Review
, Volume: 19( 1) DOI: 10.37532/0974-7516.2025.19(1).002Synthesis of Nitrogen-Containing Heterocyclic Compounds by Using Green Solvents
- *Correspondence:
- Manisha Malviya
Department of Chemistry, Indian Institute of Technology (BHU) Varanasi, Varanasi, India
E-mail: manisha.apc@itbhu.ac.in
Received: October 12, 2023, Manuscript No. TSOC-23-116392; Editor assigned: October 16, 2023, PreQC No. TSOC-23-116392 (PQ); Reviewed: October 31, 2023, QC No. TSOC-23-116392; Revised: December 27, 2024, Manuscript No. TSOC-23-116392 (R); Published: January 04, 2025, DOI:10.37532/0974-7516.2025.19(1).002
Citation: Malviya M, Singha M, Kumara N, et al. Synthesis of Nitrogen-Containing Heterocyclic Compounds by Using Green Solvents. Org Chem Ind J. 2025;19(1):002
Abstract
N-heterocyclic compounds are biologically active compounds providing incredibly attractive scaffolds in drug discovery efforts. In this review, we discussed several synthetic methods that have used green solvents for an environment-friendly synthesis of N-heterocycle compounds. The present synthetic methodology involves non-hazardous, easy-to-handle, cheap, non-toxic, and reusable solvents. The scope of each method is well discussed and analyzed in each section.
Keywords
Energy efficient; Environment-friendly; Green solvents
Highlights
• In this review, we have disclosed facile conventional solvent construction of pharmaceutically fascinating biologically important heterocyclic compounds.
• In this review, we discussed synthesising heterocyclic compounds using green solvents.
• The scope of each solvent is defined, and the green aspects of each synthetic approach are described.
• Here, this review fills the criteria of green chemistry, such as high functional group tolerance and high yield of the products.
Introduction
Nowadays, the area of synthetic chemistry has experienced various pioneering work accompanied by enhanced and proficient synthetic strategies that stay away from the use of hazardous chemicals, especially organic solvents, which play a very crucial role in synthetic organic chemistry. Organic solvents possess more lipophilicity and volatility as their molecular structures are mainly carbon and hydrogen atoms, sometimes with heteroatoms like THF, CH3CN, DMF, DMSO, etc. Lipophilic compounds have the permissibility to diffuse into many body organs and can be toxic.
On the other hand, due to high volatility, organic solvents can affect the lungs. Researchers have become more concerned about the dangers of organic solvents and are trying to adopt methods to reduce associated health effects. Globally, organic laboratories are well equipped with good ventilation, proper disposal systems and recyclability of organic solvents. There is an emergent need for more benign protocols in the chemical industry (Figure 1).
FIG 1. The above figure synthesis of nitrogen-containing heterocyclic compounds by using green solvents.
Green chemistry involves a series of design which is environmentally benign in nature using principles of green chemistry [1]. Here, the discussion involves using green solvents as a reaction medium in several organic reactions. These reactions play a vital role in synthetic chemistry. Anastas of the US Environmental Protection Agency introduced (EPA) Green Chemistry [2]. Since the early 1990’s, Italy and the United Kingdom have propelled essential drive-in green chemistry. This concept is epitomised in the twelve principles of green chemistry, which are as follows:
- Prevention of waste instead of remediation,
- Less hazardous/toxic chemicals,
- Atom efficiency,
- Eco-friendly solvents and auxiliaries,
- Safer products by design,
- Preferably renewable resources,
- Small pathway for synthesis,
- Energy efficient by design,
- Reagents should be used in small amounts,
- Design products for degradation,
- Analytical strategies for pollution impediment,
- Inherently secure protocols.
Green solvents as reaction medium
Green or universal solvents are known for their non-toxicity, easy availability, and high specific heat capacity. These are environment-friendly solvents, i.e., their low toxicity and low or negligible emissions towards the environment. They are generally obtained from secondary crops and, therefore, readily available. Usually, they have high specific heat capacity, which can be defined as the amount of heat required by one gram of material to raise its temperature by 1°C or 1 K. There are many green solvents, like ionic liquids, supercritical fluids (like supercritical CO2), water and other organic solvents like glycerol, glycol, PEG, ethyl acetate etc. These green solvents are eco-friendlier, less toxic, and less hazardous than traditional Volatile Organic Compounds (VOCs) as solvents. Solvents play a considerable position in the solubility of chemicals and the extraction of final products. Researchers have committed to replacing toxic and detrimental reaction mediums with non-toxic alternatives for the last decades. Polyethylene Glycol (PEG) [3], supercritical carbon dioxide [4], various ionic liquids [5], and water [6] are among the extensively probed solvents.
Widely, solvents play an essential role in solution-phase organic synthesis based on their broad classification into non-polar, polar aprotic and polar protic solvents. It finds a role in the dissolution of reactants, synthesis, extraction and recrystallisation. Organic solvents help to homogenise a reactant mixture, speeding up reactions through improved mixing and, in addition, reducing energy consumption. Moreover, organic solvents act as a heat sink for exothermic reactions. Besides solvating the solute particles, it affects other aspects like.
- Thermodynamics of the response by preferential stabilisation of chemical species;
- The kinetics of the reaction provide the required threshold energy, the activation energy for the specific reaction to occur.
Thus, the solvent is known to control the diffusion and rate of encounter of reactants, thereby influencing the overall kinetics and formation of products. Undoubtedly, the dielectric constant of an organic solvent plays an inevitable role in the whole process. We can predict the solubility of a solute molecule by using the dielectric constant (ε) of the solvent. The solvent is more suitable for dissolving non-polar (non-ionic) substances if ε is low. Polar and ionic substances are generally more likely to dissolve in a solvent with a high ε value. The dielectric constant ε represents the bond strength between solvent molecules [7].
