Research
, Volume: 15( 2) DOI: 10.37532/0974-7435.2019.15(2).182Development and Validation Method for the Determination of Sildenafil Citrate Tablets by using UV-Spectrophotometer in Pharmaceutical Formulation
- *Correspondence:
- Alrasheed AW Mohammed , Central Laboratory, Shambat Campus, Khartoum University, Sudan, E-Mail: vocard@hotmail.com
Received: November 28, 2018; Accepted: December 10, 2018; Published: April 22, 2019
Citation: Mohammed AAW, Mohammed SE, Boshra HS, et al. Development and Validation Method for the Determination of Sildenafil Citrate Tablets by using UV-Spectrophotometer in Pharmaceutical Formulation. Biotechnol Ind J. 2019;15(2):182.
Abstract
The objective of this paper is to describe the optimization, validation, and application of spectrophotometric technique for determination of Sildenafil Citrate in their pharmaceutical formulation (tablets). In this paper, a simple, rapid, accurate and sensitive spectrophotometric method has been developed and validated. The method is a direct spectrophotometric method based on thawed of sildenafil citrate in diluted hydrochloric acid. The maximum absorption wavelength for the determination of Sildenafil citrate was found to be 295 nm. Under the optimized condition, Beer’s law was obeyed in the concentration range from 5.0-40.0 μg/ml.
Introduction
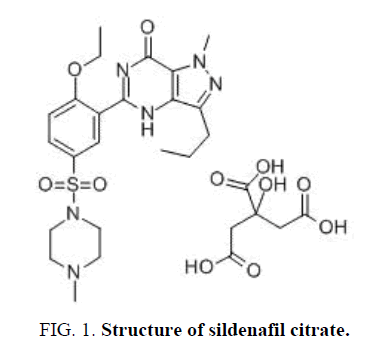
Sildenafil (SC) is a compound of the Pyrazolo-pyrimidinyl-methylpiperazine class. It is 5-[2-ethoxy-5-(4-methyl
piperazin-1-yl sulphonyl) phenyl] -1-Methyl-3-propyl-1, 6-dihydro-7Hpyrazolo [4,3-d] pyrimidin-7-one, having empirical
formula C22H30N6O4S and molecular Weight 661.71 [1]. Sildenafil Citrate is a white to off-white crystalline powder [2]. It is used not only for erectile dysfunction treatment but also for pulmonary arterial hypertension [3].
In healthy volunteers, sildenafil is extensively absorbed; however, rapid first-pass metabolism limits the absolute bioavailability to approximately 40%. Cmax is reached within 30-120 minutes of oral administration in the fasted state, and the terminal half-life is about 4 hours [4].
The most common side effects of Sildenafil citrate are a headache, facial flushing, and upset stomach. [5].
Its structural formula is given in FIG. 1.
Sildenafil citrate, a specific phosphodiesterase-5 inhibitor [6], is increasingly used for pulmonary hypertension in pregnancy [7]. Sildenafil Citrate is also emerging as a potential candidate for the treatment of intra-uterine growth retardation and for premature labor [8]. Its effects in the fetoplacental circulation are not known [9]. Our objectives were to determine whether phosphodiesterase-5 is present in the human fetoplacental circulation and to characterize the effects and mechanisms of action of Sildenafil Citrate in this circulation [10,11].
Validation is defined as finding or testing the truth of something. The objective of validation of an analytical procedure is to demonstrate that it is suitable for its intended purpose [12].
Analytical methods need to be validated, verified, or revalidated in the following instances:
• Before initial use in routine testing
• When transferred to another laboratory
• Whenever the conditions or method parameters for which the method has been validated change (for example, an instrument with different characteristics or samples with a different matrix) and the change is outside the original scope of the method [12]
Materials and Methods
Collection of the sample
All pharmaceutical formulation and samples were taken from local markets.
Reagents and solutions
All chemicals used are Analytical Grade (AG), and spectroscopic organic solvents. Deionized water was used for preparing solutions. Sildenafil citrate working standard, (purity 99.57%) was purchased from Rakshit Drugs Private Limited. Hydrochloric Acid (SDFCL, India), sp.gr:1.18 g/ml, percentage: 37.5% Hydrochloric acid 0.1 M.
