Short communication
, Volume: 14( 3)Facile Synthesis of E and Z Isomers by the Propyloxime Form
- *Correspondence:
- Guofan Jin , School of Pharmacy, Jiangsu University, Zhenjiang, 212013, P. R. China, Tel: +86-511-85038451; E-Mail: organicboron@ujs.edu.cn
Received: September 09, 2018; Accepted: September 25, 2018; Published: October 05, 2018
Citation: Dawei He, Aolin He, Guofan Jin, et al. Facile Synthesis of E and Z Isomers by the Propyloxime Form. Org Chem Ind J. 2018;14(3):129
Abstract
Keywords
Celosia; Drought stress; DUF538; Gene expression; Real-Time PCR
Introduction
Methyl-(E-Z)-2-(2-(2,5-dimethyl-4-(E-Z)-1-(propoxyimino)ethyl)phenoxy)methyl)phenyl)-3-methoxyacrylate was synthesized using different pathways, called A and B. Through a clever synthetic method, the high selective oxime form of E- and Z- isomers were obtained using methods A and B, respectively, with corresponding yields of 96% and 94%.
E-Z isomers often occur in the chemical industry, materials, and pharmaceutical manufacturing fields but it is difficult to separate E and Z isomers. In this study, the E-Z isomers of oxime derivatives could be synthesized selectively using a different pathway involving two methods.
Experimental
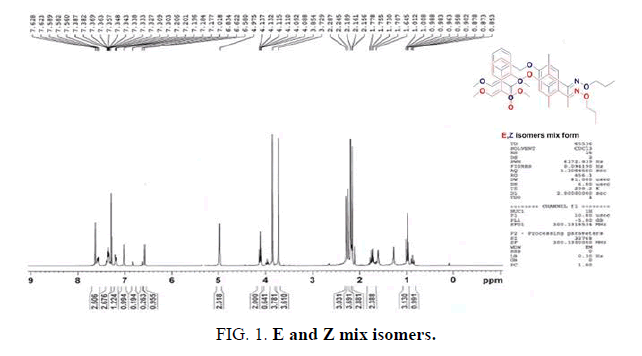
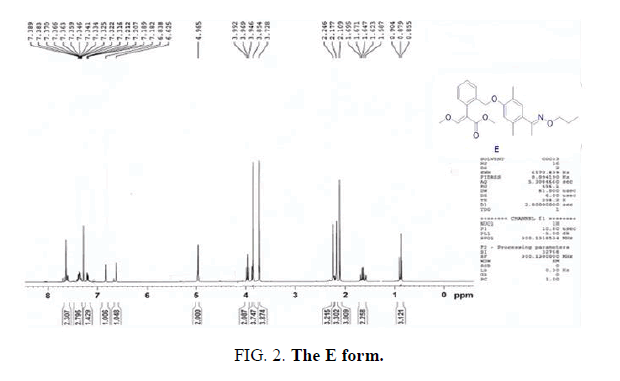
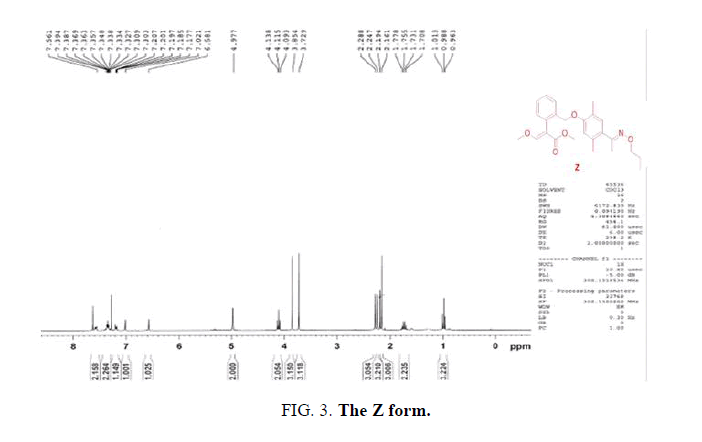
All manipulations were performed under a dry nitrogen atmosphere using standard Schlenk techniques. Acetonitrile (ACN) and methanol was purchased from Aladdin Pure Chemical Company. The reactions were monitored on Merck F-254 pre-coated TLC plastic sheets using hexane as the mobile phase. All yields refer to the isolated yields of the products after column chromatography using silica gel (200-230 mesh). All glassware, syringes, magnetic stirring bars, and needles were dried overnight in a convection oven. The 1H-NMR spectra were recorded on a Bruker 300 spectrometer operated and the chemical shifts were measured relative to the internal residual peaks from the lock solvent (99.9% CDCl3), and then referenced to Si(CH3)4 (0.00 ppm). The elemental analyses were performed using a Carlo Erba Instruments CHNS-O EA1108 analyzer (FIG. 1-3).
Results and Discussion
This paper reports the synthesis of the methyl-2-(2-(bromomethyl) phenyl)-3-methoxyacrylate moiety, such as 2,6-dimethylphenol, hydroxylamine, and 1-bromopropane, on carbon-oxygen or carbon-nitrogen combined with chemical bonding. A higher selective isomer structure of these compounds was obtained using methods A and B.