Water has recently gained considerable attention as a solvent in several chemical reactions. Water as a reaction medium in synthetic chemistry was revisited by many scientists who showed that hydrophobic effects are responsible for enhancing the rate of various synthesis processes. Remarkably, water at high temperatures was considered valuable in synthetic chemical conversions. Due to its dielectric constant, water acts as a ‘‘pseudo-organic solvent’’ in the near-critical and supercritical environment. It is also cost-effective, profusely available, and non-flammable. The unusual and versatile properties of the aqueous medium are high surface tension, high specific heat capacity, and high dielectric constant [8-9]. Water is more feasible than organic solvents since it is inexpensive, easily accessible, non-flammable and environmentally safe. Because of the remarkable and unique properties mentioned, a series of organic conversions has been reported in the presence of water.
Another unique, affordable, and environmentally friendly solvent utilised in many chemical syntheses is Polyethene Glycol (PEG). PEG is not highly hazardous compared to other organic solvents and gives pure yield. PEGs can be purchased commercially up to an average molar mass of 20,000 gmol-1. Their derivatives with larger average molecular weights can be bought as Polyethylene Oxide (PEO).
Literature Review
In the present review, we have presented low average molecular weight derivatives of PEGs (<1000 g.mol−1) that are liquid at room temperature and readily available, for example, PEG-200, PEG-400, and PEG-600. Different PEGs can be inferred as solvents depending on how hydrophobic or hydrophilic the reactants are. PEG can be easily recovered and environmentally benign; various metal-catalyzed carbon-carbon bond formation processes can be carried out in PEG. Excellent compatibility between reactions under thermal and microwave radiations is demonstrated. PEG systems made of metal are easily retrieved and reused. PEGs exhibit high solubility in polar and non-polar solvents but remain insoluble in aliphatic hydrocarbons. Using these substances as solvents in chemical processes is an intriguing application. PEGs do not produce Volatile Organic Compounds (VOCs) and have a very low vapour pressure, which makes them stable in the environment. They are an excellent reaction medium for oxidation and reduction reactions because they are stable in basic and acidic environments.
A molten salt in a liquid state is referred to as an Ionic Liquid (IL), or alternatively, salts whose melting point is below a given temperature, such as 100°C, are called Ionic Liquids (IL). Ionic liquids have poorly coordinated ions, which make them liquid below 100°C or even at room temperature, where at least one ion has a delocalised charge, and one component is organic, which prevents the formation of a stable crystal lattice. Ionic liquids are moderate to poor conductors of electricity, non-ionizing, highly viscous and exhibit low vapour pressure. They have low combustibility, are highly thermally stable and have broad liquid regions. They have favourable solvating properties for many polar and non-polar compounds. Ionic liquids can also serve as solvents for biocatalysts. Ionic liquids are immiscible with organic solvents or water for liquid-liquid extraction processes. Due to the stability of ionic liquids at temperatures above 300°C, they provide an opportunity to carry out high-temperature reactions at low pressure. Changing the cation/anion ratio, type and alkyl chain length properties like acidity/melting temperature and viscosity can be altered to meet particular demands.
Since solvents play a crucial role in reaction and many different types genres are in use, to attain manageability issues in the present review article, we have focussed on water, PEG and ionic liquids only as solvents and discussed their role critically at the end of every research group’s work which was otherwise unavailable.
Chanda and co-workers have described various types of organic synthesis, such as Passerini and Ugi, Diels–Alder, Claisen rearrangements, and 1,3-dipolar cycloadditions [10]. Sharpless et al. disclosed the rarefaction of quadricyclane and azodicarboxylates into 1,2-diazetidines [11]. The reaction was completed within ten minutes, and the yield of the products was also good. The scope of this protocol is also investigated. Tiwari and co-author reported the Wittig reaction of phosphorus ylides with aromatic and aliphatic aldehydes in the presence of water [12]. Chakraborti et al. disclosed the formation of various substituted benzothiazoles and benzothiazolines in aqueous medium [13]. Bigi and co-workers have described a Knoevenagel condensation of Meldrum’s acid with several types of aldehydes by using water as a solvent. The yield of products is suitable to excellent [14]. Moghaddam et al. have reported the synthesis of thiopyran coumarin and its derivatives from 4-hydroxy dithiocoumarin and O-acrylate salicylaldehyde in the presence of water [15]. Nageswar et al. disclosed the synthesis of quinoxalinone derivatives from a-keto esters and benzene-1,2-diamine in water [16]. The yield of products was excellent. Similarly, water is used as a green solvent in many syntheses of heterocyclic compounds [17-20].
Results and Discussion
In the following Figures, we have discussed the role of green solvents used:
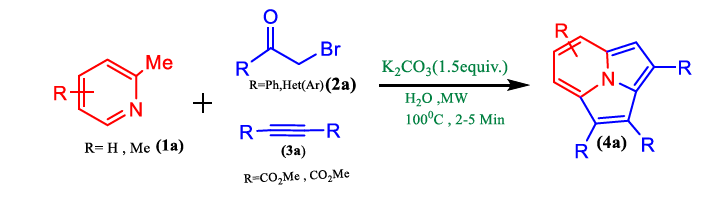
Gogoi et al. described (Figure 2) a green method using water as a green solvent for the synthesis of cycl [3.2.2] azine through 2- picoline, α-bromoacetophenone, and alkyne under microwave irradiation without using any catalyst with the help of various 2- alkyl pyridines, acyl bromides, and alkynes modified at the pyridyl ring, an aromatic moiety of acetophenone, and alkyne substituents, the reaction's adaptability was assessed in an aqueous medium under microwave irradiation (Tables 1 and 2).
