Original Article
, Volume: 16( 14)Degradation Study of Benalaxyl Fungicide Residues in Different pH Waters Using Ferrous Doped Titanium Dioxide Nanoparticles
Nageswara Rao T1, Benarji Patrudu T2*, Basaveswara Rao MV1 and Apparao K1
1Department of Chemistry, Krishna University, Machilipatnam, Andhra Pradesh, India
2Department of Chemistry, GITAM University, Hyderabad, Telangana, India
- *Correspondence:
- Benarji Patrudu T, Department of Chemistry, GITAM University, Hyderabad, Telangana, India, E-mail: benarjipatrudu@gmail.com
Received: June 01, 2016; Accepted: July 15, 2016; Published: July 25, 2016
Citation: Rao TN, Patrudu TB, Rao MVB, et al. Degradation Study of Benalaxyl Fungicide Residues in Different pH Waters Using Ferrous Doped Titanium Dioxide Nanoparticles. Anal Chem Ind J. 2016;16(14):108.
Abstract
Studied the dissipation behavior of benalaxyl beneath direct sunlight by using Fe doped TiO2 nanoparticles (FeTiO2) as catalyst. FeTiO2 nanoparticles are synthesized and characterized by way of Scanning Electron Microscopy (SEM) combined with Energy Dispersive X-ray evaluation (EDX) and Fourier Transform Infrared Spectroscopy (FT-IR). The photo catalytic studies were conducted by the 1 l of Milli-Q water, pH 4.0, 7.0 and 9.0 buffer were spiked with 1 mL of 1000 mg/L stock solution of benalaxyl 35% WP to get 1 μg/ml concentration of pesticide active in water. Three sets of such samples in triplicates were prepared and sonicated for 10 min to get homogeneous concentration of pesticide active in water and labeled as S1, S2, and S3. Two sets of samples (S1 and S2) were added FeTiO2 nanoparticles to get 0.06 g/L content of photo catalyst. S1 and S3 sets of samples were exposed to sun light. S2 set of samples were kept in an oven at 40°C in dark. The Milli-Q water spiked with stock solution of pesticide and without addition of FeTiO2 nanoparticles (S3) were also exposed to sun light for the measurement of photolytic degradation of pesticide in water. The FeTiO2 added Milli-Q water without spiking of pesticides (S4) was also maintained as untreated control for the identification of absence of pesticide. The collected samples were quantified using a validated HPLC-UV method. Kinetic parameters such as rate constant (k), DT50 and DT90 were calculated using the dissipation data of benalaxyl.
Keywords
Fe doped TiO2; Benalaxyl; HPLC-UV; SEM; TEM; DT50; DT90
Introduction
Anilides are a myriad group of fungicides. The earliest advent turned into anilazine (dyrene), as a leaf spot fungicide from Bayer and Nissan, accompanied by using the seed treatment carboxin (vitavax), that has great effect on bunts, smuts and various Basidiomycetes including Rhizoctonia sp. Later, the dicarboximides iprodione (rovral) from Rhone-Poulenc, vinclozolin (ronilan) from BASF and procymidone (sumisclex) from Sumitomo followed. These fungicides all had first-rate protectant effect on the genera Botrytis, Monilinia and Sclerotinia [1]. Preventing resistance has become an issue with the extensive scale use of these fungicides. Benalaxyl as a xylem-systemic fungicide is normally directly sprayed on the soil surface that has ability to harm the animals living in the soil. But, the stereo selectivity of benalaxyl in reptiles is hardly ever been studied [2]. Chinese language lizards (Eremias argus) have been firstly used to assess the stereo selectivity in biodegradation and toxicity of racemate and character enantiomers of benalaxyl [3,4]. Within the current years, using heterogeneous picture catalyst nano Fe doped TiO2 (FeTiO2) in the degradation and mineralization of herbicide, insecticide, N-heterocyclic compounds, saturated fatty acids, exceptional organic dyes in water and gaseous pollutant in air the use of UV-visible light has received extensive interest