 times for 15 min -Wash with distilled water 3 times -Dry at 60°C for 12 h |
-Mannitol 10 g/L -Tryptone 10 g/L -MgCl2 0.1 g/L -KH2PO4 0.4 g/L -K2HPO4 0.3 g/L -NaCl 0.5 g/L |
feathers 2 g/L | -500 mL Erlenmeyer flask -medium 100 mL -inoculum 1 mL (106 UFC) -37°C -160 rpm -pH 8.5 |
1.5 d/100% | Keratinolytic: 3.8 U/mL | [26] |
| Bacillus pumilis A1 | -Wash with tap water 3 times -Wash with distilled water 3 times -Dry at 90°C for 22 h |
-KH2PO4 0.5 g/L -K2HPO4 0.5 g/L -NaCl 2 g/L -KCl 0.1 g/L -MgSO4•7H2O 0.1 g/L |
feathers 50 g/L | -1 L Erlenmeyer flask -medium 100 mL -45°C -250 rpm -pH 10 |
5 d/100% | Caseinolytic: 560 U mL | [1] |
| Bacillus altitudinis GVC11 | Without pretreatment | Nutrient broth | feathers 10 g/L | -250 mL Erlenmeyer flask -medium 100 mL -inoculum 2% v/v -200 rpm -Temperature and-pH: not reported |
White feathers: 2d/100%Black feathers: 4d/100% | Keratinolytic: 157 U/mL | [19] |
| Bacillus megaterium | -Cut into 5 mm segments -Dry with air |
-Phosphate buffer solution 0.5 mMol/L -LB medium |
feathers 8 g | -1 L Erlenmeyer flask -37°C -127 rpm -pH: not reported |
8 d/ degradation not reported |
Not reported | [17] |
| Bacillus subtilis MTCC 441 | -Wash with tap water -Dry at 40°C for 2 d |
-K2HPO4 0.3 g/L -KH2PO4 0.4 g/L -NaCl 0.5 g/L -MgCl2•6H2O 0.1 g/L |
feathers 10 g/L | -250 mL Erlenmeyer flask -inoculum 5%v/v (4 × 107 UFC/mL) -medium 100 mL -pH 7.5 -Temperature and pH: not reported |
5 d/97.8% | Keratinolytic: 68 U/mL | [23] |
| Bacillus cereus Wu2 | Withoutpretreatment | -NH4Cl 2 g/L -MgSO4•7H2O 0.2 g/L -K2HPO4 1 g/L -CaCl2 0.1 g/L -KH2PO4 0.4 g/L |
feathers 10 g/L | Performed on agar plates -40°C -pH 5.3 -rpm: not reported |
4 d/100% | Keratinolytic: 1750 U/mL | [22] |
| Brevibacillussp.AS-S10-II | -Wash -Dry off -Cut thinly -Suspend in a buffer solution (100 mMTris-HCl) |
Meal broth | feathers 10 g/L | Volume not reported -45°C -200 rpm -pH 8.0 |
2 d/78 to 82% | Keratinolytic: 1.3 mg/L | [32] |
| Alcaligenes sp. AQ05-001 |
Withoutpretreatment | -NaCl 0.5 g/L -K2HPO4 0.7 g/L -KH2PO4 1.4 g/L -MgSO4•6H2O 0.001 g/L |
feathers 1 g/Lplus a source of carbon (sucrose and whey) and nitrogen (ammonium bicarbonate and urea) | -Agar plates -inoculum 1% v/v-25-30°C -pH 7.5 -rpm: not reported |
2 d/100% | Keratinolytic: 8.85 U/mL | [44] |
| Pseudomonas microphilus | -Cut into small pieces -Wash with tap water -Sterilize with 0.1% mercuric chloride and alcohol -Wash with distilled water -Dry with hot air at 45°C for 24 h |
-NaCl 0.5 g/L -NH4Cl 5.5 g/L -K2HPO4 0.3 g/L -KH2PO40.4 g/L -MgCl20.24 g/L -Yeast extract 0.1 g/L |
feathers 1 g | -250 mL Erlenmeyer flask -37°C -pH 7.5 -rpm: not reported |
30 d/70% | Keratinolytic: 0.884 U/mL | [21] |
| Pseudomonas mendocinaPM2 | -Wash with running water -Dry at 60°C for 2 days |
-K2HPO4 0.3 g/L -KH2PO4 0.4 g/L -NaCl 0.5 g/L -MgCl2•6H2O 0.1 g/L |
poultry feathers 10 g/L | -250 mL Erlenmeyer flask -Inoculum 5% v/v (4 × 107 CFU/mL) -medium 100 mL -pH 7.5 -Temperature and-pH: not reported |
5 d/91.07% | Keratinolytic: 54.8 U/mL | [23] |
| Pseudomonas stutzeri | Withoutpretreatment | -K2HPO4 0.3 g/L -KH2PO4 0.4 g/L -NaCl 0.5 g/L -MgCl2•6H2O 0.1 g/L |
feathers 10 g/L | -100 mL Erlenmeyer flask -inoculum 5% v/v (1x 108 CFU/mL) -medium 100 mL -30°C -125 rpm -pH: not reported |
5 d/100% | Keratinolytic: 42 U/mL | [24] |
| Leuconostocsp. | -Cut into small pieces -Wash with tap water -Sterilize with 0.1% mercuric chloride and alcohol -Wash with distilled water -Dry with hot air at 45°C for 24 h |