FIG 2. Synthesis of cycl[3.2.2]azine.
| S. no | Solvent | 1 h | 2 h | 8 h | 24 h |
|---|---|---|---|---|---|
| Yield | Yield | Yield | Yield | ||
| 1 | CH2Cl2 | 0 | 0 | 0 | 0 |
| 2 | DMF | 0 | 5 | 8 | 8 |
| 3 | DMSO | 0 | 0 | 0 | 0 |
| 4 | Toluene | 0 | 0 | 0 | 0 |
| 5 | CH3CN | 2 | 4 | 4 | 8 |
| 6 | MeOH | 10 | 20 | 25 | 35 |
| 7 | EtOH | 8 | 15 | 26 | 40 |
| 8 | H2O | 70 | 78 | 84 | 80 |
| Note: Reactions were carried out at 100 | |||||
TABLE 1. Effect of different solvents on respective yields of cycl [3.2.2] azine at different time intervals.
| Entry | Alkyl pyridine | Acyl bromide | Alkyne | Products | Yield |
| 1 | 
1(a) |
 2(a) 2(a) |
 3(a) 3(a) |
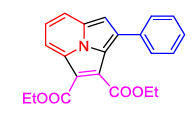 4(a) 4(a) |
90% |
| 2 | 1(a) | 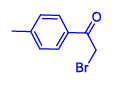 2(b) 2(b) |
3(a) |  4(b) 4(b) |
92 |
| 3 | 1(a) | 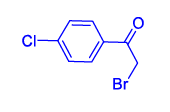 2(c) 2(c) |
3(a) | 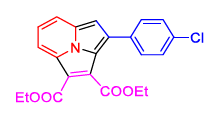 4(c) 4(c) |
60 |
| 4 | 1(a) | 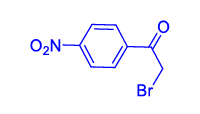 2(d) 2(d) |
3(a) | 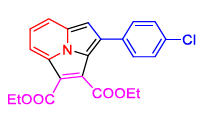 4(d) 4(d) |
20 |
| 5 | 1(a) | 2(a) |  3(b) 3(b) |
 4(e) 4(e) |
78 |
| 6 |  1(b) 1(b) |
2(a) | 3(a) | 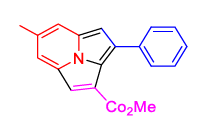 4(f) 4(f) |
80 |
| 7 | 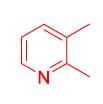 1(c) 1(c) |
2(b) | 3(b) | 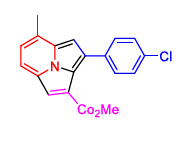 4(g) 4(g) |
74 |
| 8 | 1(c) | 2(c) | 3(b) | 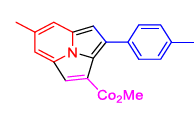 4(h) 4(h) |
65 |
| 9 | 1(c) | 2(d) | 3(b) | 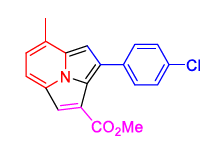 4(i) 4(i) |
22 |
| 10 | 1(a) | 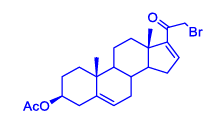 2(e) 2(e) |
3(a) |  4(j) 4(j) |
78 |
| 11 | 1(a) |  2(f) 2(f) |
3(a) | 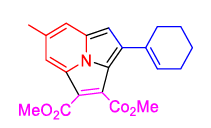 4(k) 4(k) |
74 |
| Note: aConditions: 1 (1 mmol), 2 (1.2 mmol), 3 (1.5 mmol), K2CO3 (1.5 mmol), water (10 ml). bReactions were conducted under microwave irradiation for 2–5 min at 100oC. cIsolated yields. | |||||
TABLE 2. Synthesis of cycl [3.2.2]azines 4a–k in an aqueous medium.
In conclusion, there has been a lot of interest in using water as a solvent to accomplish chemical transformations under thermal and microwave heating as a green option. It has also been shown that cycl[3.2.2]azine can be produced from steroidal and carbocyclic acyl bromides via this process. This process in an aqueous media doesn't use metal or hazardous solvents. Critically, it has been noted that this work unannounced the role of water. The reason for the reaction in water may be the high dielectric constant of water, which provides more solvation energy and, thereby, requires energy for the formation of the intermediates to boost the reaction yield in the specific experiment (Figure 3).
Kumar et al. accomplished a method synthesis of diarylazoles, using water as a solvent to react with α-tosyloxy ketones with amidines variable (Table 3). This method uses no metal reagents, toxic, volatile, good-yield corrosive organic solvent, etc.
FIG 3. The Synthesis of diarylazoles 3a and 4a in water solvents.
| Entry | Solvent | Yield at 60°C | Yield at 80°C |
|---|---|---|---|
| 1 | EtOH | 47 | 23 |
| 2 | MeOH | 40 | 18 |
| 3 | DMF | 15 | 10 |
| 4 | CH3CN | 27 | No Product |
| 5 | THF | 18 | 30 |
| 6 | Xylene | 23 | No Product |
| 7 | Toluene | 20 | No Product |
| 8 | CHCl3 | 30 | 55 |
| 9 | CH2Cl2 | 35 | 25 |
| 10 | Distilled water | 87 | 70 |
| 11 | Tap Water | 84 | 68 |
| Note: Reaction conditions: tosyloxyacetophenone (1 mmol), thiobenzamide/benzamidine (1 mmol), and solvent (0.5ml) | |||
TABLE 3. Optimization of solvents.