because of its low price education, low toxicity, high balance and effectiveness than TiO2 [5]. When FeTiO2 nanoparticles are subjected to UV, VIS or solar light, it gains electricity from mild and sell electrons (e–) from the valence band (VB) of TiO2 to the conduction band (CB) leaving a fantastic hole (h+). Fe in FeTiO2 lure electrons (e–) and fantastic holes (h+) from TiO2 since the electricity levels of Fe2+/Fe3+ lies close to that of Ti3+/Ti4+, and reduce the recombination of image-generated electron and hole pair in TiO2 and enhance the provision of electrons (e–) and wonderful holes (h+) in FeTiO2. These electrons (e–) and fine holes (h+) are concerning inside the degradation of natural molecules through oxidation/discount process [6-8]. Escherichia coli can be destroyed in water completely within an hour. This can be observed through deposited Escherichia coli microorganism in water on FeTiO2 skinny film and irradiating it in visible radiation [9,10]. Based on these studies, the objective of our study was to investigate the dissipation behaviour of benalaxyl in 3 unique buffers by the use of FeTiO2 as catalyst under natural climatic conditions in solar light.
Materials and Methods
Reference analytical standard of benalaxyl (purity 99.6%), titanium tetrachloride and iron nitrate were obtained from Sigma Aldrich. The test item benalaxyl 35% wettable powders (WP) were purchased from local market. Acetonitrile, water HPLC grade, sodium hydroxide LR grade, potassium chloride GR grade, boric acid GR grade, potassium biphthalate GR grade, hydrochloric acid AR grade and potassium phosphate AR grade were obtained from the Merck India Limited. Distilled water was purified by using the Milli-Q Plus apparatus (Millipore, Bedford, MA, USA).
Preparation of FeTiO2 nanoparticles
The TiO2 nanoparticles have been organized by using the drop wise addition of 5 mL of TiCl4 (Sigma Aldrich) in a 100 mL distilled water containing 0.2 M HCl (AR Grade Purity-36.6%) at 5°C ± 0.5°C and ultra-sonicated for 1 h at 82°C and kept for 18 h at 82°C in a thermostat controlled oven (TiCl4+2H2O → TiO2+4HCl). The received white precipitate became washed with distilled water ten times by way of the usage of refrigerated centrifuge and ultimately washed with methanol. The methanol changed into then decanted and the precipitate (TiO2 nanoparticles) become dried at 120°C for 4 h. A 100 ml boiling iron nitrate (Sigma Aldrich) added drop wise to the boiling distilled water containing 2 g of TiO2 nanoparticles. The resultant turned into sonicated at 100°C about 30 min, in step with the following chemical equation:
TiO2 (aqua)+Fe(NO3)3·9H2O (aqua) → Fe–TiO2
The obtained brown colour FeTiO2 nanoparticles were washed with distilled water six times by using refrigerated centrifuge and finally washed with methanol. The FeTiO2 nanoparticles dried at 120°C for 4 h after decanted the methanol.
Standard stock solution
The stock solution of reference standard was prepared by weighing about 10 mg of benalaxyl of known purity into a 10 ml volumetric flask using an analytical balance having accuracy of 0.01 mg. The content of each flask were dissolved using HPLC grade acetonitrile and made up to the mark.
Sample stock solution
Accurately 283.85 mg of test item (purity 35.23%) of benalaxyl was taken into a 100 ml volumetric flask. The content was dissolved in 5 ml of acetonitrile, sonicated and made up to the mark with the acetonitrile. The concentration was 1000 mg/L solution. The stock sample solution was used for preparation of dose samples in different aqua’s buffers.
Acidic buffer
The buffer solution of pH 4.0 was prepared by dissolving 4.0 g of disodium hydrogen orthophosphate in 1.0 L Milli-Q water and the pH was adjusted to 4.0 using 1.0 mole/L hydrochloric acid solution.
