Original Article
, Volume: 12( 3)Methane Sulphonic Acid Catalyzed Efficient Protocol for Synthesis of 2-Hydroxy Chalcones
- *Correspondence:
- Pramod Kulkarni, Department of Chemistry, Hutatma Rajguru Mahavidyalaya, Rajgurunagar, MH 410505, India, E-mail: pramodskulkarni3@gmail.com
Received: June 06, 2016; Accepted: June 20, 2016; Published: June 25, 2016
Citation: Ramesh G, Sunil J, Pramod K. Methane Sulphonic Acid Catalyzed Efficient Protocol for Synthesis of 2-Hydroxy Chalcones. Org Chem Ind J. 2016;12(3):101.
Abstract
Methane sulphonic acid was found an efficient catalyst for synthesis of 2’-hydroxy chalcone via aldol condensation of 2-hydroxy acetophenone and substituted benzaldehyde. The merits of this protocol are efficient, eco-friendly, and inexpensive catalyst, short reaction time, high yield, and easy workup procedure. The catalyst is also well work with electron donating as well as electron with drawing group present aromatic ring. The structures of products were confirmed by melting point, IR, and 1H NMR.
Keywords
2-hydroxy acetophenone; Substituted benzaldehyde; Chalcone; Claisen-Schmidt condensation
Introduction
Chalcones are found in natural products which belong to the class of open chain flavonoids in which two aromatic rings are linked by a three carbon α, β-unsaturated carbonyl skeleton. The chalcones are main chemical intermediates for synthesis of flavonoids like flavanone, flavones, isoflavones and synthesis of bioactive heterocyclic [1,2] as well as these compounds are main synthons for the preparation of five and six member ring systems [3] and use for the synthesis of medicinal intermediates [4,5].
Chalcones shows broad spectrum of medicinal properties due to the presence of α, β-unsaturated carbonyl skeleton. The chalcone exhibits biological activities like antimalarials [6], anti-inflammatory, antioxidant and antiulcer [7], anti HIV [8], antiviral [9], antibacterial [10], antituberculosis [11], anticancer [12], and antileishmanials activity [13].
Due to its importance medicines and key intermediate for the synthesis of bioactive molecules, chalcone attracted many researchers for their synthesis. Numerous methods are reported for the synthesis of chalcones, the widely used method is the base catalysed Clasien-Schmidt reaction in which the condensation of aromatic ketone with an aldehyde is carried out in the presence of catalyst like KOH [14], Basic Al2O3 [15], ZnCl2 [16], AlCl3/CS2 [17], BF3 [18], KOH/TEBA/EtOH [19], Mg-Al- OtBu [20], NaNO3/EtOH , using ultrasound [21], NaOH/EtOH [22], Ba(OH)2 grinding [23], CaO /microwave [24]. However, some of these reported methods suffer from drawbacks like use of toxic and hazardous solvent, longer reaction time, low yield, not applicable to acid and base sensitive functional group, the addition of reactant and catalyst in cooling condition. Due to these limitations, hence, there is a scope to develop new methods.
Methane sulphonic acid is a clear colourless liquid available as a 70% solution in water and anhydrous form. Its pKa value is -1.9 and low molecular weight. Due to low pKa value, methane sulphonic acid is strong acid. Methane sulphonic acid is easily available, inexpensive, and biodegradable forming sulphate and carbon dioxide. Due to this all properties methane sulphonic acid attracts many chemists to use as catalyst in many organic transformations [25]. In this paper, we report methane sulphonic acid catalyzed Claisen-Schmidt condensation reaction between 2’-hydroxy acetophenone and substituted benzaldehyde to afford corresponding 2’-hydroxychalcone in good yield.
Experimental
General
All purchased chemicals were of analytical grade and used without further purification. The 1HNMR spectra were obtained on a Bruker DRX-300 Avance instrument using CDCl3 as solvent and TMS as internal standard at 300 MHz. All products are known compounds; their physical and spectroscopic data were compared with those reported in the literature and found to be identical.
