Editorial
, Volume: 15( 1) DOI: 10.37532/0974-7435.2019.15(1).186Efficient One-Pot Three-Component Mannich Reaction for the Synthesis of Barbituric Acid
- *Correspondence:
- S Ravichandran Lovely Professional University, Phagwara, Punjab, India, E-Mail: ravichanduru@yahoo.com
Received: January 04, 2019; Accepted: January 29, 2019; Published: January 31, 2019
Citation: Ravichandran S, Murugesan C. Efficient One-Pot Three Component Mannich Reaction for the Synthesis of Barbituric Acid. Biotechnol Ind J. 2019;15(1):186.
Abstract
An environmentally friendly organic synthesis of novel Barbituric acid using SnCl2.2H2O under microwave irradiation herein described. The reaction time is very short with reasonable yield enhancement. Further the role of SnCl2.2H2O is studied in the reaction under microwave irradiation and concluded that microwave assisted SnCl2.2H2O catalyzed reaction is the best in terms of catalysis as well as reaction yield improvement.
Keywords
Barbituric acid; Microwave heating; Mannich base
Introduction
Microwave-assisted Organic Reaction Enhancement (MORE) reactions are extremely fast, cleaner than conventional reactions and lead to higher atom economy (less chemical waste) [1-8]. Because of the short time requirement, ease of workability and environmentally friendliness, microwaves provide an alternative green approach to conventional methods. It can be termed as ‘e-chemistry’ because it is easy, effective, economical and eco-friendly and is believed to be the next step towards Green Chemistry for the attainment of sustainable development [4-22].
Over the past few decades, Mannich bases of heterocyclic molecules have been grabbing the attention of the synthetic organic chemists for their wide applications of biological activities. The manic reaction is widely used for the construction of a nitrogen-containing molecule. In this three component transformation, compounds possessing β-hydrogen atom, an aldehyde, and an amine react to form β-aminoketone derivative. Recently, the use of inorganic solid supports as catalysts has been developed for solvent-free reactions resulting in milder conditions and simple experimental procedures [4]. SnCl2.2H2O catalyzed organic reactions are gaining importance due to their inexpensive nature and special catalytic attributes in heterogeneous reactions for the preparations of heterocyclic molecules. Several Mannich bases have diverse biological activities. In continuation of our research work [5-9], we herein developed a new synthetic procedure for the synthesis of Barbituric acid catalyzed by SnCl2.2H2O [22]. The synthesized compounds have been characterized by TLC, Elemental analysis, IR and 1H-NMR Spectroscopy.
Results and Discussion
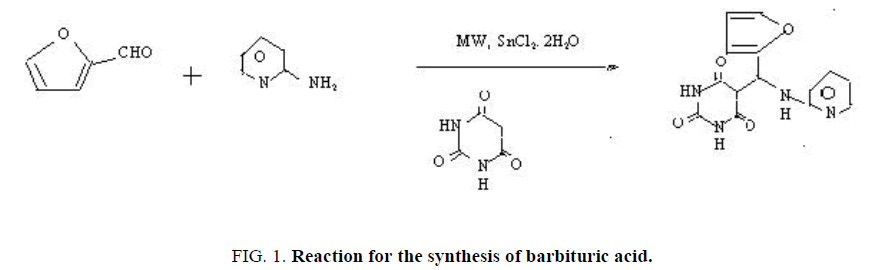
Condensation of furfural, 2-aminopyridine with uracil in the presence of SnCl2.2H2O under microwave affording the desired product in reasonable yield (FIG. 1).
Experimental
Synthesis of barbituric acid
In the preparation of Barbituric acid, an acidic SnCl2.2H2O was added to the equimolar (0.01 mole) mixture of furfural, 2-aminopyridine with uracil and the reaction mixture bath placed inside the microwave oven (Samsung model at 1050 W, 70% of total power) and irradiated for 5 minutes. Now, the progress of the reaction was carefully monitored through TLC. On completion of the reaction, the reaction mixture was allowed to cooled at room temperature and rendered basic (pH 8) with 10% NaHCO3 and then extracted with ethyl acetate. The organic layer was washed with brine, dried over anhydrous Na2SO4 and evaporated to leave behind the crude product, which was further purified by column chromatography over silica gel (hexane: ethyl acetate 4:1). Satisfactory Elemental analysis, IR, NMR spectral data are obtained. Yield: 68% Mp 290-292°C.
1H NMR (CDCl3+DMSO) δ8.1-8.5 (m, 5H, C-H-pyridine), 4.5-4.8 (d, 1H), 5.0 (brs, 1H, NH), 6.2-6.4 (m, 3H, C-H furan ring), 3.0 (d, 1H, C-H).