Instrumentation
The following instruments, equipment or apparatus were used during the course of this work.
UV-Visible Spectrophotometer model UV-1800 (SHIMADZU, KYOTO, JAPAN) equipped with 1 cm quartz cell, was used in the quantitative analysis of assay, the absorption spectra of test and reference solution were recorded in 1 cm quartz cells at many λ max. FTIR (Fourier Transform Infra-Red spectrophotometer), (SHIMADZU, KYOTO, JAPAN), Model FTIR-8400s. Electronic Sensitive balance (SHIMADZU, KYOTO, JAPAN), Type A × 120, Capacity 120 g, Readability 0.1 mg.
Preparation of 0.1M hydrochloric acid solution
8.0 ml of concentrated hydrochloric acid was taken accurately and transferred to 1000 ml volumetric flask containing about 300 ml of deionized water, mixed well, cooled, and completed the volume to the mark with the same diluent and mixed.
Solubility studies of Sildenafil Citrate for UV-Spectrophotometer analysis: Solubility of SIL was determined at (28 ± 2)°C. An excess amount of the drug was taken into 25 ml volumetric flasks each containing different concentrations of diluted hydrochloric acid such as (0.01, 0.1, 0.5, 1.0) M , suitable solvent for sildenafil citrate (SIL) chosen (0.1 M HCl) according to solubility of drug and high intensity (absorbance) of the drug.
Determination of maximum absorption
Determination of the wavelength of maximum absorption (λ max) of sildenafil citrate: 10 mg of Sildenafil citrate working standard was accurately weighed and transferred to a 100 ml volumetric flask, 5.0 ml of 0.1N HCl was added to the weight and sonicated for 2 minutes for dissolution. The solution was kept aside to cool and completed to the mark with the same solvent to give a 100 μg/ml stock solution which was diluted suitably to produce (0.6, 1.0, 10.0, 25.0 and 50.0) μg/ml of SILC. This solution was scanned in the spectrum mode from 200-800 nm. From the spectrum of the drug obtained λ max of SIL was determined at 295 nm.
Determination of the wavelength of maximum absorption (λ max) of placebo of sildenafil citrate: From the total weight of tablet placebo PLC composition 274.83 mg accurately weighed 10.0 mg of PLC Placebo of Sildenafil Citrate tablets compositions and transferred to 100 ml volumetric flask, minimum amount (about 5.0 ml) of 0.1N HCl was added to the weight of PLC and dissolved by sonication for 2.0 minute for dissolution, cooled in room temperature and completed to the mark with the same solvent to give 100 μg/ml stock solution which was diluted suitably to produce (5.0, 10.0, 25.0 and 50) μg/ml of PLC . This solution was scanned in the spectrum mode from 200-800 nm. From the spectrum of the PLC composition, there is no spectrum obtained in the λ max of Sildenafil Citrate drug 271 nm (FIG. 2).
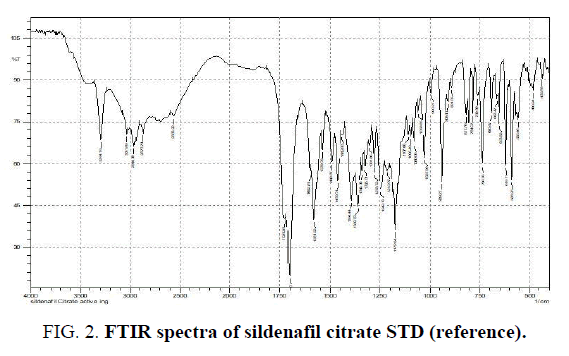
Determination of the wavelength of maximum absorption (λ max) of 0.1N HCl (Sildenafil Citrate solvent): 3.5 ml of 0.1N HCl scanned in the UV-Spectrophotometer in the range of 200-800 nm, the maximum absorption wavelength of the diluted acid was found to be 208 nm (FIG. 3).
Preparation of standard stock solution of Sildenafil Citrate for UV-spectrophotometer analysis
50 mg of sildenafil citrate working standard (99.57% purity) was accurately weighed and dissolved in 0.1M HCl, sonicated for 60 seconds for dissolution, transferred quantitatively into 50 ml volumetric flask, completed the volume to the mark with the same solvent and mixed well, 1.0 ml of this solution was transferred to 100 ml volumetric flask, completed to the mark with 0.1M HCl to get (15,20,25,30,35,40,105) μg/ml and absorbance was measured at 295 nm.