Neutral buffer
The buffer solution of pH 7.0 was prepared by dissolving 4.0 g of potassium dihydrogen orthophosphate in 1.0 L Milli-Q water and the pH was adjusted to 7.0 using 1.0 mole/L sodium hydroxide solution.
Basic buffer
The buffer solution of pH 9.0 was prepared by dissolving 1.25 g of boric acid and in 1.0 L milli-Q water and the pH was adjusted to 9.0 using 1.0 mole/L sodium hydroxide solution.
Photo catalytic studies
The photo catalytic studies were carried out in a borosil glass bottle under sunlight at GITAM University, Hyderabad. To the one-litre of Milli-Q water, pH 4.0, 7.0 and 9.0 buffer were spiked with 1 ml of 1000 mg/L stock solution of pesticide formulation to get 1 μg/mL concentration of pesticide active in water (each pesticide was spiked into separate one-litre glass bottle). Three sets of such samples in triplicates were prepared and sonicated for 10 min to get homogeneous concentration of pesticide active in water and labelled as S1, S2, and S3. Two sets of samples (S1 and S2) were added FeTiO2 nanoparticles to get 0.06 g/L content of photo catalyst (optimum amount). The sample suspension of FeTiO2 was sonicated in the dark for 10 min before exposure to the sunlight, to get even disperse of FeTiO2 particles in water and attain adsorption equilibrium. S1 and S3 sets of samples were exposed to sun light from morning 8 AM to evening 5 PM in the month of February. S2 set of samples were kept in an oven at 40°C in dark. The unexposed to sun light samples (S2) were maintained for the measurement of nonphotocatalytic degradation of pesticide active in water. Milli-Q water spiked with stock solution of pesticide and without addition of FeTiO2 nanoparticles (S3) were also exposed to sun light for the measurement of photolytic degradation of pesticide in water. The FeTiO2 added Milli-Q water without spiking of pesticides (S4) was also maintained as untreated control for the identification of absence of pesticides. The day temperature during the exposure period of soil samples under sunlight varied from 28°C to 45°C. The intensity of the sunlight and temperature were measured during the exposure time using LUX meter.
Sampling data
Water samples were collected from the bottle at different depth on different occasion after exposure under sun light (0, 6, 12, 18, and 24 h for photo catalytic experiment. The collected water sample was centrifuged and filtered thoroughly 0.2 μ filters and analysed in HPLC.
Chromatographic separation parameters
The HPLC-UV system used, consisted Shimadzu high performance liquid chromatography with LC-20AT pump and SPD-20A interfaced with LC solution software, equipped with a reversed phase Column Eclipse XDB-C18 (150 mm × 4.6 mm i.d. × 3.5 μm particle size), oven temperature was maintained at 30°C. The injected sample volume was 20 μl. Mobile Phases A and B was Acetonitrile and HPLC Water (60:40 v/v). The flow-rate used was kept at 1.0 ml/min with a detector wavelength at 210 nm. The external standard method of Calibration was used for this analysis.
Method validation
Method validation ensures analysis credibility. Recovery studies were conducted by fortifying three different concentrations of each fungicide at 0.03, 0.15 and 0.3 μg/g levels in four different buffers. Three replicates determinations were made at each concentration level along with two controls. Based on the recovery study the limit of quantification was established. Linearity was determined by different known concentrations (0.01, 0.05, 0.1, 0.5, 1.0 and 2.0 μg/ml) which were prepared by diluting the stock solution. The Limit of Detection (LOD, μg/ml) was determined as the lowest concentration giving a response of 3 times the baseline noise defined from the analysis of control sample. The Limit of Quantification (LOQ, μg/ml) was determined as the lowest concentration of a given fungicide giving a response of 10 times the baseline noise.