IR (KBr): 1030 (C=O), 1600 (C=C), 1650 (C=O), 2960 (C-H), 3050 (Py C-H), 3390 (N-H) cm-1 Anal. Calculated for C14H11N4O4: C 53.33, H 3.49, N 17.77. Found: C 53.35, H 3.48, N 17.79.
Conclusion
We have developed a facile, convenient and efficient synthetic procedure for the synthesis of Barbituric acid. The reported procedure clearly highlights the versatility of solid supports when coupled with microwaves. In conclusion, this condensation method offers an alternative route for the novel synthesis of Mannich reaction in reasonable yield making the process more simple and economic than any other conventional methods.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mogilaiah K, Reddy GR. Microwave-assisted solvent-free synthesis of trans-cinnamic acids using lithium chloride as a catalyst. Synth Commun. 2004;34(2):205-10.
- Buszek KR, Brown N. N-Vinylpyridinium and-ammonium tetrafluoroborate salts: new electrophilic coupling partners for Pd (0)-catalyzed Suzuki cross-coupling reactions. Org Lett. 2007;9(4):707-10.
- Andersson H, Almqvist F, Olsson R. Synthesis of 2-substituted pyridines via a regiospecific alkylation, alkynylation, and arylation of pyridine N-oxides. Org Lett. 2007;9(7):1335-37.
- Bram G, Loupy A, Villemin D. In solid supports and catalysts in organic synthesis. K. Smith. 1992;12.
- Ravichandran S, Subramani K, Arun Kumar R. Microwave-assisted solvent free Friedlander synthesis of 1, 8-naphthyridines. Int J Chem Sci. 2009;7(2):993.
- Raman N, Ravichandran S. Synthesis and structural characterisation of some transition metal complexes of piperidinobenzyl semicarbazide and their antibacterial study. Int J Chem Sci. 2004;2:489.
- Emelda AR, Jeyachandramani N, Ravichandran S. Synthesis, characterization and antimicrobial activity of Cu(II), Co(II), Ni(II) and Zn(II) complexes derived from a new mannich base, n-(1-morpholinobenzyl)benzamide and 1,2-diaminobenzene. Asian J Chem. 2008;20(1):337.
- Raman N, Ravichandran S. New neutral schiff base and its metal complexes derived from mannich base, n-(1-morpholinobenzyl) acetamideraman, N. Polish J Chem. 2005;79(7):1107-14.
- Raman N, Ravichandran S. Synthesis and characterization of a new Schiff base and its metal complexes derived from the mannich base, N-(1-piperidinobenzyl) acetamide. Synth React Inorg M. 2005;35(6):439-44.
- Shirodkar PY, Vartak MM. Synthesis biological and qsar evaluation of mannich bases of 6-nitro-quinazolones. Ind J Heterocyclic Chem. 2000;9(3):239-40.
- Varma R. Solvent-free organic syntheses using supported reagents and microwave irradiation. Green Chem. 1999;1(1):43-55.
- Kidwai M, Venkataramanan R, Garg RK, et al. Novel one pot synthesis of new pyranopyrimidines using microwaves. J Chem Res. 2000;12:586-87.
- Kidwai M, Misra P. Ring closure reactions of chalcones using microwave technology. Syn Comm. 1999;29(18):3237-50.
- Kidwai M, Misra P, Bhushan KR. Alumina-supported synthesis of thiadiazolyl thiazolothiones. Syn Comm. 2001;31(6):817-22.
- Kidwai M, Sapra P, Bhushan KR, et al. Microwave-assisted solid-support synthesis of pyrazolino/iminopyrimidino/thioxopyrimidino imidazolines. Synthesis. 2001;10:1509-12.
- Li TS, Li AX. Montmorillonite clay catalysis part 10.1 K-10 and KSF-catalysed acylation of alcohols, phenols, thiols and amines: scope and limitation. J Chem Society. 1998;12:1913-18.
- Ramchandani PK, Upnade BS, Vinod MP, et al. Pd-Cu-exchanged montmorillonite K10 clay: an efficient and reusable heterogeneous catalyst for vinylation of aryl halides Chem. Commun. 1997;21:2071-72.
- Varma RS, Saini RK. Solid state dethioacetalization using clayfen. Tetrahedron Lett. 1997;38(15):2623-24.
- Villemin D, Alloum AB. Dry reaction under microwave: condensation of sulfones with aldehydes on KF-alumina. Syn Comm. 1991;21(1):63-68.
- Kidwai M. Dry media reactions. Pure Appl Chem. 2001;73(1):147-51.
- Kidwai M, Sapra P. An efficient synthesis of benzopyranopyrimidines using inorganic solid support. Syn Comm. 2002;32(11):1639-45.
- Prakash M, Krishnaswamy G, Ravi D, et al. Synthesis and characterisation of novel knoevenagel condensation product of naphthofuran-2-carbaldehyde with barbituric acid and ethylcyanoacetate. J Chem Pharm Res. 2018;10(5):157-62.