Preparation of sample solution of sildenafil citrate for UV-spectrophotometer analysis
Equivalent to 50 mg of Sildenafil citrate from 20 tablets were finely powdered Sildenafil citrate tablets drugs and weighted, dissolved in 0.1M HCl, sonicated for 60 seconds for dissolution, then transferred quantitatively into 100 ml volumetric flask and completed the volume to the mark with the same solvent, mixed well and filtered through Whatman filter paper No. 41. 1 ml of this solution was transferred to 100 ml volumetric flask, diluted to the mark with 0.1M HCl to get 5.0 μg/ml and measured the absorbance at 295 nm.
Results
Method development
The method is a direct spectrophotometric method based on thawed of sildenafil citrate in diluted hydrochloric acid.
Identification of sildenafil citrate STD
The below spectral of Sildenafil Citrate was found to exhibit characteristics absorption bands at 3240 cm-1, 1627 cm-1, 1620 cm-1, 1180 cm-1, 1100 cm-1, 3600 cm-1, 828 cm-1, showing N-H, C=O, C=C, C-O, C-N, O-H, and aromatic substitution bands respectively for Sildenafil Citrate.
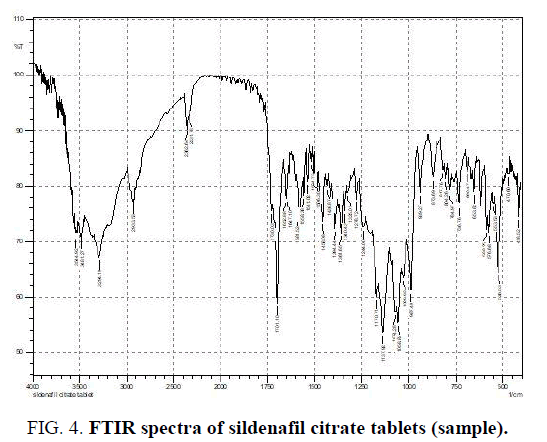
The Infrared (IR) absorption spectrum of SIL tablets was generated using a Spectrum 100 Fourier transform-infrared attenuated total reflectance spectrometer (SHIMADZU 8400s). The spectrum, obtained at a wave-number range of 4000-418 cm-1 and a resolution of 4 cm-1 is described in FIG. 4. Band assignments for the resultant spectrum are summarized in TABLE 1 below [13].
| No. | Functional group(s) | Characteristic Absorption(s) range (cm-1) | Identified in this study (cm-1) |
|---|---|---|---|
| 1 | SO2 stretch | 1200-1100 | 1172.74 |
| 2 | Aromatic C=C bond | 1700-1500 | 1582.62 |
| 3 | C=O stretch | 1750-1680 | 1702.21 |
| 4 | Saturated C-H stretch | 2950-2850 | 2962.71 |
| 5 | Unsaturated C-H stretch | 3100-3010 | 3029.26 |
| 6 | Secondary N-H stretch | 3500-3300 | 3299.30 |
| 7 | O-H stretch | 3550-3200 | 3617.00 |
Table 1: Distribution of underreporting bullying experiences among students? demographical variables.
Method
For validation of analytical methods, the guidelines of the International Conference on the Harmonization (ICH) of Technical Requirements for the Registration of Pharmaceuticals for Human Use have recommended the accomplishment of accuracy tests, precision, specificity, the linearity of the method. ICH of Technical Requirements for the Registration of Pharmaceutical for Human Use (ICH) Q2B, 1996, validation of analytical procedures and methodology [12].