-NaCl 0.5 g/L -NH4Cl 5.5 g/L -K2HPO4 0.3 g/L -KH2PO4 0.4 g/L -MgCl2 0.24 g/L -Yeast extract 0.1 g/L |
feathers 1 g | -250 mL Erlenmeyer flask -37°C -pH 7 -rpm: not reported |
30 d/31% | Keratinolytic: 0.425 U/mL | [21] |
| Chryseobacteriumsp. kr6 | -Wash threefold with tap water -Wash with distilled water -Dry at 48°C for 48 h -Stored at room temperature |
-KH2PO4 0.4 g/L -NaCl 0.5 g/L -CaCl2 0.015 g/L |
feathers 50 g/L | -1 L Erlenmeyer flask -medium 100 mL -30°C -125 rpm -pH: not reported |
Not reported | Azocaseinolytic: 160-170 U/mL | [25] |
| StenotrophomonasmaltophiliaR13 | -Wash with water -Sterilize |
-KH2PO4 0.2 g/L -K2HPO4 1.4 g/L -NaCl 1.5 g/L -CaCl2•2H2O 0.05 g/L -MgSO4• 7H2O 0.3 g/L |
-feathers 1 g/L -Glucose 1 g/L -Polypeptone1.2 g/L |
-250 mL Erlenmeyer flasks -30°C -200 rpm -pH: not reported |
6 d/100% | Keratinolytic: 82.3±1.0 U/mL | [14] |
| ActinomadurakeratinilyticaCpt29 | Without pretreatment | -NaCl 1.5 g/L -KH2PO4g/L -K2HPO41 g/L -KCl 0.5 g/L -MgSO4• 7H2O 1.5 g/L |
feathers 40 g/L | -9 Ml Tubes -45°C -pH 9.0 -rpm: not reported |
3 d/100% | Keratinolytic: 24,000 U/mL | [27] |
| Coprinopsissp. | -Wash with running tap water -Rinse with water+Tween 80 0.1% (v/v) -Rinsed several times with distilled water -Dry with air -Cut into shorter lengths (1 and 2 cm) -Defatted with Diethyl ether (99.7%) -Powderedand sieved |
-K2HPO4•3H2O 100 mg/L -MgSO4•7H2O 500 mg/L -ZnSO4•7H2O5 mg/L -FeSO4•7H2O 10 mg/L |
feather powder1.5 g | -40°C -pH 7 -pH: not reported |
42 d/79% | Keratinolytic: 32, 000 U/mL | [28] |
| Aspergillusniger | -Wash with water and detergent -Dry at 60°Cand milled |
30 mL of a solution 0f 0.9% (NH4)2SO4 in 0.1 mol/L HCl | 0.4 g of whole chicken feather was mixed with 40 g of a wheat bran mixture |
-Solid-state fermentation -32°C -pH 5 -rpm: not reported |
7 d/ degradation not reported |
Keratinolytic: 172.7 U/mL | [29] |
| Trichosporonloubieri | Chicken feathers and human nails were washed with water and detergent. All keratin substrates were previously delipidated with chloroform ⁄methanol (1:1, v ⁄ v). The media were incubated for 7 days at 28°C, centrifuged at 2,000 g for 20 min and concentrated 20 fold by dialysis (cut off 9 kDa) using PEG 4000 overnight at 4°C. |
0.01% yeastextract | 1% of the different keratin substrates: human hair, animal hair (bovine hide), chicken feather and human nails in 0,1 M acid citric buffer pH 5.5 with 0.01% yeast extract. | -28°C pH: 5.5 -rpm: not reported |
7 days/ -Human nail (61.3%) -Animal hair from bovine hide (32.7%). -Human hair (16.4%) -Chickenfeathers (8.9%) |
Nail keratine: 193.3 U/mL Human hair: 171.8 U/mL Human hair: 145.7 U/mL Chiken feathers: 46.7 U/mL | [46] |
| Streptomycessp. | -Wash with warm distilled water -Dried at 45°C in an incubator for 2 days. -Autoclaved at 121°C.for 45 min -Stored at room temperature |
-CaCO35 g/L,NaCl 5 g/L, K2HPO4g/L | Feathers 5.0 g/L | -250 ml Erlenmeyer flasks -28°C -pH: 8 -150 rpm |
5.5 days/degradation not reported | 487 U/mL | [47] |
| Actinomycetes consortium (3H, 8H y M4) | -Wash with hot water -Sterilize at 134°C for 50 min to 2 atm -Dry at 30°C for 72 h -Cut in pieces |
-NaCl 5 g/L -CaCO35 g/L -K2HPO4•3H2O 3.5 g/L |
Feather 5-7 g/L | -100 mL Erlenmeyer flask -55°C -130 rpm -pH 7.5-8.5 |
3 d/87-91% | Hydrolytic in solid medium, measured in a range of 10 to 28 mm |
[31] |
Table 2: Biological degradation processes for the treatment of chicken feathers.