Procedure for synthesis of 2’-hydroxychalcone
In a 50 mL round bottom flask, substituted benzaldehyde (1 mmol), 2-hydroxyacetophenone (1 mmol) dissolved in 10 mL toluene, 0.1 mmol of methane sulphonic acid dissolved in 1 mL of ethanol added to the reaction mixture and the resulting reaction mixture was refluxed for 4 hours. The progress of the reaction was monitored by TLC. After completion of the reaction, the solvent was removed under reduced pressure and oily product was separate out. The oily product was dissolved in ethanol and added crushed ice to separate out solid product. The crude product was filtered on suction pump, followed by washing with water and ice cold ethanol. The resulting product was purified by recrystallisation from ethanol. The physical constant and spectral data IR, 1HNMR , 13CNMR for 2’-hydroxy chalcone (Table 1), were identified with those of authentic samples.
| Entry | Aldehyde | Product | % Yieldb | M.P. °C [Ref] |
|---|---|---|---|---|
| 1 | 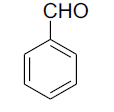 |
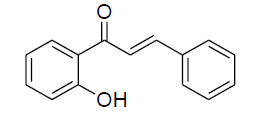 |
87 | 78 [26] |
| 2 | 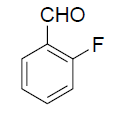 |
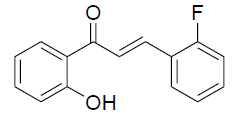 |
80 | 57[27] |
| 3 | 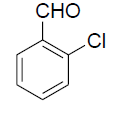 |
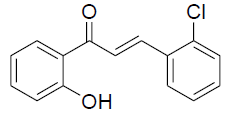 |
82 | 80[28] |
| 4 | 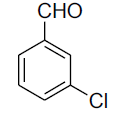 |
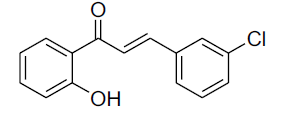 |
86 | 61[29] |
| 5 | 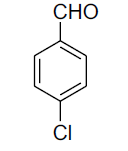 |
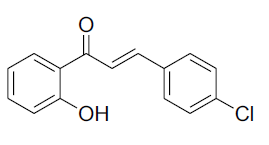 |
81 | 47[26] |
| 6 | 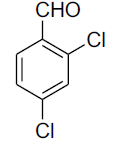 |
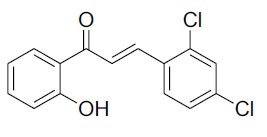 |
85 | 150-153[26] |
| 7 | 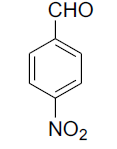 |
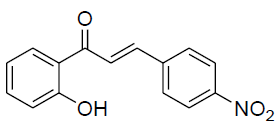 |
87 | 205[24] |
| 8 | 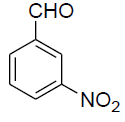 |
 |
90 | 172-174[30] |
| 9 | 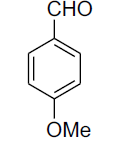 |
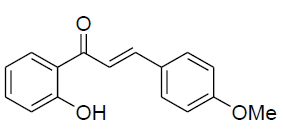 |
83 | 91-92[25] |
| 10 |  |
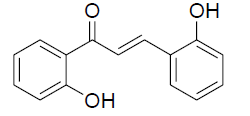 |
72 | 161-163[25] |
aReaction condition: 2-hydroxy acetophenone (1 mmol), substituted benzaldehyde (1 mmol), dissolved in 10 mL toluene in 50 mL RB, 0.1 mmol methane sulphonic acid dissolved in 1 mL EtOH added to this solution, and reflux the reaction mixture for 4 hr b: isolated yield after purification.
Table 1: Synthesis of substituted 2’-hydroxy chalcone using methane sulphonic acid as a catalysisa.
Results and Discussion
The present protocol describes a new efficient and environmentally benign procedure for the synthesis of 2’-hydroxy chalcone using strong Brønsted –Lowry acid that is methane sulphonic acid. The reaction of benzaldehyde (1 mmol), 2- hydroxy acetophenone (1 mmol), was carried out in the presence of methane sulphonic acid (0.1 mmol in 1 mL of ethanol) in toluene (10 mL) at reflux temperature. The reaction proceeded smoothly to afford the corresponding 2’-hydroxy chalcone in 87% yield within 4 hours. After completion of the reaction, reaction mixture was cooled and toluene was removed under reduced pressure. The oily product was separated from reaction mixture. Oily product was dissolved in ethanol and added crushed ice to separate out crude solid product. The solid was filtered on suction pump, followed by washing with water and ice cold to give the desired product. Also reaction of aromatic aldehydes bearing electron donating and withdrawing groups on aromatic ring underwent smooth conversion to afford the corresponding 2’-hydroxy chalcones in good to moderate yield without affecting the functional group (Table 2). The obtained products were characterized by spectroscopic methods (IR, 1HNMR , 13CNMR) and by comparison of physical constant with authentic samples ( Figure 1).