Determination of maximum absorption
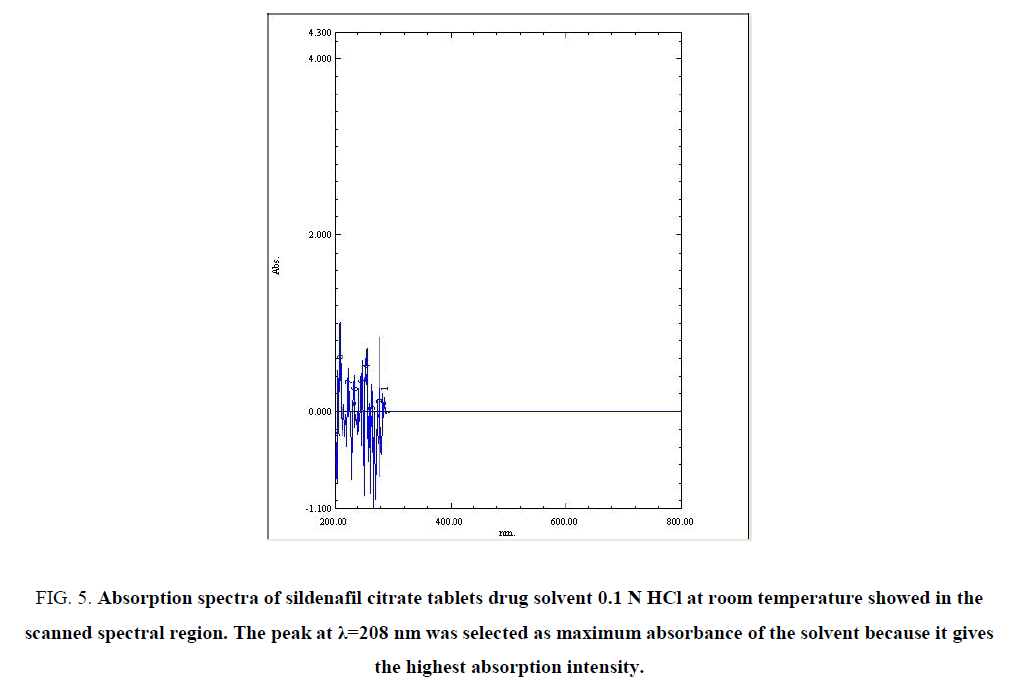
Maximum absorption spectra of drug solvent (0.1N HCl): The absorption spectra of the SILC solvent (0.1 N HCl) which shown in FIG. 5 shows maximum absorbance of 0.1 N HCl at λ=208 nm, this maximum absorbance show there is no any interference between the excipients of the drug and active ingredient of the drug under study in the scanned spectral peaks region, while the absorption spectrum of diluted hydrochloric acid (0.1 N) shows no absorption. The peak at λ=208 nm was selected at maximum lambda for the solvent because it gives the highest absorption intensity, as indicated by the ε values (TABLE 2).
Figure 5: Absorption spectra of sildenafil citrate tablets drug solvent 0.1 N HCl at room temperature showed in the scanned spectral region. The peak at λ=208 nm was selected as maximum absorbance of the solvent because it gives the highest absorption intensity.
| NO. | Wavelength (nm) | Absorbance |
|---|---|---|
| 1 | 286.00 | 0.032 |
| 2 | 276.00 | -0.102 |
| 3 | 262.00 | -0.180 |
| 4 | 254.00 | 0.292 |
| 5 | 246.00 | 0.112 |
| 6 | 233.00 | 0.030 |
| 7 | 223.00 | 0.107 |
| 8 | 208.00 | 0.395 |
| 9 | 201.00 | -0.477 |
| 10 | 280.00 | -0.272 |
| 11 | 268.00 | -0.493 |
| 12 | 260.00 | -0.217 |
| 13 | 249.00 | 0.001 |
| 14 | 238.00 | -0.085 |
| 15 | 229.00 | -0.208 |
| 16 | 217.00 | -0.112 |
| 17 | 214.00 | -0.096 |
| 18 | 203.00 | -0.803 |
Table 2: The values of multi-wavelength absorbance of the drug Solvent (0.1 N HCl) shows many different absorbance values.