For the biological degradation process, feathers must be subjected to a pre-treatment, which consists of washing them with tap water to eliminate wastes from the birds, in some cases they are disinfected with detergents (Tween 80), a buffer solution (100 mM Tris HCl buffer) and sometimes sterilized with a solution of mercury chloride and 1% alcohol. The next step is to dry the feathers at a temperature of 40°C to 60°C. Finally, they are crushed or cut into small pieces aimed at obtaining a larger area of contact of the carbon source with the microorganisms [9,13,16, 21,23,25,26,28-30,32].
For the growth of the microorganisms in charge of the degradation of the feathers, most authors use mineral media constituted by phosphates, sulfates and chlorides, which are necessary for their metabolism. As nitrogen source, authors use yeast extract, tryptone and nitrates [9,13,14,26]. Glucose, mannitol and wheat bran are used as easy digestible carbon sources. It must be pointed out that the main carbon source used is the chicken feather itself, which contains keratin in the highest proportion (Table 2). Many works have mentioned the relevance of the keratinolytic activity of microorganisms during the degradation of feathers, standing out the work by Al-Musallam et al. [28] with a maximal keratinolytic activity of 32, 000 U/mL produced by Coprinopsis sp.
Among the microorganisms mostly studied to accomplish degradation of chicken feathers is the genus Bacillus, which is able to degrade completely the feathers at different times, depending on the used species [1,13,22]. This genus is capable of using the feather as the sole source of carbon to achieve fermentation, adding to the medium only mineral salts that supply other microelements for the adequate growth of the microorganism Fakhfakh et al. [1] and Lo et al. [22], in some cases, adding an additional nitrogen source like a yeast extract and tryptone [13,23, 26]. As a product of the degradation of feathers, enzymes like keratinase are obtained, whose enzymatic activity is in the range of 3.8 to 1750 U/mL [22,26].
Other microorganisms studied as degraders of chicken feathers are Leuconostoc sp. and Pseudomona microphilus, however their degrading processes are slower, reaching up to 30 days and they present a low keratinolytic activity of 0.425 and 0.884 U/mL, respectively [21].
Results and Discussion
Byproducts of the biological degradation
The biological degradation process of chicken feathers is environmentally friendly as it does not generate wastes, aside from being economically feasible because it allows obtaining diverse byproducts, from an industrial waste, with a high added value as are soluble proteins and amino acids at diverse proportions (Table 3) [21,23], standing out the works of Kani et al. [21] and Chaturvedi et al. [24] who used a Pseudomonas strain for the degradation of feathers obtaining a high concentration of amino acids (1.992 mg/mL) and soluble protein (0.546 mg/mL), respectively. These products have applications in the degradation of malachite green absorbed by feathers (about 99.5% degradation after 24 h) and the shaving of goat skins with raw extract of keratinase.
| Strain | Byproduct | Application | Reference |
|---|---|---|---|
| Pseudomonas mendocina PM2 | Protein: 468.2 mg/L Keratinase | Degradation of malachite green absorbed by feathers (about99.5% degradation after 24 h) | [21] |
| Bacillus subtilis MTCC 441 | Protein: 546.7 mg/L | Do not metabolize feathers with malachite green | [23] |
| Pseudomonas stutzeri | Protein: 784.2 µg/mL Keratinase |
Shaving of goat skins with raw extract of keratinase | [24] |
| Scopulariopsisbrevicaulis (Sacc.) | Keratinase | The purified enzyme hydrolyzes different materials such as chicken feathers, nails and human hair | [18] |
| StenotrophomonasmaltophiliaR13 | Total amino acids: 2298.8 µM Protein: about 43 µg/mL |
Obtaining indoleacetic acid (IAA) (327.7±3.9 µg/mL) without l-tryptophanin the medium with antifungal activity | [14] |
| Bacillus licheniformisER-15 | Keratinase | Hair removal | [16] |
| FervidobacteriumislandicumAW-1 | Amino acids:Histidine, cysteine, lysine,tryptophan and methionine Keratinase | Applications of keratinase enzyme at high temperatures Study of the structural stability of thermostable enzymes at high temperatures |
[11] |
| Chryseobacteriumsp. kr6 | Feather hydrolyzate | Antioxidant and antihypertensive activity | [25] |
| Kokuriaroseae | Feather meal with lysine, methionine and histidine | Nutritional supplement (Alternative source of protein for bird feed) | [12] |
| Fervidobacteriumpennivorans | Methane gas | Biofuel | [20] |
| Actinomycetes consortium (3H, 8H y M4) | Biohydrolyzed | Source of soluble protein, amino acids, enzyme and other add products | [31] |
| consortium of anaerobic bacteria | Methane gas | Biofuel | [30] |
| Aspergillusniger | Keratinase | Enzymatic enhancement of foods and feed additives made of feathers. Production ofamino acids and high molecular weight peptides, which are substrates for cosmetics |
[29] |
| Paenibacilluswoosongensis TKB2 | Keratinase | Promoter of plant growth. Promotes seed germination (germination rate 87.5%) and seedling growth of Cicerarietinum. Nodule formation (3-fold) is induced and soil fertility increase by the alteration of N, P, K and the C/N ratio by 1.2-fold. Improves the amount of fixed nitrogen of free-life (2-fold) and phosphate solubilizers (5.8-fold) |
[48] |
| Bacillussubtilis DB 100 | Soluble proteins and NH2-free amino groups Keratinolyticalkaline protease |
Production of enzyme of keratin degradation, it works as a proteaseand as a keratinase | [3] |
Table 3: Byproducts and applications of biological treatment
The produced amino acids and peptides of high molecular weight can be used as substrates for cosmetics [29]. Some other interesting byproducts are used in food supplements for example from a bio-hydrolysate obtained of the degradation of feathers with an actinomycetes consortium, can be used as a source of soluble proteins, amino acids, enzymes and other valuable products [31]. Among the byproducts of greatest interest obtained in this process are the enzyme keratinase, which has been used for the degradation of diverse materials, like nails and human hair [18] and to shave off goat skins [24].