| S.N. | Structure | Spectral analysis |
|---|---|---|
| 1 | 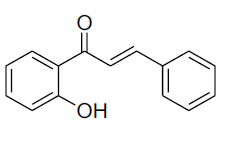 |
IR: (KBr) ν/cm-1 3531 (-OH), 1650 (C=O), 1613 (C=C), 13555 (O-C), 683 (Ar-H); 1H NMR(CDCl3, 300MHz): δ12.75 (s, 1H, -OH), 7.92 (d, 1H, J=8.06) 7.89 (d 1H J=7.85Hz) 7.85 (d 1H J=14.89 Hz) 7.80 (d, 1H, J=14.88Hz), 7.34-7.81 (m 5 H), 7.00-7.09(m 2H); 13CNMR (CDCl3, 75MHz):δ 194.22, 164.52, 160.70, 142.47, 135.52, 134.94, 129.93, 129.88, 128.21, 127.24, 115.78, 112.13, 111.58, 110.6 |
| 2 | 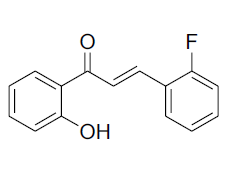 |
IR:(KBr) ν/cm-1 3453 (-OH), 1651 (C=O), 1617 (C=C), 1354 (O-C), 688(Ar-H); 1H NMR (CDCl3, 300MHz): δ12.94 (s,-OH), 8.23 (d,1H, J=15.56Hz) 7.93 (dd, 1H, J=8.06 Hz, J=1.46Hz),7.79 (d 1H, J=15.58Hz)7.66(dd 1H J=7.79 Hz J=1.45Hz) 7.49 (m 1H) 7.4 (m 1H) 7.09-6.8 (m 4H); 13CNMR (CDCl3, 75MHz):δ 192.91, 164.22, 159.09, 141.19, 136.12, 133.50, 129.42, 129.32, 124.65, 120.91, 120.30, 117.48, 117.32, 112.29 |
| 3 | 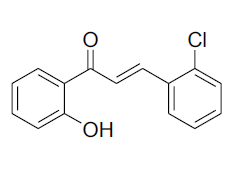 |
IR: (KBr) ν/cm-1 3468 (-OH), 1653 (C=O), 1615 (C=C), 1322 (O-C), 680 (Ar-H); 1H NMR (CDCl3, 300 MHz): δ 12.89 (s, -OH),8.21 (d, 1H J=15.54 Hz), 7.90 (dd, 1H J=8.07 Hz, J=1.47 Hz) 7.78 (d 1H J=15.19 Hz), 7.68 (dd 1H J=7.72 Hz, J=1.42 Hz) 7.45 (m 1H),7.40 (m, 1H), 7.10-6.85 (m, 4H);13CNMR (CDCl3, 75 MHz): δ193.12, 163.98, 159.04, 140.37, 138.05, 132.18, 129.24, 129.11, 122.32, 120.52, 118.9, 118.56, 111.31 |
| 4 | 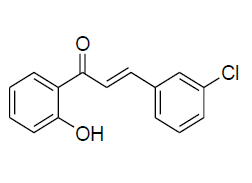 |
IR: (KBr) ν/cm-1 3538 (-OH),1665 (C=O), 1613 (C=C), 1328 (C-O), 676 (Ar C-H); 1H NMR (CDCl3, 300 MHz): δ 12.81 (s –OH) 7.91 (dd 1H J=8.06 Hz, J=1.44 Hz), 7.89 (d 1H J=15.50 Hz) 7.65 (d 1H J=15.51 Hz) 7.55 (m,1H), 7.36 (d 1H J=8.05) 7.25 (d, 1H J=7.85 Hz) 7.15 (m 1H) 7.02 (d 1H J=8.1 Hz) 7.01-6.93 (m 2H); 13CNMR (CDCl3, 75 MHz): δ 194.73, 162.75,163.01, 148.38,135.02, 137.02, 129.95, 128, 122.02, 121.08, 120.19, 119.07, 118.08, 110.68, 114.15 |
| 5 | 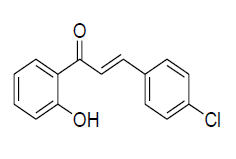 |
IR: (KBr) ν/cm-1 3351 (-OH), 1635 (C=O), 1552 (C=C), 1204 (C-O), 642 (Ar C-H);1H NMR (CDCl3, 300 MHz): δ12.74 (s 1H, -OH), 7.90 (d 1H J=8.06 Hz), 7.86 (d 1H J=15.60 Hz), 7.64 (d 1H J=15.61 Hz), 7.61 (d 2H J=8.40 Hz),7.51 (m 1H) 7.40 (d 2H J=8.41 Hz), 7.04 (d1H J=8.43 Hz),6.90 (m 1H); 13CNMR (CDCl3, 75 MHz): δ 190.92, 164.56, 144.01, 137, 136.02,133,130.19, 129.85, 129, 120.50, 119.80, 118.19, 117.95. |