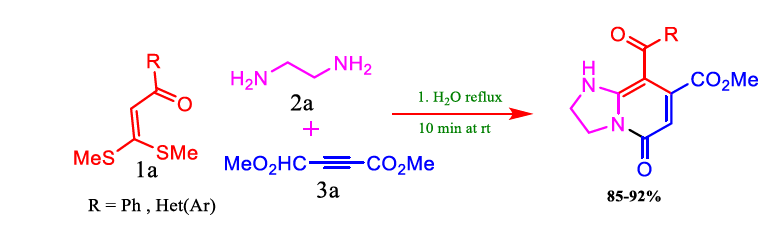
In summation, Kumar et al. established a straightforward and environmentally friendly method for producing diarylazoles, which are bioactive compounds, in high yields. The protocol is incredibly effective and transparent when using water as a solvent and reaction promoter. The absence of metal reagents, the avoidance of poisonous, flammable, and corrosive organic solvents, and the relative simplicity of safe experimental techniques are benefits of the reaction circumstances. Interestingly, the tap water and Distilled water gave almost similar results. The protic solvent was chosen because the intermediate is involved in intermolecular H-bonding (Figure 4). This process is a practical and alluring way to produce a variety of bioactive diarylazole under environmentally favourable circumstances. Chanu et al. have shown that water accomplished a green synthesis to obtain tetrahydro imidazopyridines by α-oxoketene dithioacetals, diamines, and DMAD. The study of this synthesis uses water as a green solvent in neutral conditions (Table 4).
FIG 4. Synthesis of tetrahydro imidazopyridines in green solvent.
| Entry | Base | Solvent | Time(min)/yield |
|---|---|---|---|
| 1 | Et3N | CH3CN | 10/(50%) |
| 2 | - | CH3CN | 10/(70%) |
| 3 | Et3N | EtOH | 10/(45%) |
| 4 | - | EtOH | 10/(75%) |
| 5 | NaOBut | DMF | 10/(60%) |
| 6 | Et3N | THF | 10/(65%) |
| 7 | - | DMSO | 10/(60%) |
| 8 | - | DMF | 10/(75%) |
| 9 | - | - | 10/(77%) |
| 10 | - | H2O | 10/(85%) |
| Note: All reactions were performed by refluxing the mixture of 1a (0.5 mmol) and 2a (0.5 mmol), then further addition of DMAD (0.5 mmol) at 0-25°C to get imidazo[1,2-a] pyridine. | |||
TABLE 4. Optimization of solvent for the synthesis of tetrahydro imidazopyridines.
Overall, Chanu et al. have presented a simple method of producing tetrahydro imidazopyridines from easily accessible oxoketene S, S-acetals. It can be observed from Figure 5 and Table 5. that when the temperature increased, notable modification decrease in polarity, polarizability and degree of hydrogen bonding, and reduced surface tension and viscosity were observed. The water solvent's characteristics undergo special modifications due to these significant alterations, which set it apart from other solvents. Since the polarity of water decreases with increased temperature, the solvent characteristics of water can be precisely adjusted for each case by varying the temperature. Consequently, a set of bicyclic pyridine derivatives with nitrogen at the ring junction was produced under benign and eco-friendly reaction conditions. The method involves the synthesis of three new C-N bonds and one C-C bond, leading to the development of two heterocyclic systems.
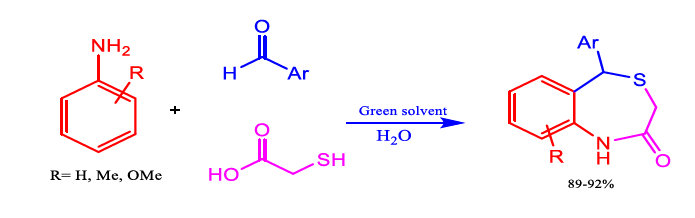
In 2008, Tu et al. demonstrated a green synthesis of benzothiazepinone in water solvent; this reaction afforded the desired products with high yield to aromatic aldehydes, various aniline, and mercaptoacetic acid.
FIG 5. Synthesis of benzothiazepinone.
| Entry | Solvent | Time(min) | Yield (%) |
|---|---|---|---|
| 1 | Benzene | 13 | Trace |
| 2 | DCM | 13 | Trace |
| 3 | DMF | 13 | Trace |
| 4 | THF | 13 | Trace |
| 5 | Glycol | 13 | 10 |
| 6 | Ethanol | 13 | 17 |
| 7 | HOAc | 13 | 55 |
| 8 | H2O | 13 | 92 |
TABLE 5. Solvent effect on the synthesis of compounds at 90°C.
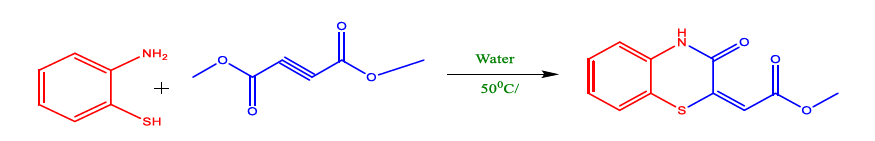
In summation, Tu et al. showed the development of an effective multi-component tandem reaction with MW assistance to produce benzothiazepine. The process is simple, requires low-cost starting materials, and provides high-quality products with chemoselectivity that depends on the electronic effects of the substituted aromatic aldehydes and amines. This new heterocycle synthesis technique is appealing and is very promising for accessing molecules with potential biological interest due to its polar and ionic compounds that dissolve quickly in water at room temperature and become less polar when heated. Water is also inexpensive, readily available in pure form, harmless, and non-flammable (Figure 6). In addition, it exists in a liquid state at room temperature at 1 atmospheric pressure. Water is essential to synthesise benzothiazepinone because of its strong solvation capabilities. Zhang et al. developed a green approach to synthesise 1,4-benzothiazine-3-one in aqueous medium. These desirable products have good yields in a very short time and no use of any catalyst (Table 6).