It is important to point out that the degradation process can be coupled to the detoxification of some other contaminant in the environment, such as malachite green, which can be absorbed by chicken feathers and degraded jointly by Pseudomonas mendocina PM2 [23]. It is also possible to obtain growth promoters like indole-acetic acid (327.7 ± 3.9 μg/mL) to improve seeds germination and seedlings growth (germination rate 87.5%) and seedling growth of Cicer arietinum [14, 48].
Other biotechnological applications relevant nowadays due to the energy problems arising from the depletion of fossil fuels, are the generation of biofuels such as methane a byproduct of the degradation of chicken feathers by Fervidobacterium pennivorans and anaerobic bacteria consortia [20,30].
Physicochemical processes in the treatment of chicken feathers
Among the methods of feathers degradation are the physicochemical ones, which include alkaline hydrolysis (with sodium sulfide, sodium or calcium hydroxide) and alkaline-acid hydrolysis (with sodium hydroxide and citric acid), microwaving, polymerization and carbonization; aside from mixture of feathers with oleic substances like soy and glycerol to form polymers (Table 4).
| Process | Conditions | Byproducts | Applications | Feather Pre-treatment | Reference |
|---|---|---|---|---|---|
| Alkaline (sodium hydroxide) and acid (citric acid) hydrolysis | Feathers were treated with various concentrations of NaOH For the preparation of the thermoplastic was mixed with glycerol and compressed between aluminum foils. | Bio-thermoplastic | Thermoplastic film for biomedical applications | -Commercial chicken feather fiber (NIXA, MO) -Wash, dry and grind |
[35,36] |
| Alkaline hydrolysis | Samples of 2 g ofchicken feathers were immersed in solutions of NaOH and Na2S in the range 0.05–0.4 N and 0.05–0.3 N, respectively |
Adsorbents | Adsorbents to remove copper and zinc from wastewater |
Some of the materials attached to the feathers were first removed, through several washings with tap water and detergent and were then left in the open air for several days to get rid of odors. have been frozen by liquid nitrogen before cutting into small sizes using an electrical cutter |
[34] |
| Mixture of feather keratin With graphite oxide |
About 70 g of feather powder,30 g plasticizer mixture (15 g glycerol and 15 g propylene glycol), 3 g sodium sulfite prepared in 15 mL deionized water and 23 mL graphite oxide dispersión prepared in deionized water |
plasticsnano-reinforced | Agricultural films, composting bags and biodegradable containers |
-washed with hot water -Dry a for three days and then dried at 50°C -Grinding of Feather and size sieve of 0.25 mm perforations. |
[41] |
| Mixture of feathers with glycerol | The deoxidation of the feathers is carried out with 2-mercaptoethanol. Then, the extracted keratin is modified with chloroacetic acid. At the end, glycerol is added as plasticizer | Protein films | For administration and control of drug release Widely used in food, biotechnology, thermoplastics, packaging and other applications | The feathers were washed, dried and ground in small filaments with a length of 1-2 mm. 3 g of feathers were refluxed in hydrated ethanol. They were then pretreated with a solution of HCl Ethanol and HCl were filtered and the cleaned and pretreated feathers | [53] |
| Mixture of feathers with soybean oil and glass fibers | The plates were formed with modified soybean oil with glass fibers E and 30% feathers. The material was compressed with copper plates and an anti-inflammatory agent | Printed circuit boards (PCBs) | All Computer Circuits | The feather fibers were dried in an oven at 50°C for at least 4 h and then stored in a desiccator prior to use. | [56] |
| Feathers mixed with epoxy | Feathers were mixed with different percentages of epoxy and glass. The best mixture was with percentages of 5 and 10% epoxy | Dielectric materials | They are used in various applications, including Insulation, encapsulation, printed circuit boards, capacitors and other devices. |
The chicken feathers were cleaned with a polar solvent (ethanol) and dried. The quills were removed and short fibers 10-15 mm in length were obtained | [57] |
| Mixture of chicken feather fibers with LDPE (Low Density Polyethylene) | Feathers=0 to 50% by weight Melting temperature=171-168°C Mixing time=15 min The mixture is sandwiched between Teflon coated with aluminum paper and cooled under an aluminum block |
Polyethylene reinforced with chicken feather fibers | All products made with polyethylene | The feathers are cleaned and separated from the rachis through a process developed and patented by USDA. They were milled and sieved. | [60] |
| Mixture of chicken feather protein, melanin, sodium pyrophosphate and glyoxal | Keratin powder was dissolved with melanin and sodium pyrophosphate (1:8:5) in distilled water. Glyoxal was then added at 80°C. The pH was adjusted to 8 at a temperature of 90°C for 2 hours |
Flameretardantonflame | For fire prevention of different materials | The feathers were cleaned and dissolved with 10g/L NaOH and 4g/L urea at 90°C for 3 hours. Later keratin solution was neutralized with hydrochloric acid and subsequently filtered. The filtrate was again treated with hydrochloric acid. The protein was obtained by filtration and dried at 50°C. |
[59] |
| Microwave treatment | Hydrolyzed at mild high temperature from 433 to 473 °K. Hydrolisis was carried out with a self-deasing batch-type autoclave reactor power of 1200 W. The reaction about 500 mL Teflon spraying cylinder vessel with a height of about 6 and a diameter of 10 cm |