| 6 | 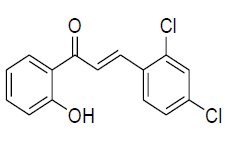 |
IR: (KBr) ν/cm-1 3432 (-OH), 1635 (C=O), 1598 (C=C), 1337 (C-O), 686 (Ar C-H); 1H NMR (CDCl3, 300 MHz): δ 12.95 (s, 1H -OH) 8.24 (d 1H J=15.60 Hz), 7.95 (m 1H), 7.80 (d 1H J=15.61 Hz), 7.65 (m 1H), 7.51 (m 1H) 7.42 (m 1H) 7.04-6.9 (m 3H); 13CNMR (CDCl3, 75 MHz): δ 119.2, 120.4, 120.9, 123.3, 128.1, 128.9, 129.7,130.2,136, 139.29, 136.9,140,163.7,193.2 |
| 7 | 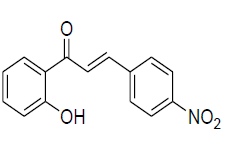 |
IR: (KBr) ν/cm-1 3505 (-OH), 1697 (C=O), 1529 (C=C), 1345 (C-O), 671 (Ar C-H);1H NMR (CDCl3, 300 MHz): δ12.92 (s 1H –OH), 7.94 (d 1H J= 8.01 Hz) 7.89 (d 1H J=14.9 Hz), 7.65 (d 1H J=14.8 Hz);7.63 (d 2H J=8.2 Hz),7.53 (m 1H), 7.44 (d 2H J=8.1 Hz), 7.03 (d 1H J= 8.4 Hz) 6.92 (m1H); 13CNMR (CDCl3, 75 MHz): δ196.14, 165.78, 145.96, 136, 136.91,133.25, 130.19, 129.12, 124, 121, 120.17, 118.85, 118.11 |
| 8 | 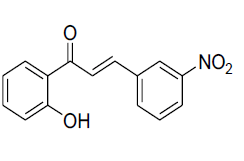 |
IR: (KBr) ν/cm-1 3428 (-OH), 1688 (C=O), 1593 (C=C), 1294 (C-O), 693 (Ar C-H);1H NMR (CDCl3, 300 MHz): δ 12.85 (s 1H –OH), 7.95 (d 1H J= 8.02 Hz), 7.90 (d 1H J=15.5 Hz), 7.66 (d 1H J=15.4 Hz), 7.52 (m 1H), 7.35 (d 1H J=8.05 Hz), 7.27 (d 1H J=7.78 Hz), 7.21 (m 1H), 7.04 (d 1H 7.98), 7.01-6.93 (m 2H);13CNMR (CDCl3, 75 MHz): δ 196.12, 163.78, 160.02, 145.18, 136.91, 136.03, 130.05, 129.68, 121.12, 120.57, 118.85, 117.11, 116.61, 115.72 |
| 9 | 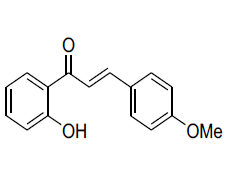 |
IR: (KBr) ν/cm-1 3542 (-OH), 1645 (C=O), 1608 (C=C), 1338 (O-C), 675 (Ar-H); 1H NMR (CDCl3, 300 MHz): δ 12.61 (s 1H, -OH), 7.74 (d 1H J=8.1 Hz), 7.71 (d 1H J=15.62 Hz), 7.61 (d 1H J=15.62 Hz), 7.52 (d 2H J=8.14 Hz),7.44 (m 1H) 7.32 (d 2H J=8.14 Hz), 7.02 (d,1H J=8.1 Hz),6.86 (m 1H), 3.84 (s, 3H); 13CNMR (CDCl3, 5 MHz): δ190.23, 164.51,163.3 143.94, 137.11, 136.22,133.42,130.12, 129.20, 128.9, 120.45, 119.32, 118.10, 117.53, 55.33 |
| 10 |  |
IR: (KBr) ν/cm-1 3462 (-OH), 1648 (C=O), 1610 (C=C), 1327 (O-C), 670 (Ar-H); 1H NMR (CDCl3, 300 MHz): δ 12.72 (s, -OH), 11.15 (s, OH), 7.95 (d, 1H J=15.41 Hz), 7.86 (dd, 1H J=8.37 Hz, J=1.45 Hz) 7.72 (d 1H J=15.41 Hz), 7.56 (dd 1H J=8.3 Hz, J=1.45 Hz) 7.34 (m, 1H),7.29 (m 1H), 7.03-6.82 (m, 4H);13CNMR (CDCl3, 75 MHz): δ193.16, 163.85, 162.97, 140.36, 137.97, 132.13, 128.93, 128.5, 121.93, 120.42, 118.67, 118.10, 111.21 |
Table 2: Spectral data of chalcone.