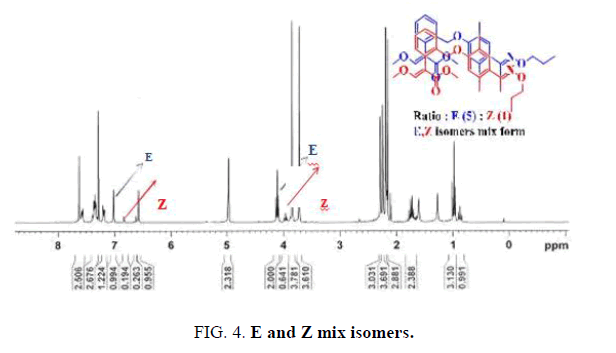
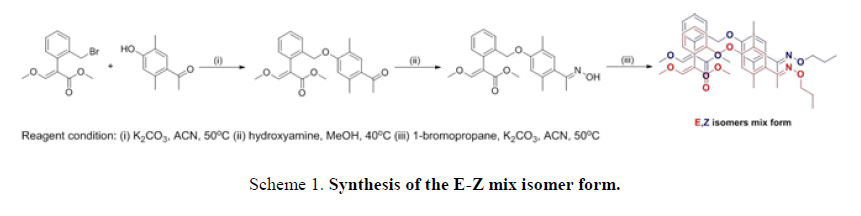
general procedure for the preparation of methyl-2-(2-(2,5-dimethyl-4-(E)-1-(propoxyimino)ethyl)phenoxy)methyl)phenyl)-3-methox yacrylate consisted of a serial reaction, such as electrophilic substitution, Friedel-Crafts, and amination under basic conditions to produce a mixture product (Scheme 1 and FIG. 4)[1-6].
The mixed isomer was obtained by a conventional method, and the E-Z isomer ratio was 5:1 according to the 1H-NMR spectrum, as shown in FIG. 4. The following reports the detail steps to solve the above problems using a different method (Scheme 2 and Scheme 3).
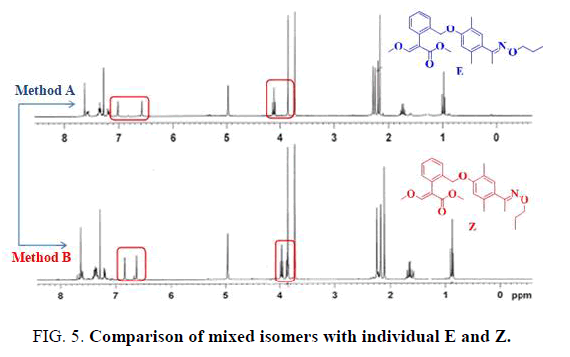
The E and Z target compounds were synthesized separately through a clever design route. 1H-NMR spectroscopy revealed values between the two single peaks of the aromatic ring in the range of 7.0-6.5 ppm with a triplet and single peak in the range of 4.4-3.8 ppm. Different degrees of the E and Z isomers were obtained in moderate yields (E 96% and Z 94%), as shown in FIG. 5.
In summary, the E and Z isomers of oxime derivatives were synthesized using a simple synthetic route considering the structural properties, feasibility, and analytical properties of the target compounds. The knowledge gained from the modified synthetic route will facilitate final product selection and evaluations.
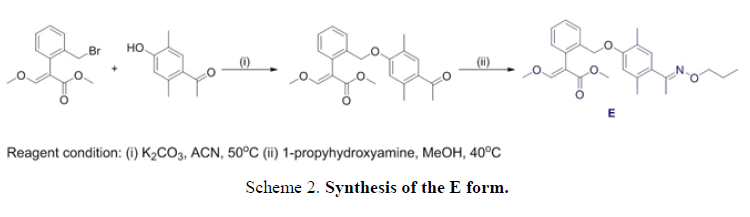
Method A
Synthesis of methyl-2-(2-(2,5-dimethyl-4-(E)-1-(propoxyimino)ethyl)phenoxy)methyl)phenyl)-3-methoxyacrylate (monomer E). A solution of methyl 2-(2-((4-acetyl-2,5-dimethylphenoxy)methyl)phenyl)-3-methoxyacrylate (1.6 g, 4.3 mmol) and O-propylhydroxylamine (0.4 g, 5.2 mmol) in 15 mL of methanol was stirred at room temperature for 30 min, and then heated under 40°C for 3 h. The crude product was then concentrated, and the residue was purified by flash column chromatography (ethylacetate/hexane 1:6) to give monomer E: Yield: 1.8 g (96 %). 1HNMR (CD3Cl), δ, ppm: 7.61 (s, 1H), 7.59-7.54 (m, 1H), 7.39-7.33(m, 2H), 7.20-7.18 (m, 1H), 7.02 (s, 1H), 6.58 (s, 1H), 4.97 (s, 2H), 4.13-4.09 (t, J=6.9 Hz, 2H), 3.85 (s, 3H), 3.72 (s, 3H), 2.28 (s, 3H), 2.24 (s, 3H), 2.19 (s, 3H), 1.77-1.70 (m, 2H), 1.01-0.96 (t, J=7.5 Hz, 2H). Found, %: C70.35; H 7.40; N 3.43. C25H31NO5. Calculated, %: C 70.33; H 7.38; N 3.41.