Amino acid (arginine, alanine, threonine, glycine, praline, serine, glutamic acid, aspartic acid, cystine y tyrosine). The total yield of amino acid at 54.72% with feather containing about 71.83% keratin |
By-product with high nutritional value | Duck feather were washed with water, dread and cut into small pieces | [50] |
| Pyrolysis by thermal | A constant N2 flow (100 mL/min) was proved for 3 h prior temperature ramps to minimize the oxygen concentration. The temperature was increased to 215°C at a 3°C/min and kept constant for 2, 4, 10, 15 and 24 h | Keratin fibers with aryl carbons and cyclic amines | Keratin fibers for the production of materials with high melting point, catalysts of high performance and for textile use | Chicken feather fiber were provided by Feather fiber Corporation (Nixa, MO) | [55] |
| Polymerization | Deoxygenating by passing N2 . Polymerization was initiated by adding the oxidant (K2S2O8) at pH 5.5 at 60°C for 4 h. The grafting reaction was terminated by adding 1 ml of 2% paradioxybenzene. Afther neutralizing, filtered, washed and dried |
Bio-thermoplastic | For biomedical applications | Chicken feather fiber were provided by Feather fiber Corporation (Nixa, MO) | [35] |
| Carbonization of feathers | The feathers were carbonized by heating to 800°C and activated with potassium hydroxide in repeatedly. Subsequently placed in acetylene black and polyvinylidene fluoride (PVDF) which was dissolved in N-methyl pyrolidinone solution and pressed into 1 square centimeter steel mesh and a thickness of 0.2 mm. |
Feather electrode | Electrode for super capacitors | The feather fibers were dried in an oven at 50°C for at least 4 h and then stored in a desiccator prior to use. | [58] |
Table 4: Physicochemical processes: byproducts and applications.
Among the alkaline hydrolysis methods is that report by Coward-Kelly et al. [49] in which the authors indicate that, after a digestion with calcium hydroxide at 150°C and 100 rpm, they identified the amino acids that remained after the degradation, these were alanine, arginine, cysteine, leucine, histidine, methionine, among others, some of these were essential amino acids and they were used as supplements for cattle feed. The authors emphasize the importance of degrading the feather to keratin and of this to free amino acids, because keratin is a protein that is not degraded by proteolytic enzymes and is only digested in 18%. They also demonstrate that keratin is deficient in amino acids like arginine, histidine, lysine, methionine and threonine; therefore, it is not recommended for monogastric animals but is adequate for ruminants. On the other side, Chen et al. [50] developed a novel method using microwaves for the degradation of feathers and, like Coward-Kelly et al. [49] identified several amino acids that can be used as nutrient source and to obtain high commercial value products, reporting that from the total content of protein, 71.83% is keratin; in addition, they obtained a high yield in easily separated amino acids and the method is environmentally friendly [50].
From alkaline hydrolysis, some polymers can be obtained and used as basis of a varnish to treat and preserve archaeological wood. Endo et al. [51] obtained a polymer based on duck, chicken and hen feathers hydrolyzed with sodium hydroxide at 70°C for 3 h and found that the best structures of the keratin hydrolysate were those obtained from duck feathers; this because they were characterized by greater crystallinity and anti-alkali structures that contribute to a good dimensional stability. Popular preservation methods include the addition of polyethylene glycols of up to 90% compared to the polymer obtained from duck feathers, of which up to 40% is used for good wood preservation.
Al-Asheh et al. observed the capacity of the feathers treated with alkaline solutions of 0.2 N NaOH as adsorbents of heavy metals of copper and zinc in wastewater [34]. The feathers treated chemically with alkaline solutions have twice the sorption capacity of the untreated feathers. Another hydrolysis used to treat feathers is the alkaline-acid (sodium hydroxide and citric acid) for the production of biothermoplastics, which are poor in water stability and mechanical properties; therefore, authors mixed the keratin extracted from the feathers with acrylate monomers to improve these characteristics. Biothermoplastics are used for the elaboration of biomedical scaffolds; this is one of the most novel applications because they are used for support [35,36].
Another area of opportunity is the development of biomaterials of natural origin, as for example proteins, including collagen, albumin, gelatin, fibroin and keratin. Of these proteins, materials based on keratin are a promise to revolutionize the world of biomaterials because of their biocompatibility, biodegradability capacity, mechanical durability and natural abundance [52]; however keratin is insoluble in many common solvents like diluted acids, alkalis, water and organic solvents [53]. Some authors consider that the challenge in the production of biopolymers or biothermoplastics is to develop a technique to dissolve keratin to produce solutions capable of re-crosslinking, having a minimal degradation of primary protein chains and being able to be escalated at the industrial level [54]. Currently, a wide array of these techniques is available for the dissolution of hard keratin, often reactions of reduction or oxidation are used and, more recently, ionic liquids are being tested [31].