Maximum absorption spectra of drug excipients (placebo)
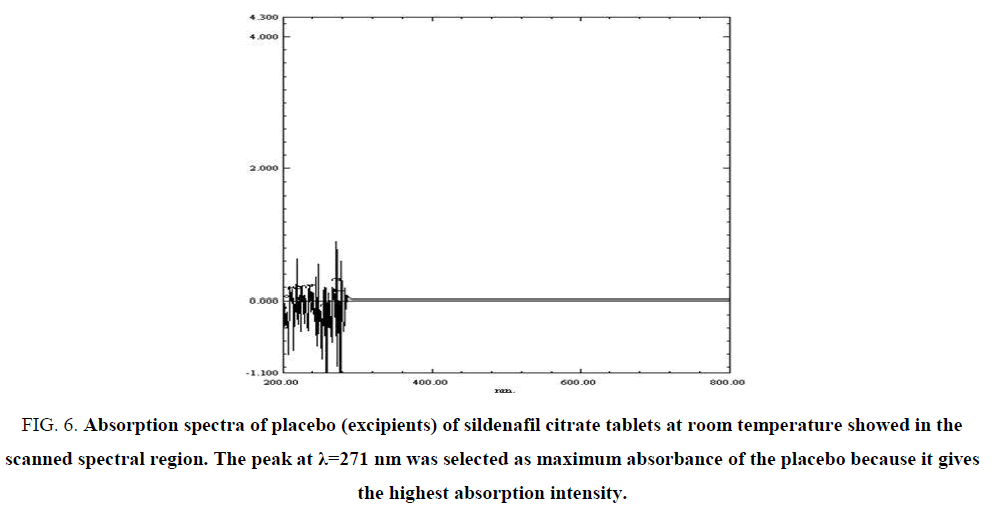
Maximum absorption spectra of the SIL excipient (placebo) in FIG. 6 shows maximum absorbance λ=271 nm, this maximum absorbance show there is no any interference between the excipients of the drug and active ingredient of the drug under study in the scanned spectral peaks region, while the absorption spectrum of placebo shows no absorption. The peak at λ=271 nm was selected at maximum lambda for the placebo because it gives the highest absorption intensity, as indicated by the ε values (TABLE 3)
Figure 6: Absorption spectra of placebo (excipients) of sildenafil citrate tablets at room temperature showed in the scanned spectral region. The peak at λ=271 nm was selected as maximum absorbance of the placebo because it gives the highest absorption intensity.
| NO. | Wavelength (nm) | Absorbance |
|---|---|---|
| 1 | 278.00 | -0.080 |
| 2 | 271.00 | 0.096 |
| 3 | 255.00 | -0.281 |
| 4 | 237.00 | 0.013 |
| 5 | 226.00 | -0.010 |
| 6 | 218.00 | -0.029 |
| 7 | 211.00 | -0.032 |
| 8 | 201.00 | -0.154 |
| 9 | 282.00 | -0.108 |
| 10 | 276.00 | -0.217 |
| 11 | 258.00 | -0.353 |
| 12 | 252.00 | -0.355 |
| 13 | 231.00 | -0.137 |
| 14 | 214.00 | -0.164 |
| 15 | 206.00 | -0.331 |
| 16 | 203.00 | -0.366 |
Table 3: Absorbance values of excipient (placebo) of SIL drug.
Maximum absorption spectra of drug standard solution
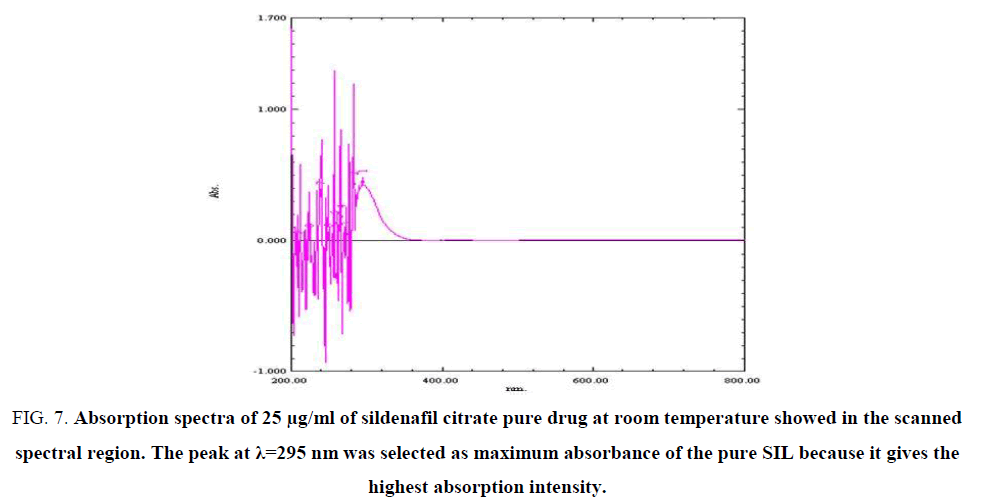
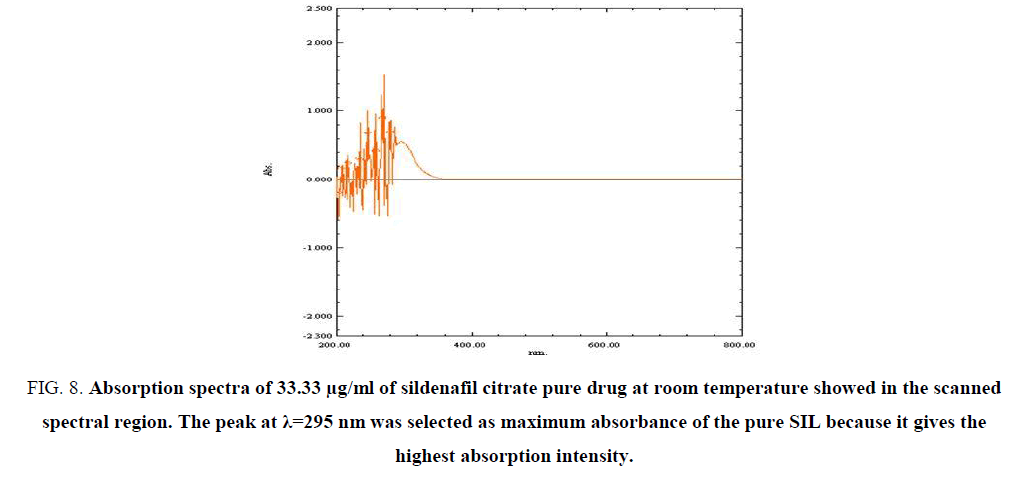