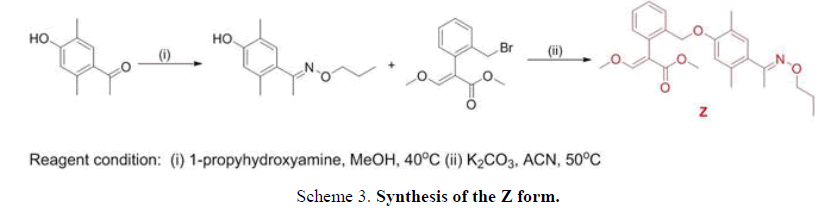
Method B
Synthesis of methyl-2-(2-(2,5-dimethyl-4-(Z)-1-(propoxyimino)ethyl)phenoxy)methyl)phenyl)-3-methoxyacrylate (monomer Z). A solution of methyl-2-(2-(bromomethyl)phenyl)-3-methoxyacrylate (2.3 g, 8.1 mmol) and potassium carbonate (1.3 g, 8.9 mmol) in 20 mL of acetonitrile was stirred at room temperature for 30 min. Subsequently, 1-(4-hydroxy-2,5-dimethylphenyl)ethan-1-one O-propyloxime (3.6 g, 8.5 mmol) was added at room temperature, and then heated under 50°C for 5 h. The crude product was then concentrated, and the residue was purified by flash column chromatography (ethylacetate/hexane 1:6) to give monomer Z: Yield: 2.8 g (94 %). 1HNMR (CD3Cl), δ, ppm: 7.68-7.59 (m, 1H), 7.62 (s, 1H), 7.38-7.31(m, 2H), 7.21-7.18 (m, 1H), 6.83 (s, 1H), 6.62 (s, 1H), 4.96 (s, 2H), 3.99-3.94 (t, J= 6.9 Hz, 2H), 3.85 (s, 3H), 3.72 (s, 3H), 2.24 (s, 3H), 2.17 (s, 3H), 2.10 (s, 3H), 1.69-1.58 (m, 2H), 0.90-0.85 (t, J= 7.5 Hz, 2H). Found, %: C70.36; H 7.42; N 3.47. C25H31NO5. Calculated, %: C 70.31; H 7.36; N 3.42.
Highlights
1. Difficult to separate E and Z isomers
2. Different pathways, A and B
3. Through a clever synthetic method
4. Their highly selective oxime form
5. Individual E and Z
Graphic Abstract
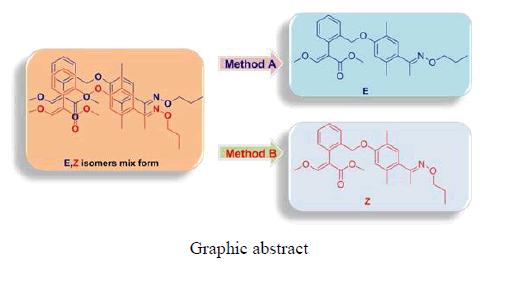
This paper reports the synthesis of the E- and Z-isomers of methyl-(E-Z)-2-(2-(2,5-dimethyl-4-(E-Z)-1-(propoxyimino)ethyl)phenoxy)methyl)phenyl)-3-methoxyacrylate to study their highly selective oxime form.
Acknowledgment
This study was supported by a Grant (No. 5501290005) from the scientific research foundation of Jiangsu University.
References
- Fuse S, Otake Y, Mifune Y, et al. A Facile Preparation of a-Aryl Carboxylic Acid via One-Flow Arndt?Eistert Synthesis. Aust J Chem. 2015;68:1657-61.
- Cvijetic IN, Tanç M, Juranic IO, et al. 5-Aryl-1H-pyrazole-3-carboxylic acids as selective inhibitors of human carbonic anhydrases IX and XII. Bioorg Med Chem. 2015;23:4649-59.
- Bezlada A, Szewczyk M, Mlynarski J. Enantioselective hydrosilylation of imines catalyzed by chiral zinc acetate complexes. J Org Chem. 2015;81:336-42.
- Sathyanarayana P, Ravi O, Muktapuram PR, et al. Copper catalyzed oxygen assisted C (CNOH)?C (alkyl) bond cleavage: a facile conversion of aryl/aralkyl/vinyl ketones to aromatic acids. Org Biomol Chem. 2015;13:9681-5.
- Wang HY, Mueller DS, Sachwani RM, et al. Carbon-carbon bond formation and pyrrole synthesis via the [3,3] sigmatropic rearrangement of o-vinyl oxime ethers. Org Lett. 2010;12:2290-3.
- Blake, JA, Ingold KU, Lin SQ, et al. Org Bio Chem. 2004;2:415-20.