FIG 6. Synthesis 1,4-benzothiazine-3-one.
| Entry | Solvent | Time/min | Yield (%) |
|---|---|---|---|
| 1 | n- Hexane | 30 | 41 |
| 2 | THF | 30 | 37 |
| 3 | Toluene | 30 | 58 |
| 4 | Dioxane | 30 | 61 |
| 5 | MeOH | 30 | 88 |
| 6 | EtOH | 30 | 82 |
| 7 | MeCN | 30 | 83 |
| 8 | H2O | 30 | 97 |
| Reaction condition: 2-Aminothiophenol (10 mmol), DMAD (10 mmol), solvent (20 mL) at 50°C. | |||
TABLE 6. Optimization of solvent in the synthesis of 1,4-benzothiazine-3-one.
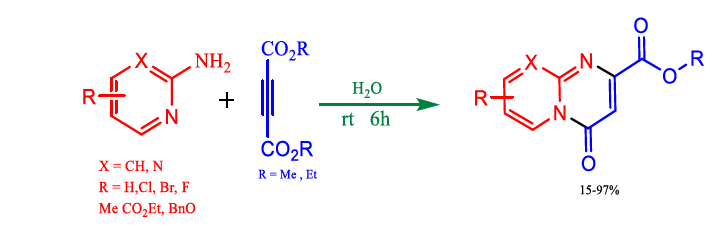
Zhang et al. demonstrated a simple and efficient method for producing 1,4-benzothiazine-3-one, high regioselectivity, and excellent yield (Figure 7). This technique has a wide range of substrates, mild operating conditions, readily available starting materials, and ease of operation due to the solvent properties of water, such as the molecular structure of water and its capability to form intermolecular hydrogen bonds resulting in unique physicochemical properties, making water a fascinating solvent. Yan et al. succeed in preparing alkyl 4-oxo-4H-pyrido[1,2-a] pyrimidine-2-carboxylate derivatives in the presence of water, with 2-aminopyridines and dialkyl ethylenedicarboxylates (Table 7).
FIG 7. Green method for alkyl 4-oxo-4H-pyrido[1,2-a] pyrimidine-2-carboxylate synthesis.
| Entry | T(°C) | t(h) | Solvent | Yield (%) |
|---|---|---|---|---|
|
1 |
Rt |
6 |
H2O |
82 |
|
2 |
Rt |
6 |
Toluene |
0 |
|
3 |
Rt |
12 |
Toluene |
0 |
|
4 |
Rt |
6 |
DMF |
15 |
|
5 |
Rt |
6 |
MeOH |
0 |
|
6 |
Rt |
6 |
Acetone/H2O (1:1) |
70 |
|
7 |
Rt |
6 |
Without solvent |
0 |
|
Reaction conditions: 1a (0.32 mmol), 2a (0.38 mmol), catalyst-free, on 30 mL water for six h in air. |
||||
TABLE 7. Influence of solvent in the synthesis of alkyl 4-oxo-4H-pyrido[1,2-a] pyrimidine-2-carboxylate at room temperature.
Yan et al. showed that an environmentally friendly and catalyst-free technique may create alkyl 4-oxo-4H-pyrido[1,2-a]2- aminopyridine acetylene dicarboxylate derivatives of pyrimidine-2-carboxylate at water. Many substrates can be used with this process, producing yields ranging from acceptable to good. In this result, we found that it gave 70 % yield in the presence of acetone: water mixture (1:1) while a higher product in H2O only. This may be due to the high dielectric constant for the water, which provides appropriate solvation and sufficient activation energy for the generation of intermediates and thus, the products. This simple and environmentally friendly route to synthesise alkyl 4-oxo-4H-pyrido[1,2-a] pyrimidine-2-carboxylate derivatives has great potential in future medicinal chemistry (Figure 8).
Nagarapu et al. have reported a green method for synthesising quinoxaline from orthophenylene diamines with ketone derivatives using a green solvent, polyethene glycol-400, and their toxic product form, and no catalyst was used in this reaction (Table 8).
FIG 8. Synthesis of quinoxaline using green solvent polyethylene glycol-400.
| Entry | Solvent (5 ml) | Yield (%) |
|---|---|---|
| 1 | Toluene | 25 |
| 2 | CH3CN | 50 |
| 3 | MeOH | 54 |
| 4 | DMSO | 51 |
| 5 | Water | 45 |
| 6 | PEG | 90 |
| Reaction condition: O-Phenylenediamine (1.1 mmol), α-bromoketone (1.0 mmol), 80°C, 8 h. | ||
TABLE 8. Screening of solvent for the synthesis of quinoxaline.
On critical analysis, Nagarapu et al. have developed a simple and effective method for producing quinoxaline derivatives using PEG as a recyclable medium without using any additives or organic co-solvents. Despite the important role of solvent, it was sneaked. They have used average molecular weight PEG-400 for the required specific heat capacity and dielectric constant at 80°C. Later characteristic is mainly responsible for the solvation of orthophenylene diamines and ketone with more hydrophobic behaviour. At the same time, the former property is evitably given high-temperature reactions (Figure 9).
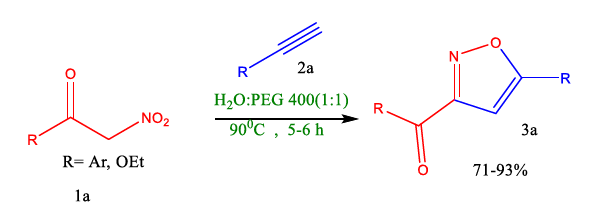
Chary et al. successfully developed a green synthesis of isoxazoles. These reactions were conducted with a 1,3-dipolar cycloaddition in green solvent aqueous polyethylene glycol from benzol nitromethane/ethyl 2-nitroacetate and terminal alkynes (Table 9).