Results and Discussion
Description of FeTiO2 nanoparticles
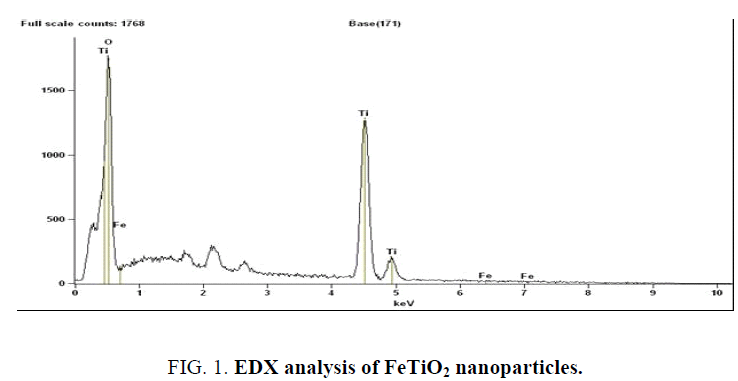
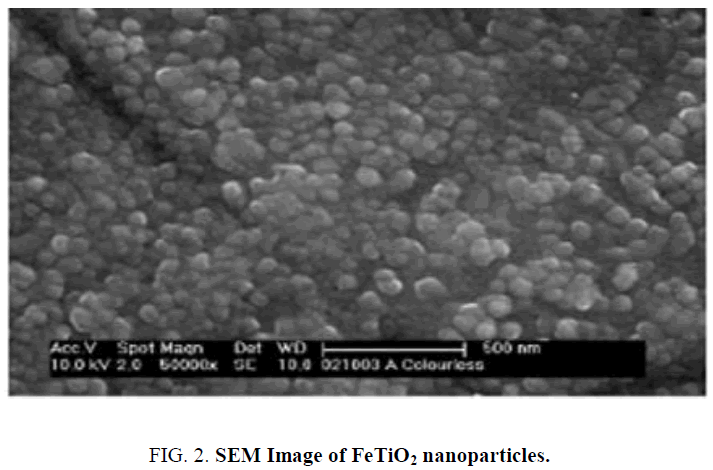
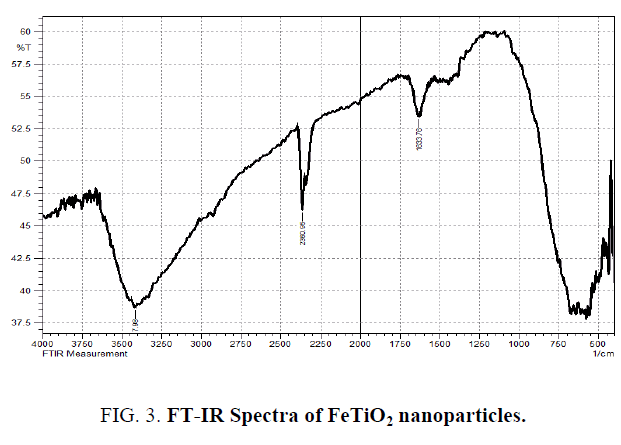
The scanning electron microscope (SEM) images of FeTiO2 nanoparticles and energy dispersive X-ray analysis (EDX) for the quantitative determination and elemental composition of Fe/Ti ratio presented in Figure 1 and 2 which indicates the Fe content was 0.5% and Ti, O and Fe are the elemental compositions and the size of the particle was observed to be 20 nm to 24 nm. The Fourier transform Infrared Spectroscopy was showed peaks at (652 cm-1 to 551 cm-1), (1632 cm-1) and (2235 cm-1) indicated for molecular water, Ti–O and Ti–O–Fe stretching vibration band respectively and presented in Figure 3.
Specificity
Specificity was confirmed by injecting the Mobile phase solvents i.e., Acetonitrile and 0.1% Orthophosphoric acid, HPLC water, sample solution standard solution and buffer controls (acidic, neutral, basic) there were no matrix peaks in the chromatograms to interfere with the analysis of fungicide residues shown in Figure 4-6. Furthermore, the retention time of benalaxyl was constant at 5.3 ± 0.2 min.