Some of the most important biomedical applications of natural polymers include drug carriers that are biodegradable and have the quality of controlling the administration of the drug. In these systems, an active therapeutic agent is incorporated into a structure of polymeric network so that the drug is released from the material in a predetermined manner [52].
Another technique for the treatment of chicken feathers is pyrolysis at high temperatures; one of the most recent studies is that reported by Senoz et al. [55] who evaluated the structural changes of the chicken feathers after a pyrolysis process by a constant flow of N2 , posterior to a thermal treatment at 25°C to 600°C. Changes were determined by mass spectrometry, solubility tests and gel chromatography. The thermal treatment below the fusion point provided enough reticulations of the protein matrix to maintain intact the fibrous structure; on the other side, the treatment above the fusion point provided cycling and aromatization reactions to the protein matrix. Release of aromatic carbons and free amines were observed during these transformations, finding that the adjustment of the thermal profile of pyrolysis provides conditions that lead to the fabrication of materials that are useful at high temperatures, like fibers or macromolecules with textile application and in high yield catalyzers.
Some other applications of chicken feathers based on chemical reactions are those mentioned by Zhan and Wool [56] who mixed feathers (30%) with soy oil and glass fibers to produce printed circuit boards used for all types of computational circuits. Mishra and Nayak [57] obtained dielectric materials to be used as isolation, for printed circuit boards, for encapsulators and condensers, by mixing 5% of feathers with epoxy and glass. On the other hand, Zhan et al. [58] carbonized feathers to obtain electrodes for super-condensers. Sun et al. [37] solubilized feathers with 1-butyl-3-methylimidazolium chloride attaining a solubilization of up to 23% to obtain micro and nanoparticles of keratin to eliminate chromium. Among the characteristics of some byproducts obtained by physicochemical methods, stand out the high fusion point and the procurement of high yield condensers and materials with flame retardant properties [55,59].
Future Outlooks
According to what was reviewed in this research on biological and physicochemical treatments of feathers and feather waste, it is observed that studies in the future will be focused on the following points.
• Scaling and optimization of bioprocess, obtaining products with high added value, emphasizing the production of enzymes for use in the medical, food, cosmetic and industrial areas mainly.
• The future research in the physicochemical treatments of feathers is focused in the use of green technology, evolving group of methods and materials environmental friendly for to modify the properties of feathers
• Improvement of nanomaterials for its application in the healthcare sector (For example, as carriers and as excipients to obtain different drug delivery systems and orthopedic devices, etc).
Conclusion
Chicken feathers are potential raw materials to obtain byproducts of high economical and scientific value; this is due to its low cost as waste, large availability and chemical composition, The biotechnological processes for the degradation of feathers pose some advantages over the physicochemical methods like their low energetic costs, being environmentally friendly and the procurement of considerable amounts of enzymes applicable to the chemical, cosmetics and food industries. It is considered necessary to escalate studies because most of the developed physicochemical and biological processes are currently limited to the laboratory scale.
References
- Fakhfakh N, Ktari N, Haddar A, et al. Total solubilisation of the chicken feathers by fermentation with a keratinolytic bacterium, Bacillus pumilus A1 and the production of protein hydrolysate with high antioxidative activity. Process Biochem. 2011;46:1731-7.
- Kumar EV, Srijana M, Chaitanya K, et al. Biodegradation of poultry feathers by a novel bacterial isolate Bacillus altitudinis GVC11. Indian J Biotechnol. 2011;10:502-7.
- Zaghloul TI, Embaby AM, Elmahdy AR. Biodegradation of chicken feathers waste directed by Bacillus subtilis recombinant cells: Scaling up in a laboratory scale fermentor. Bioresour Technol. 2011;102:2387-93.
- Mazotto AM, Ascheri JLR, de Oliveira Godoy RL, et al. Production of feather protein hydrolyzed by B. subtilis AMR and its application in a blend with cornmeal by extrusion. LWT-Food Sci Technol. 2017;84:701-9.
- Agrahari S, Wadhwa N. Degradation of chicken feather a poultry waste product by keratinolytic bacteria isolated from dumping site at ghazipur poultry processing plant. Int J PoultSci 2010;9:482-9.
- SAGARPA. Secretaria de Agricultura, ganaderia, desarrollo rural, pesca y alimentación. Escenario base: Proyeccionespara el sector Agropecuario de México. Mexico DF 2009:51-3.
- Kowalska T, Bohacz J. Dynamics of growth and succession of bacterial and fungal communities during composting of feather waste. Bioresour Technol. 2010;101:1268-76.
- Queiroga AC, Pintado ME, Malcata FX. Potential use of wool-associated Bacillus species for biodegradation of keratinous materials. IntBiodeterior Biodegradation. 2012;70:60-5.
- Coello N, Vidal L, Bretaña A. Aislamiento de unacepa de Kocuriaroseadegradadora de plumas de aves de corral. Rev Cientific FCV-Luz. 2000;10:107-13.
- Toni CH, Richter MF, Chagas JR, et al. Purification and characterization of an alkaline serine endopeptidase from a feather-degrading Xanthomonasmaltophilia strain. Can J Microbiol. 2002;48:342-8.