FIG 9. Preparation of isoxazoles using a green solvent.
| Entry | Solvent | Time(h) | Yield (%) |
| 1 | CH3CN | 20 | 0 |
| 2 | 1,4-dioxane | 24 | 0 |
| 3 | DMF | 20 | 0 |
| 4 | DMSO | 20 | 0 |
| 5 | PEG 400 | 12 | 30 |
| 6 | H2O | 48 | 0 |
| 7 | H2O/1,4-dioxane (1:1) | 12 | 0 |
| 8 | H2O/CH3CN (1:1) | 12 | 0 |
| 9 | H2O/DMF (1:1) | 12 | 0 |
| 10 | H2O/DMSO (1:1) | 12 | 0 |
| 11 | H2O/PEG-400 (1:1) | 4 | 80 |
| 12 | H2O/PEG-400 (1:1) | 6 | 88 |
| 13 | H2O/PEG-400 (1:1) | 12 | 82 |
| 14 | H2O/EG (1:1) | 6 | 70 |
| 15 | Ethylene Glycol | 6 | 35 |
| Reactions were carried out using 1a (1.0 mmol), phenylacetylene(2a) (1.0 mmol), and solvent (5 ml) at 90°C. | |||
TABLE 9. Optimization of solvent for the synthesis of isoxazoles.
In conclusion, Chary et al. research shows the application of aqueous PEG-400 in enabling the 1,3-dipolar cycloaddition of benzoyl nitromethane/ethyl 2-nitroacetate with terminal alkynes or alkenes that results in isoxazolines under environmentally friendly circumstances. PEG 400, an affordable and non-toxic reaction medium, was demonstrated to have preceded the reaction in high yield. This method synthesises complex compounds without hazardous solvents, bases, catalysts, or dehydrating agents. Noteworthily, they used a mixture of protic solvent water and PEG-400 (1:1); the cause was undiscussed. It might be used due to the role of solvation of the hydrophilic part by H2O. In contrast, the hydrophobic part with PEG-400 provided the exact amount of activation energy for the formation of the product (Figure 10).
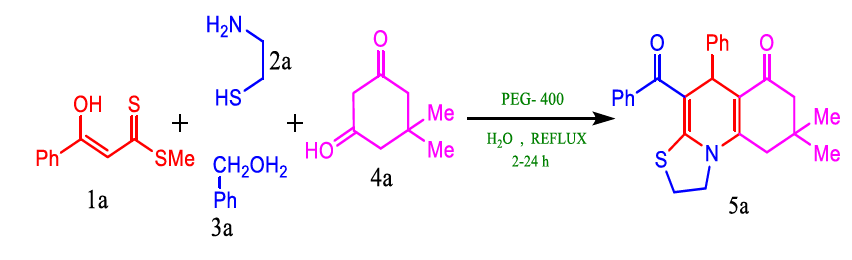
For the synthesis of thiazoloquinoline, Nagaraju et al. employed a green approach synthesis. The compounds were synthesised by reacting water-PEG 400 with cyclic 1,3-diketones, aldehydes, cysteamine, and enolic thioesters. This environmentally benign method offers a high yield while avoiding conventional chromatographic separation (Table 10).
FIG 10. Synthesis of thiazoloquinoline.
| Entry | Solvent (mol%) | Temp. (°C) | Time | Yield (%) |
|---|---|---|---|---|
| 1 | None | 100 | 11 | 45 |
| 2 | MeOH | Reflux | 24 | 20 |
| 3 | EtOH | Reflux | 24 | 30 |
| 4 | CH3CN | Reflux | 24 | 15 |
| 5 | Acetone | Reflux | 24 | 10 |
| 6 | DMF | 110 | 9 | 55 |
| 7 | DMSO | 110 | 3 | 70 |
| 8 | H2O | Reflux | 24 | Trace |
| 9 | PEG 400 | 110 | 6 | 75 |
| 10 | PEG-400(50) | Reflux | 4 | 81 |
| 11 | PEG-400(33) | Reflux | 3 | 85 |
| 12 | PEG-400(10) | Reflux | 2 | 92 |
| 13 | PEG-400(15) | Reflux | 2 | 89 |
| 14 | PEG-400(5) | Reflux | 6 | 50 |
| 15 | PEG-EtOH (50) | Reflux | 12 | 40 |
| 16 | EtOH-H2O | Reflux | 12 | 30 |
| Reaction conditions: 1a (0.5 mmol) was treated with 2a (0.5 mmol), followed by the addition of 3a (0.5 mmol) and 4a (0.5 mmol). | ||||
TABLE 10. Study of the effect of solvent on the synthesis of thiazoloquinoline.
According to the research group, this convergent and adaptable technique offers exceptional functionality tolerance, a broad substrate range, and abnormally high bond-forming efficiency in addition to structural and step economies. This strategy exemplifies reconciling complexity and simple operations in an environmentally friendly, efficient, and cost-effective way (Figure 11).
Siddiqui et al. synthesised benzofuran at 31°C using an ionic liquid as a green solvent, which has been used as an organocatalytic for the hetero-cyclization reaction of o-alkylphenol with [bmIm]OH in an aqueous base giving of benzofuran with high atom economy and-chemoselectivity up to 97% (Table 11).