Conclusion
In conclusion here we report, an efficient synthesis of 2’-hydroxy acetophenone using methane sulphonic acid is catalysis. The merits of this protocol is high yield, easily available and inexpensive, biodegradable acid catalysis, simple reaction condition, easy work procedure, high yield, short reaction time (Table 3).
| Entry | Catalyst | Time | Temperature | % yield | Ref. |
|---|---|---|---|---|---|
| 1 | KOH | 24 h to 47 h | Room Temp. | 41-50 | [14] |
| 2 | Basic Al2O3 | 2.5 h | Room Temp. | 72-83 | [15] |
| 3 | ZnCl2 | 5 min. | M. W. | 75-90 | [16] |
| 4 | AlCl3/CS2 | 6 h | Room Temp. | 91 | [17] |
| 5 | BF3 | 3 h | Room Temp. | 61 | [18] |
| 6 | KOH/TEBA/C2H5OH | 24 h | 30°C | 71-92 | [19] |
| 7 | Mg-Al-Ot Bu | 18 h | Reflux | 77-93 | [20] |
| 8 | NaNO3/CH3OH | 16 h to 48 h | Room Temp. | 40-98 | [20] |
| 9 | KOH/C2H5OH | 4 min to 300 min | U. Sonication | 52-97 | [21] |
| 10 | KF/Al2O3 | 4 min to 900 min | U. Sonication | 83-98 | [22] |
| 11 | NaOH/C2H5OH | 2 min | M.W. | 85-96 | [23] |
| 12 | Ba(OH)2 grinding | 2 min to 5 min | Room Temp. | 83-92 | [24] |
| 13 | Methane sulphonic acid | 4h | Reflux | 72-90 | In this Paper |
Table 3: Comparison of this method with other reported methods.
Acknowledgement
One of the authors, Ramesh Gawade thankful to Principal of Yashavantrao Chavan Institute of Science, Satara, MH 415001, India for providing necessary infrastructure facility.
References
- Harborne J, MabryT, Mabry H.The Flavonoids. London: Chapmann and Hall; 1975.p: 127.
- Dhar D. The Chemistry of Chalcones and Related Compounds, New York: Wiely and Sons; 1981.
- Powers DG, Casebier DS, Fokas D, Ryan WJ, Troth JR, Coffen DL. Automated Parallel Synthesis of Chalcone-Based Screening Libraries. Tetrahedron. 1998;54:4085-96.
- Rohrmann E, Jones R, Shonle H. The Use of Chalcones in the Synthesis of Medicinal Intermediates. J Am Chem Soc. 1944;66(11):1856-7.
- Bhunia A, Thorat S, Gonnade R. Reaction of N-heterocyclic carbenes with chalcones leading to the synthesis of deoxy-Breslow intermediates in their oxidized form. Chem Commun.2015;51:13690-3.
- Yadav N, Dixit SK, Bhattacharya A, et al. Bhasin Antimalarial activity of newly synthesized chalcone derivatives in vitro. ChemBiolDrug Des. 2012;802:340-7.
- Choudhary AN, Kumar A, Juy V. Design, Synthesis and Evaluation of Chalcone Derivatives as Anti- Inflammatory, Antioxidant and Antiulcer Agents.Lett Drug Des Discov. 2012;9(5):479-88.
- Wu JH, Wang XH, Yi YH, et al.Anti-AIDS agents 54. A potent anti-HIV chalcone and flavonoids from genus Desmos. BioorgMedChemLett. 2003;13(10):1813-5.