Linearity
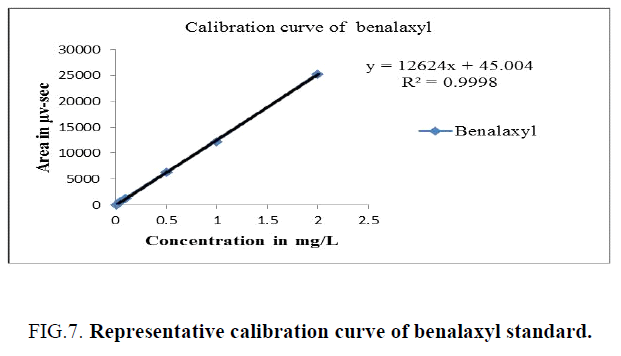
Different known concentrations of benalaxyl (0.01, 0.05, 0.1, 0.5, 1.0 and 2.0 μg/ml) were prepared into a distinct 10 ml volumetric flasks by means of diluting the stock solution. These calibration solutions had been immediately injected into a HPLC. A calibration curve has been plotted for concentration of the standards injected versus areas observed and the linearity of method evaluated by analysing six standard concentration solutions. The details had been provided in Table 1. The peak areas acquired from exclusive concentrations of requirements were used to calculate linear regression equation. This was Y=12624X+45.004 with correlation coefficient of 0.9998 respectively. A calibration curve is showed in Figure 7.
| Concentration in (mg/L) | Peak area of benalaxyl (µv-sec) |
|---|---|
| 2 | 25431 |
| 1 | 12384 |
| 0.5 | 6355 |
| 0.1 | 1386 |
| 0.05 | 753 |
| 0.01 | 166 |
Table 1. Calibration details - benalaxyl.
Recovery
The methods were observed in precision with an acceptable range <20% of RSD when injected 10X LOQ recovery sample five times consecutively into the HPLC. The statistical parameters such as standard deviation (SD) and percentage of relative standard deviation (% RSD) were presented in Table 2.
| Statistical Parameters | Compound Name |
|---|---|
| Benalaxyl | |
| Precision | |
| Mean | 99 |
| SD | 1.28 |
| % RSD | 1.32 |
| Recovery | |
| Mean | 98 |
| SD | 1.35 |
| % RSD | 1.41 |
Table 2. Precision details - benalaxyl.
The method had an acceptable recovery range (70% to 110%) for fungicide in four different soils. The Limit of Quantification (LOQ) was established as 0.03 mg/L from 10:1 peak to noise height ratio. The statistical parameters for recovery such as mean recovery percentage, standard deviation (SD), percentage of relative standard deviation (% RSD) and Horwitz Limit are presented in Table 2. The formula for calculation residue and statistical parameters are presented below the equation:

Where, A: Peak area of active content in sample (μV*sec); C: Concentration of the standard solution μg/ml); D: Peak area of active content in standard solution (μV*sec)

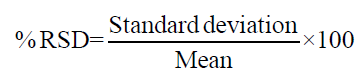
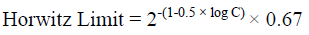
Photo catalytic decontamination of pesticide in water
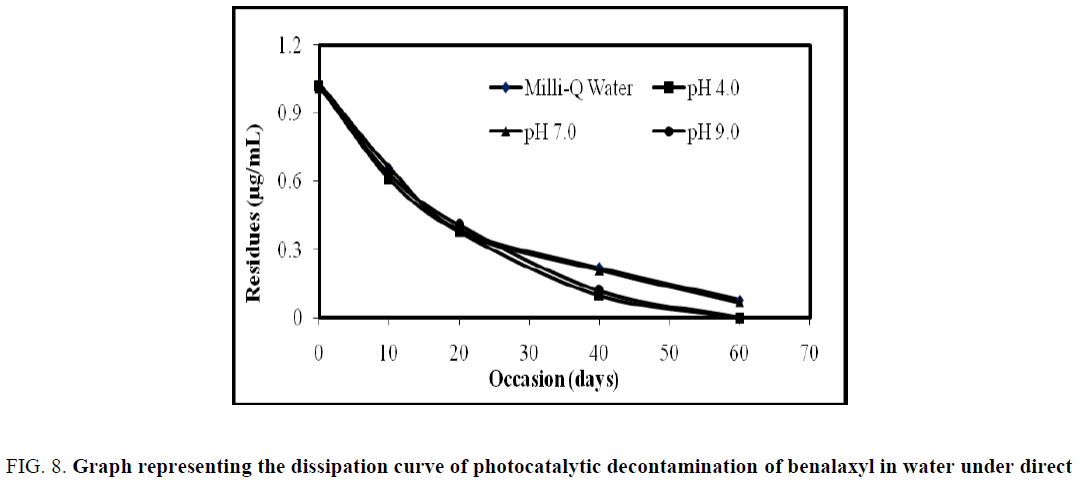