FIG 11. [bmIm]OH catalysed the synthesis of benzofuran at 31°C.
| S. no. | Base | Solvent | Time (h) | Yield |
|---|---|---|---|---|
| 1. | Pyridine | H2O | 24 | No reaction |
| 2. | Liquid ammonia | DMF | 24 | No reaction |
| 3. | Diethyl amine | ethanol | 24 | No reaction |
| 4. | DMAP | 1,4 dioxane | 24 | No reaction |
| 5. | NaOH | H2O | 24 | No reaction |
| 6. | KOH | DMF | 24 | No reaction |
| 7. | Li2CO3 | ethanol | 24 | No reaction |
| 8. | Na2CO3 | 1,4 dioxane | 24 | No reaction |
| 9. | K2CO3 | DMF | 24 | No reaction |
| 10. | Cs2CO3 | DMF | 36 | No reaction |
| 11. | — | (bmIm)Br | 36 | No reaction |
| 12. | Pyridine | (bmIm)Br | 9 | 13 |
| 13. | Liquid NH3 | (bmIm)Br | 9 | 15 |
| 14. | Diethyl ammine | (bmIm)Br | 9 | 17 |
| 15. | DMAP | (bmIm)Br | 9 | 21 |
| 16. | NaOH | (bmIm)Br | 6 | 39 |
| 17. | KOH | (bmIm)Br | 6 | 43 |
| 18. | Li2CO3 | (bmIm)Br | 3 | 57 |
| 19. | Na2CO3 | (bmIm)Br | 3 | 65 |
| 20. | K2CO3 | (bmIm)Br | 3 | 75 |
| 21. | Cs2CO3 | (bmIm)Br | 3 | 81 |
| 22. | — | (bmIm)OH | 2.5 | 97 |
| Reaction conditions: 1a (0.5 mmol) was treated with 2a (0.5 mmol), followed by the addition of 3a (0.5 mmol) and 4a (0.5 mmol). | ||||
TABLE 11. The below table shows Base, solvent, Time and yield.
According to the research group, the reaction was encouraged by the successful cyclisation using an ionic liquid, resulting in benzofuran with high yield; the reaction was done for the synthesis of a broad range of benzofurans containing both electrondonating and electron-withdrawing substituents. This mechanism involves two different ionic liquids ([bmIm]OH and [bmIm]Br). Still, a better result is obtained when using ([bmIm]OH in the presence of different bases (Cs2CO3) due to the difference in nucleophilicity. Both the ionic liquids have nucleophiles (Br- and OH-), where OH- being a stronger nucleophile (weaker the acid, the stronger the conjugate base and the stronger the nucleophilicity and order of nucleophilicity is C2H5O– > OH– > Cl– > Br– > I–), abstracts H from OH of phenol (Tables 12 and 13). Further, a facile attack of phenoxide ion on the C-C bond leads to hetero-cyclization, as described in Figure 11.
| Property | Organic solvents | Ionic liquid |
|---|---|---|
|
Number of Solvents |
>1000 |
>1000000 |
|
Selectivity |
Only one function |
Multi-function |
|
Catalytic activity |
Rare |
Common and tuneable |
|
Vapor pressure |
the Clausius-Clapeyron Equation must be obeyed |
The low Vapour pressure in typical circumstances |
|
Chirality |
Rare |
Common and Tuneable |
|
Flammability |
Usually, flammable |
Usually, non-flammable |
|
Polarity |
Conventional polarity |
Questionable |
|
Adaptability |
Limited range of solvents available |
Virtually unlimited content, such as designer solvents, |
|
Price |
Cost Effective |
Very costly |
|
Reusability |
Green imperative |
Economic necessity |
|
Viscosity |
0.2-100 |
22-40,000 |
|
Density, g/cm3 |
0.6-1.7 |
0.8-3.3 |
|
Refractive index |
1.3-1.61 |
1.5-2.2 |
TABLE 12. Difference between organic solvents vs. ionic liquid.
| S. n. | Name of the green solvent | Specific heat capacity |
|---|---|---|
| 1 | PEG 200 | not available |
| 2 | PEG 400 | 2.350 kJ/kg K (at 298.15 K) |
| 3 | PEG 600 | 2.490 kJ/kg K (at 298.15 K) |
| 4 | PEG 1000 | 2.142 kJ/kg K (at 298.15 K) |
| 5 | (TMG)(Lac), | 3.132 J. g−1K−1 ( 310 K) |
| 6 | (TMG)(BF4), | 3.135 J. g−1K−1 ( 310 K) |
| 7 | (TMG)(SUB), | 3.342J. J. g−1K−1 ( 310 K) |
| 8 | (TMG)(DOD), | 3.371 J. g−1K−1 ( 310 K) |
| 9 | (Bmim)(Ac) | 3.372 J. g−1K−1 ( 310 K) |
| 10 | (Bmim) (BF4) | 3.433 J. g−1K−1 ( 310 K) |
| 11 | H2O | 4.174 J. g−1K−1 ( 310 K) |
TABLE 13. Thermophysical properties of several forms of green solvents, as identified in the literature.
(TMG) (Lac),=1,1,3,3-tetramethylguanidium lactate, (TMG)(SUB), (TMG)(DOD),=Tetramethyl guanidium-based ionic liquids.
Conclusion
Green chemistry is expanding in a wide range of academic and commercial domains. Because the earth's resources are limited, using them responsibly is critical. In contrast, we have seen that when developing new medications, researchers and pharmaceutical companies worked for green manufacturing processes. Hence, most pharmaceutical companies are working harder than ever to cut waste and avoid air and water contamination. Greener procedures with less negative environmental impact and lower drug costs are made possible by green solvents; we anticipate that this review will provide a quick overview of the significance of green solvents that should be chosen by developing an understanding of polarity details, specific heat data and dielectric constants into consideration. With the benefits of green chemistry, the industry should change traditional approaches. The main feature of this review is the green solvent and protocols used in a synthetic organic reaction. Thus, conversions reported in the presence of green solvents are straightforward, proficient, and eco-friendly by nature. A green approach in reaction medium will undoubtedly offer remarkable benefits to the environment and mankind. Following are the challenges and suggestions for adopting green solvents:
• Though green solvents play a climacteric role in organic synthesis by influencing the reaction kinetics and shifting the chemical equilibrium, scarcely reported research on a selection of green solvents is its limitation.
• Majorly for industrial practice, green solvents have been selected based on environmental, health and safety issues and legislation only. Therefore, some green solvents with mediocre properties have been omitted, which can affect process performance. Furthermore, the library of green solvents with present properties is narrow.