- Ishitsuka H, Ninomiya YT, Ohsawa C, et al. Direct and specific inactivation of rhinovirus by chalcone Ro 09-0410. Antimicrob Agents Chemother. 1982;22(4):617-21.
- Bo?ic DD, Milenkovic M, Ivkovic B,et al. Antibacterial activity of three newly-synthesized chalcones & synergism with antibiotics against clinical isolates of methicillin-resistant Staphylococcus aureus. IndJ Med Res.2014;140(1):130-7.
- Lin YM, Zhou Y, Flavin MT, et al. Chalcones and flavonoids as anti-tuberculosis agents. BioorgMedChem. 2002;10(8):2795-802.
- Modzelewska A, Pettit C, Achanta G,et al. Anticancer activities of novel chalcone and bis-chalcone derivatives. BioorgMedChem. 2006;14:3491.
- Zhai L, Chen M, Blom J, et al. The antileishmanial activity of novel oxygenated chalcones and their mechanism of action. J Antimicrob Chemother 1999;43(6):793-803.
- Dhar DN, Lal JB. Chalcones. Condensation of Aromatic Aldehydes with Resacetophenone. II. J Org Chem. 1958;23(8):1159-61.
- Varma RS, Kabalka GW, Evans LT, et al. Aldol Condensations on Basic Alumina: The Facile Syntheses of Chalcones and Enones in a Solvent-Free Medium. SynthCommun. 1985;15:279-284.
- Reddy GV, Maitraie D, Narsaiah B,et al. Microwave Assisted Knoevenagel Condensation: A Facile Method for the Synthesis of Chalcones. Synth Commun. 2001;31:2881.
- Calloway NO, GreenLD. Reactions in the Presence of Metallic Halides. I. ß-Unsaturated Ketone Formation as a Side Reaction in Friedel-Crafts Acylations. J Am Chem Soc. 1937;59(5):809-11.
- Breslow DS, Hauser CR. Condensations.1 XI. Condensations of Certain Active Hydrogen Compounds effected by Boron Trifluoride and Aluminum Chloride2. J Am Chem Soc. 1940;62(9):2385-8.
- Sebti S, Solhy A, Tahir R, et al. Calcined sodium nitrate/natural phosphate: an extremely active catalyst for the easy synthesis of chalcones in heterogeneous media. Tetrahedron Lett. 2001;42(45):7953-5.
- Kantam ML, Prakash VB, Reddy CV.Efficient synthesis of chalcones by a solid base catalyst. Synth Commun. 2005;35:1971.
- Fuentes A, Marinas JM, Sinisterra JV.Catalyzed Synthesis of Chalcones Under Interfacial Solid-Liquid Conditions with Ultrasound. Tetrahedron Lett. 1987;28:4541.
- Li JT, Yang WZ, Wang SX. Improved synthesis of chalcones under ultrasound irradiation. Ultrason Sonochem. 2002;9:237.
- Gupta R, Gupta AK, Paul S. Indian J Chem. 1995;34B:61.
- Kumar S, Lamba MS, Makrandi JK.An efficient green procedure for the synthesis of chalcones using C-200 as solid support under grinding conditions. Green ChemLettRev2008;1:123.
- Kulkarni P. Methane Sulphonic Acid is Green Catalyst in Organic Synthesis. OrientJChem2015;31(1):447-51.
- Kulkarni P. Calcium Oxide Catalyzed Synthesis of Chalcone Under Microwave Condition. CurrMicrowave Chem. 2015;2(2):144-9.
- Albogami AS, Karama U, Mousa AA. SimpleandEfficientOneStepSynthesisofFunctionalizedFlavanonesandChalcones. OrienJChem. 2012;28(2):619-26.
- Basic J, Kalinic M, Ivkovic B, Eric S, Milenkovic M, Vladimirov S, et al. Synthesis, QSAR analysis and mechanism of antybacterial activity of simple 2'-hydroxy chalcones. DigJNanomaterBios. 2014;9:1537-46.
- Singh S, Sharma PK, Kumar N, et al.Anti-oxidant Activity of 2-hydroxyacetophenone Chalcone.JAdvSciRes. 2011;2(3):37-41.
- Vatkar BS, Pratapwar AS, Tapas AR, et al. Synthesis and Antimicrobial Activity of Some Flavanone Derivatives. IntJ ChemTech Res. 2010;2(1): 504-8.