The absorption spectra of the SIL standard in 0.1 N HCl solvent (FIG. 7-9) show maximum absorbance at λ=295 nm, this maximum absorbance for different concentrations: (25, 33.3 and 50) μg/ml, while the absorption spectrum of SIL shows no absorption peaks in the scanned spectral region. The peak at λ=295 nm was selected because it gives the highest absorption intensity, as indicated by the ε values (TABLES 4-6).
Figure 7: Absorption spectra of 25 μg/ml of sildenafil citrate pure drug at room temperature showed in the scanned spectral region. The peak at λ=295 nm was selected as maximum absorbance of the pure SIL because it gives the highest absorption intensity.
Figure 8: Absorption spectra of 33.33 µg/ml of sildenafil citrate pure drug at room temperature showed in the scanned spectral region. The peak at λ=295 nm was selected as maximum absorbance of the pure SIL because it gives the highest absorption intensity.
Figure 9: Absorption spectra of 50 μg/ml of sildenafil citrate pure drug at room temperature showed in the scanned spectral region. The peak at λ=295 nm was selected as maximum absorbance of the pure SIL because it gives the highest absorption intensity.
| No. | Wavelength (nm) | Absorbance |
|---|---|---|
| 1 | 295.00 | 0.418 |
| 2 | 283.00 | 0.402 |
| 3 | 273.00 | 0.02 |
| 4 | 265.00 | 0.152 |
| 5 | 257.00 | 0.103 |
| 6 | 249.00 | 0.004 |
| 7 | 239.00 | 0.332 |
| 8 | 224.00 | 0.004 |
| 9 | 208.00 | -0.047 |
| 10 | 287.00 | 0.326 |
| 11 | 278.00 | -0.02 |
| 12 | 270.00 | -0.072 |
| 13 | 260.00 | -0.022 |
| 14 | 252.00 | -0.105 |
| 15 | 245.00 | -0.255 |
| 16 | 229.00 | -0.224 |
| 17 | 216.00 | -0.22 |
| 18 | 203.00 | -0.728 |
Table 4: The values of multi-wavelength absorbance of pre-drug of SIL 25 µg/ml, 295 nm was selected as maximum absorbance of the pure SIL because it gives the highest absorption intensity.