On 0 h analysis of benalaxyl fortified water showed that the residue of benalaxyl as 1.04, 1.02, 1.03, and 1.02 μg/L, for Milli- Q water, pH 4, pH 7 and pH 9 buffer water with 0.06 g/L load of catalyst respectively. The residues of benalaxyl dissipated to 0.36, 0.42, 0.37, 0.12 μg/L, on 12th h for Milli-Q water, pH 4, pH 7 and pH 9 with 0.06 g/L load of catalyst respectively. On 18th h the benalaxyl concentration degraded to near the LOQ level for Milli-Q water, pH 4, pH 7 and got complete degradation on 24th h but pH 9 buffer water got complete degradation on 18th h.
The summarized results for photo catalytic studies are presented in Table 3 and Figure 8. The data clearly demonstrate that the decontamination of pesticide follows pseudo-first-order kinetics in FeTiO2 loaded water (S1) when calculated residues values with time by using below the first order kinetic formula (OECD 111). The absence of pesticide residues in S4 were observed because of no pesticide was applied and no degradation of fungicide in S2 was observed due to inactivation of the FeTiO2 in the absence of light. DT50 and DT90 values were calculated using the following formulas:
| Occasion (hours) | Residues (µg/mL) | |||
|---|---|---|---|---|
| Milli-Q Water | pH 4.0 | pH 7.0 | pH 9.0 | |
| Benalaxyl | ||||
| 0 | 1.04 | 1.02 | 1.03 | 1.02 |
| 6 | 0.63 | 0.74 | 0.61 | 0.43 |
| 12 | 0.36 | 0.42 | 0.37 | 0.12 |
| 18 | 0.13 | 0.19 | 0.15 | BDL |
| 24 | BDL | BDL | BDL | BDL |
Table 3. Dissipation data for photo catalytic decontamination of benalaxyl in water under direct sunlight.
Figure 8: Graph representing the dissipation curve of photocatalytic decontamination of benalaxyl in water under direct sunlight.
DT50=ln 2/(k) and DT90=ln 10/(k)
Where, ‘k’ is slope of the curve obtained from the dissipation data.
The calculated DT50 and DT90 values are presented in Table 4. The rate constant value was calculated by linear regression equation from the first order rate equation:
| Kinetic parameters | Water | |||
|---|---|---|---|---|
| Milli-Q Water | pH 4.0 | pH 7.0 | pH 9.0 | |
| Benalaxyl | ||||
| DT50 (h) | 6.12 | 7.42 | 6.62 | 3.89 |
| DT90 (h) | 20.32 | 24.64 | 22 | 12.91 |
| k (1 x 10-3 h-1) | 113.3 | 93.5 | 104.7 | 178.4 |
Table 4. Kinetic parameters for photocatalytic decontamination of benalaxyl in water under direct sunlight.