- Nam GW, Lee DW, Lee HS, et al. Native-feather degradation by Fervidobacteriumislandicum AW-1, a newly isolated keratinase-producing thermophilic anaerobe. Arch Microbiol. 2002;178:538-47.
- Bertsch A, Álvarez R, Nereida C. Evaluación de la calidadnutricional de la harina de plumasfermentadasporkocuriaroseacomofuentealternativa de proteínas en la alimentación de aves. Rev Cientific FCV-Luz. 2003;13:139-45.
- Park GT, Son HJ. Keratinolytic activity of Bacillus megaterium F7-1, a feather-degrading mesophilic bacterium. Microbiol Res. 2009;164:478-85.
- Jeong J-H, Lee OM, Jeon Y-D, et al. Production of keratinolytic enzyme by a newly isolated feather-degrading Stenotrophomonasmaltophilia that produces plant growth-promoting activity. Process Biochem. 2010;45:1738-45.
- Jeong JH, Park KH, Oh DJ, et al. Keratinolytic enzyme-mediated biodegradation of recalcitrant feather by a newly isolated Xanthomonassp. P5. PolymDegrad Stab. 2010;95:1969-77.
- Tiwary E, Gupta R. Medium optimization for a novel 58 kDadimerickeratinase from Bacillus licheniformis ER-15: Biochemical characterization and application in feather degradation and dehairing of hides. Bioresour Techno. 2010;101:6103-10.
- Forgács G, Alinezhad S, Mirabdollah A, et al. Biological treatment of chicken feather waste for improved biogas production. J Environ Sci. 2011;23:1747-53.
- Sharaf EF, Khalil NM. Keratinolytic activity of purified alkaline keratinase produced by Scopulariopsisbrevicaulis (Sacc.) and its amino acids profile. Saudi J of Biol Sci. 2011;18:117-21.
- Kumar E, Srijana E, Chaitanya K, et al. Biodegradation of poultry feathers by a novel bacterial isolate Bacillus altitudinis GVC11. Indian J Biochem. 2011;10:502-7.
- Costa JC, Barbosa SG, Sousa DZ. Effects of pre-treatment and bioaugmentation strategies on the anaerobic digestion of chicken feathers. Bioresour Technol. 2012;120:114-9.
- Kani TP, Subha K, Madhanraj P, et al. Degradation of chicken feathers by Leuconostoc sp. and Pseudomonas microphilus. Eur J Exp Biol. 2012;2:358-62.
- Lo WH, Too JR, Wu JY. Production of keratinolytic enzyme by an indigenous feather-degrading strain Bacillus cereus Wu2. J BiosciBioeng. 2012;114:640-7.
- Chaturvedi V, Bhange K, Bhatt R, et al. Biodetoxification of high amounts of malachite green by a multifunctional strain of Pseudomonas mendocina and its ability to metabolize dye adsorbed chicken feathers. J Environ Chem Eng. 2013;1:1205-13.
- Chaturvedi V, Bhange K, Bhatt R, et al. Production of kertinases using chicken feathers as substrate by a novel multifunctional strain of Pseudomonas stutzeri and its dehairing application. BiocatalAgricul Biotech. 2014;3:167-74.
- Fontoura R, Daroit DJ, Correa APF, et al. Production of feather hydrolysates with antioxidant, angiotensin-I converting enzyme-and dipeptidyl peptidase-IV-inhibitory activities. New Biotechno. 2014;31:506-13.
- Liu Q, Zhang T, Song N, et al. Purification and characterization of four key enzymes from a feather-degrading Bacillus subtilis from the gut of tarantula Chilobrachysguangxiensis. IntBiodeterior Biodegradation. 2014;96:26-32.
- Habbeche A, Saoudi B, Jaouadi B, et al. Purification and biochemical characterization of a detergent-stable keratinase from a newly thermophilicactinomyceteActinomadurakeratinilytica strain Cpt29 isolated from poultry compost. J BiosciBioeng. 2014;117:413-21.
- Musallam AA, Gharabally DH, Vadakkancheril N. Biodegradation of keratin in mineral-based feather medium by thermophilic strains of a new Coprinopsissp. IntBiodeterior Biodegradation. 2013;79:42-8.
- Mazotto AM, Couri S, Damaso MCT, et al. Degradation of feather waste by Aspergillusnigerkeratinases: Comparison of submerged and solid-state fermentation. IntBiodeterior Biodegradation. 2013;85:189-95.
- Xia Y, Massé DI, McAllister TA, et al. Anaerobic digestion of chicken feather with swine manure or slaughterhouse sludge for biogas production. Waste Manage. 2012;32:404-9.
- Tonkova E, Gousterova A, Neshev G. Ecologically safe method for improved feather wastes biodegradation. IntBiodeterior Biodegradation. 2009;63:1008-12.
- Mukherjee AK, Rai SK, Bordoloi NK. Biodegradation of waste chicken-feathers by an alkaline β-keratinase (Mukartinase) purified from a mutant Brevibacillus sp. strain AS-S10-II. IntBiodeterior Biodegradation. 2011;65:1229-37.