| No. | Wavelength (nm) | Absorbance |
|---|---|---|
| 1 | 342.00 | 0.044 |
| 2 | 338.00 | 0.061 |
| 3 | 323.00 | 0.173 |
| 4 | 313.00 | 0.33 |
| 5 | 301.00 | 0.516 |
| 6 | 299.00 | 0.537 |
| 7 | 295.00 | 0.562 |
| 8 | 294.00 | 0.558 |
| 9 | 292.00 | 0.538 |
| 10 | 290.00 | 0.501 |
| 11 | 283.00 | 0.119 |
| 12 | 282.00 | -0.079 |
Table 5: The values of multi-wavelength absorbance of the drug of SIL 33.33 µg/ml, 295 nm was selected as maximum absorbance of the pure SIL because it gives the highest absorption intensity.
| S.NO | Wavelength (nm) | Absorbance |
|---|---|---|
| 1 | 340.00 | 0.076 |
| 2 | 331.00 | 0.151 |
| 3 | 326.00 | 0.211 |
| 4 | 798.00 | 0.008 |
| 5 | 315.00 | 0.434 |
| 6 | 310.00 | 0.576 |
| 7 | 294.00 | 0.841 |
| 8 | 299.00 | 0.797 |
| 9 | 298.00 | 0.811 |
| 10 | 295.00 | 0.846 |
| 11 | 292.00 | 0.782 |
| 12 | 289.00 | 0.760 |
Table 6: The values of multi-wavelength absorbance of pre-drug of SIL 50 µg/ml, 295 nm was selected as maximum absorbance of the pure SIL because it gives the highest absorption intensity.
Specificity and selectivity
Specificity: Specificity is the ability to assess unequivocally the analyte in the presence of components that may be expected to be present in the sample matrix for demonstrating the specificity of the method for drug formulation [14]. The excipients used in different formulation products did not interfere with the drug peak and thus, the method is specific sildenafil citrate. To further confirm the specificity of the method, UV scans of the spiked drug were taken in the range 200-400 nm and no significant change was found by comparing the absorbance of pure drug and Spiked drug at the analytical wavelength of the drug.
Sensitivity: Following ICH Q2 (R1) recommendations based on the signal to noise approach the Limit of Detection (LOD) was determined for which a signal height to noise ratio of 3 was obtained and for a Limit of Quantitation (LOQ), a signal height to noise ratio of 10 was obtained.
Linearity, LOD, LOQ, and range
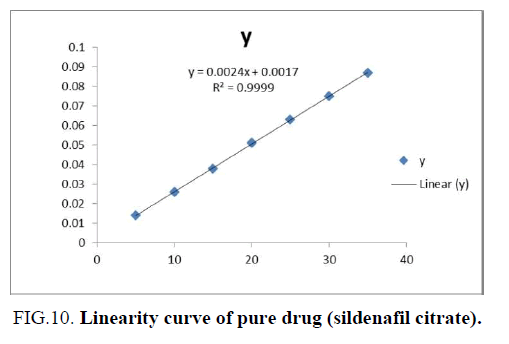
Linearity: The linearity of an analytical method is its ability to select test results that are directly, or by a well-defined mathematical transformation, proportional to the concentration of an analyte in samples within a given range. The linearity of the method was observed within the expected concentration range demonstrating its suitability for analysis. The correlation coefficient (r2) was found to be 0.999 and value of intercept was less than 40 of the response of 100% of the test concentration in all the cases indicating a functional linear relationship between the concentration of the analyte absorbance and sample absorbance (FIG. 10).
LOD: The detection limit (LOD) is the lowest amount of an analyte in a sample that can be detected, but not necessarily quantitated, under the stated experimental conditions. It may be expressed as a concentration that gives absorbance value. The lower limit of detection for sildenafil citrate is 2.40 μg/ml in reference material, formulation and 1.60 μg/ml [15].
LOQ: Limit of Quantitation (LOQ) is the lowest amount analyte in a sample that can be determined with acceptable precision and accuracy under the stated experimental conditions. Absorbance value can be taken as LOQ of the method [15]. The LOQ values were found to be less than 2.0 μg/ml.