• There still needs to be more intuitive outlook-based literature on reaction-based solvent selection and design for specific reactions.
• Green Solvents affect valuable catalysts besides reactants in a multi-component or one-pot reaction. Therefore, solvent selection based on catalyst performance should also be rationalised while planning a synthesis.
• Understanding green solvent effects can be improvised by coupling the quantum chemical method, i.e., Computer-Aided Molecular Design (CAMD), with the systematic solvent selection procedure. CAMD is a promising tool to guide the frequent solvent selection for chemical reactions and is broadly based upon reaction rates, which are solventindependent contributions. Further, some contributions arise due to interactions of a molecule with its environment in a liquid reaction mixture, and COSMO-RS can calculate these solvent-dependent contributions. Recently, a method for integrated design of reaction solvents and processes called Rx-COSMO-CAMPD enables solvent design using prediction of both solvent-dependent and independent contributions, including reaction kinetics based on quantum chemical methods. Such intuitive design of synthesis reaction considering green solvent for the specific reaction is the demand of the present synthetic chemistry.
• Nevertheless, ultra-fast and operando spectroscopy in a wide frequency range will be helpful in opening underhanded discussions on green solvents. Spectroscopic studies will be helpful in the prediction of the generation of metastable coordination states with distinct molecular identities for simple solutes in green solvents.
Acknowledgements
The authors would like to acknowledge IIT (BHU) Varanasi's financial support to MS as a Teaching Assistant Fellowship.
Conflict of Interest
The authors declare no conflict of interest.
References
- Chen J, Spear SK, Huddleston JG, et al. Polyethylene glycol and solutions of polyethylene glycol as green reaction media. Green Chem. 2005;7(2):64-82.
- Welton T, Smith PJ. Palladium catalyzed reactions in ionic liquids. Adv Org Chem. 2004;51:251-282.
- Li CJ. Organic reactions in aqueous media with a focus on carbon− carbon bond formations: a decade update. Chem Rev. 2005;105(8):3095-3166.
[Crossref] [Google Scholar] [PubMed]
- Butler RN, Coyne AG. Water: Nature’s Reaction Enforcer Comparative Effects for Organic Synthesis “In-Water” and “On-Water”. Chemical reviews. 2010;110(10):6302-6337.
[Crossref] [Google Scholar] [PubMed]
- Strauss CR, Trainor RW. Reactions of ethyl indole-2-carboxylate in aqueous media at high temperature. Aust J Chem. 1998;51(8):703-706.
- Strauss CR, Trainor RW. Developments in microwave-assisted organic chemistry. Aust J Chem. 1995;48(10):1665-1692.
- Chanda A, Fokin VV. Organic synthesis “on water”. Chem Rev. 2009;109(2):725-748.
[Crossref] [Google Scholar] [PubMed]
- Narayan S, Muldoon J, Finn MG, et al. Cover Picture:“On Water”: Unique Reactivity of Organic Compounds in Aqueous Suspension (Angew. Chem. Int. Ed. 21/2005)." Angew Chem Int Ed. 2005;44(21):3157.
- Tiwari S, Kumar A. Unusual temperature dependence of salt effects for “on water” Wittig reaction: hydrophobicity at the interface. Chem Commun. 2008(37):4445-44457.
[Crossref] [Google Scholar] [PubMed]
- Chakraborti AK, Rudrawar S, Jadhav KB, et al. “On water” organic synthesis: a highly efficient and clean synthesis of 2-aryl/heteroaryl/styryl benzothiazoles and 2-alkyl/aryl alkyl benzothiazolines. Green Chem. 2007;9(12):1335-1340.
- Bigi F, Carloni S, Ferrari L, et al. Clean synthesis in water. Part 2: Uncatalysed condensation reaction of Meldrum's acid and aldehydes. Tetrahedron lett. 2001;42(31):5203-52035.
- Moghaddam FM, Kiamehr M, Khodabakhshi MR, et al. A new domino Knoevenagel-hetero-Diels–Alder reaction: An efficient catalyst-free synthesis of novel thiochromone-annulated thiopyranocoumarin derivatives in aqueous medium. Tetrahedron. 2010;66(45):8615-8622.
- Breslow R. Hydrophobic effects on simple organic reactions in water. Acc Chem Res. 1991;24(6):159-164.
- Das D, Sikdar P, Bairagi M. Recent developments of 2-aminothiazoles in medicinal chemistry. Eur J Med Chem. 2016;109:89-98.
[Crossref] [Google Scholar] [PubMed]
- T Chhabria M, Patel S, Modi P, et al. Thiazole: A review on chemistry, synthesis and therapeutic importance of its derivatives. Curr Top Med Chem. 2016;16(26):2841-2862.
[Crossref] [Google Scholar] [PubMed]
- Kumar S, Aggarwal R. Thiazole: a privileged motif in marine natural products. Mini-Rev Org Chem. 2019;16(1):26-34.
- Sashidhara KV, Rao KB, Kushwaha P, et al. Novel chalcone–thiazole hybrids as potent inhibitors of drug resistant Staphylococcus aureus. ACS Med Chem Lett. 2015;6(7):809-813.
[Crossref] [Google Scholar] [PubMed]
- Jin Z. Muscarine, imidazole, oxazole, and thiazole alkaloids. Nat Prod Rep. 2003;20(6):584-605.
[Crossref] [Google Scholar] [PubMed]
- Gogoi S, Dutta M, Gogoi J, et al. Microwave promoted synthesis of cycl [3.2. 2] azines in water via a new three-component reaction. Tetrahedron Lett. 2011;52(7):813-816.
- Kumar D, Kumar NM, Patel G, et al. A facile and eco-friendly synthesis of diarylthiazoles and diarylimidazoles in water. Tetrahedron lett. 2011 Apr 20;52(16):1983-1986.