- Sharma R, Verma VV, Gupta R. Functional characterization of an extracellular keratinolytic protease, Ker AP from Pseudomonas aeruginosa KS-1: A putative aminopeptidase with PA domain. J MolCatal B Enzym. 2013;91:8-16.
- Al-Asheh S, Banat F, Al-Rousan D. Beneficial reuse of chicken feathers in removal of heavy metals from wastewater. J Clean Prod. 2003;11:321-6.
- Reddy N, Chen L, Yang Y. Biothermoplastics from hydrolyzed and citric acid Crosslinked chicken feathers. Mater Sci and Eng C. 2013;33:1203-8.
- Reddy N, Jiang Q, Jin E, et al. Bio-thermoplastics from grafted chicken feathers for potential biomedical applications. Colloids Surf, B Biointerfaces. 2013;110:51-8.
- Sun P, Liu ZT, Liu ZW. Particles from bird feather: A novel application of an ionic liquid and waste resource. J Hazard Mater. 2009;170:786-90.
- Hoffmann G, Völker H. Anatomía y Fisiología de lasavesdomésticas Editorial Acribia. 1969:144-7.
- Schelestow K, Troncoso OP, Torres FG. Failure of flight feathers under uniaxial compression. Mater SciEng C. 2017;78:923-31.
- Reddy N, Yang Y. Structure and properties of chicken feather barbs as natural protein fibers. J Polym Environ. 2007;15:81-7.
- Esparza Y, Ullah A, Wu J. Preparation and characterization of graphite oxide nano-reinforced biocomposites from chicken feather keratin. J Chem Techno Biotechnol. 2017;92:2023-31.
- Stilborn HL, Moran ET, Gous RM, et al. Effect of age on feather amino acid content in two broiler strain crosses and sexes. Poult Res. 1997;6:205-9.
- Pan L, Ma XK, Wang HL, et al. Enzymatic feather meal as an alternative animal protein source in diets for nursery pigs. Anim Feed Sci Technol. 2016;212:112-21.
- Yusuf I, Ahmad SA, Phang LY, et al. Keratinase production and biodegradation of polluted secondary chicken feather wastes by a newly isolated multi heavy metal tolerant bacterium-Alcaligenes sp. AQ05-001. J Environ Manage. 2016;183:182-195.
- Saarela M, Berlin M, Nygren H, et al. Characterization of feather-degrading bacterial populations from birds’ nests-Potential strains for biomass production for animal feed. IntBiodeterior Biodegradation. 2017;123:262-8.
- Medeiros IP, Rozental S, Costa AS, et al. Biodegradation of keratin by Trichosporumloubieri RC-S6 isolated from tannery/leather waste. IntBiodeterior Biodegradation. 2016;115:199-204.
- Demir T, Hame? EE, Öncel SS, et al. An optimization approach to scale up keratinase production by Streptomyces sp. 2M21 by utilizing chicken feather. IntBiodeterior Biodegradation. 2015;103:134-40.
- Paul T, Halder SK, Das A, et al. Exploitation of chicken feather waste as a plant growth promoting agent using keratinase producing novel isolate Paenibacilluswoosongensis TKB2. Biocatal. AgriculBiotechnol. 2013;2:50-7.
- Kelly G, Chang VS, Agbogbo FK, et al. Lime treatment of keratinous materials for the generation of highly digestible animal feed: 1. Chicken feathers. Bioresour Technol. 2006;97:1337-43.
- Chen J, Ding S, Ji Y, et al. Microwave-enhanced hydrolysis of poultry feather to produce amino acid. ChemEng Process Intensification. 2015;87:104-9.
- Endo R, Kamei K, Iida I, et al. Dimensional stability of waterlogged wood treated with hydrolysed feather keratin. J Archaeol Sci. 2008;35:1240-6.
- Yin XC, Li FY, He YF, et al. Study on effective extraction of chicken feather keratins and their films for controlling drug release. Biomater Sci. 2013;1:528-36.
- Yin H, Dong B, Liu X, et al. Amorphous diamond-structured photonic crystal in the feather barbs of the scarlet macaw. ProcNatlAcadSci US A. 2012;109:10798-801.
- Poole AJ, Lyons RE, Church JS. Dissolving Feather Keratin Using Sodium Sulfide for Bio-Polymer Applications. J Polym Environ. 2011;19:995-1004.
- Senoz E, Wool RP, McChalicher CWJ, et al. Physical and chemical changes in feather keratin during pyrolysis. PolymDegrad Stab. 2012;97:297-7.
- Zhan M, Wool RP. Design and evaluation of bio-based composites for printed circuit board application. Composites Part A. 2013;47:22-30.
- Mishra SC, Nayak NB. An Investigation of Dielectric Properties of Chicken Feather Reinforced Epoxy Matrix Composite. Journal of Reinforced Plastics and Composites. 2010;29:2691-7.
- Zhan M, Wool RP, Xiao JQ. Electrical properties of chicken feather fibre reinforced epoxy composites. Composites Part A. 2011;42:229-33.
- Wang X, Lu C, Chen C. Effect of chicken-feather protein-based flame retardant on flame retarding performance of cotton fabric. J ApplPolym. Sci. 2014;131.
- Hernández C, Colín-Cruz A, Santos C, et al. All Green Composites from Fully Renewable Biopolymers: Chitosan-Starch Reinforced with Keratin from Feathers. Polym. 2014;6:686.