** Limit of detection and quantitation are determined via calculations [16]
**LOD=(SD of the response/slope) × 3.3
**LOQ=(SD of the response/slope) × 10
Accuracy: The accuracy of an analytical method is the closeness of test results obtained by that method to the true value. In case of the assay of a drug in a formulated product, accuracy may be determined by application of the analytical method to the drug product components to which known amount of analyte has been added within the range of method. If it is not possible to obtain samples of all drug product components, it may be acceptable to add known quantities of the analyte to the drug product (i.e. to spike). In our studies, the later technique was adopted and sildenafil citrate was spiked in the drug product.
Precision: Precision is the degree of reproducibility or repeatability of the analytical method under normal operating conditions. The method passed the test for repeatability as determined by %RSD of the absorbance values of six replicate measurements at 100% test concentration.
Ruggedness: To determine the ruggedness of the proposed method, the test sample solution was analyzed in six replicates comparing %RSD of the measurements by two analysts in the same laboratory.
Robustness: Robustness tested through changing in solvents, temperature, and oxidizing agents which its effect on absorbance (TABLE 7).
| S.No. | Validation parameter | Atorvastatin calcium |
|---|---|---|
| 1 | Detection wave Length | 295 nm |
| 2 | Beer’s Limit | 5-40 µg/ml |
| 3 | Linearity | 5-40 µg/ml |
| 4 | R2 | 0.9999 |
| 5 | Intercept | 0.0017 |
| 6 | Slope | 0.0024 |
| 7 | LOD | 1.600 µg/ml |
| 8 | LOQ | 1.85 µg/ml |
| 9 | Precision | %RSD<2 |
| 10 | Recovery | 98-102% |
Table 7: Summary of the present study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- El-Gindy AE, Shokry E, Farouk M, et al. Validated methods for determination of sildenafil citrate in the presence of its potential impurities. J Biomed Sci Res. 2010;2:262-78.
- Badwan AA, Nabulsi N Al, Omani M, et al. Unpublished results. The Jordanian pharmaceutical manufacturing company. 2014.
- Escribano P, Jiménez C, Sáenz de la Calzada C. Hipertensión arterial pulmonar en el año 2004. Revista Española de Cardiología. 2005;5:90-103.
- Nichols DJ, Muirhead GJ, Harness JA. Pharmacokinetics of sildenafil after single oral doses in healthy male subjects: absolute bioavailability, food effects and dose proportionality. Br J Clin Pharmacol. 2002;53:5-12.
- https://pdf.hres.ca/dpd_pm/00043302.PDF
- Reddy B, Reddy YR. Validation and stability indicating RP-HPLC method for the determination of sildenafil citrate in pharmaceutical formulations and human plasma. J Chem. 2008;5(2):1117-22.
- Ahn CY, Bae SK, Bae SH, et al. Pharmacokinetics of sildenafil and its metabolite, N-desmethylsildenafil, in rats with liver cirrhosis and diabetes mellitus, alone and in combination. Xenobiotica. 2011;41(2):164-74.
- Terrert NK, Bell AS, Brown D, et al. Sildenafil a potent and selective inhibitor of type 5 cGMP phosphodiesterase with utility for the treatment of male erectile dysfunction. Bioorg Med Chem Lett. 1996;6:1819-24.
- Jaffe A, Chen Y, Kisch ES, et al. Erectile dysfunction in hypertensive subjects: assessment of potential determinants. Hypertension. 1996;28(5):859-62.
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/20895s039s042lbl.pdf
- Walker DK. Pharmacokinetics and metabolism of sildenafil in mouse, rat, rabbit, dog and man. Xenobiotica. 1999;29(3):297-310.
- https://www.fda.gov/downloads/drugs/guidances/ucm073384.pdf
- Maheshwari RK, Bishnoi SR, Kumar D, et al. Quantitative spectrophotometric determination of ornidazole tablet formulations using ibuprofen sodium as hydrotropic solubilizing agent. Digest J Nanomat Biostruc. 2010;5(1).
- www.accessdata.fda.gov/drugsatfda_docs/label/2014/20895s039s042lbl.pdf
- https://www.newhope.com/supply-news-amp-analysis/2006-edition-united-states-pharmacopeia-and-national-formulary- usp-29-nf-24
- Pharmacopeia US. The Official Compendia of Standards (USP-26-2003). InUnited States Pharmacopeial Convention, Rockville, MD 2002.