K=ln a/a-x/dt
Where, dt is the time interval between t1 and t2 and a, x are the concentration of pesticides at times t1 and t2 respectively. A plot of concentration of the residues and rate with the R2 indicates first order kinetics in dissipation of fungicide. The DT90 of benalaxyl calculated by regression analysis from the dissipation data.
Results clearly indicate that the rate constant was high when the FeTiO2 was present in water than in absence of water and no degradation of pesticide in S2 set of water which were kept in dark. The decontamination was fast when studied under sunlight in presence of FeTiO2 in water due to the formation of electrons (e–) and positive hole (h+) in TiO2 when it absorbed energy from sun light and the availability of electrons (e–) and the positive holes (h+) pairs which were contributing the simultaneous oxidation and reduction of pesticide in soil were enhanced by Fe in FeTiO2. This was confirmed by the no degradation of pesticide in water samples when store in dark. Absence of pesticide residues were also observed in water spiked (S4) water samples because of no pesticide was applied.
Conclusion
The FeTiO2 nanoparticles have been located to be excellent decontaminating catalyst for benalaxyl in unique water samples. In the absence of catalyst, the compound persists several days. The mobile phase Acetonitrile and HPLC water showed good separation and resolution and the analysis time required for the chromatographic determination of three different type of buffers is very short (round 15 min for a chromatographic run). Satisfactory validation parameters which include linearity, accuracy, precision and LOQ, DT90 and DT50 values have been installed by using following South African country wide Civic company (SANCO) and Environmental Protection Agency (EPA) guidelines. Consequently, the proposed analytical technique and dissipation data might be beneficial for regular monitoring, residue labs and research students to determine the benalaxyl residues in different commodities (crop, water and soil samples).
Acknowledgement
The authors are thankful to the Dr. K Raghu Babu, Professor, Department of Engineering Chemistry, Andhra University, Visakhapatnam for providing necessary facility to conduct the Laboratory experiment.
References
- Kundu C, Goon A, Bhattacharyya A. Persistence behaviour of fungicide mixture (benalaxyl-M 4% + mancozeb 65%) WP in grapes. Bull Environ Contam Toxicol. 2012;89(6):1253-7.
- Crisippi T, Zini G, Fabbrini R. Gas chromatographic determination of benalaxyl residues in different crops and water. J AOAC Int. 1993;76(3):650-6.
- Rosso I, Giraudi G, Gamberini R, et al. Application of an ELISA to the determination of benalaxyl in red wines. J Agric Food Chem. 2000;48(1):33-6.
- Liu D, Wang P, Zhou W, et al. Direct chiral resolution and its application to the determination of fungicide benalaxyl in soil and water by high-performance liquid chromatography. AnalChim Acta. 2006;555(2):210-6.
- Carey JH, Lawrence J, Tosine HM. Photodechlorination of PCB's in the presence of titanium dioxide in aqueous suspensions. Bull Environ Contam Toxicol. 1976;16(6):663-8.
- Joung SK, Amemiya T, Murabayashi M, et al. Mechanistic studies of the photocatalytic oxidation of trichloroethylene with visible-light-driven N-doped TiO2 photocatalysts. Chemistry. 2006;12(21):5526-34.
- Sun H, BaiY, ChengY, et al. Preparation and Characterization of Visible-Light-Driven Carbon−Sulfur-Codoped TiO2 Photocatalysts. Ind Eng Chem Res. 2006;45(14):4971-6.
- GhoraiTK. Synthesis of spherical mesoporous titania modified iron-niobate nanoclusters for photocatalytic reduction of 4-nitrophenol. J Mater Res Technol. 2015.4(2):133-43.
- Ruzmanova Y, Stoller M, Chianese A. Photocatalytic Treatment of Olive Mill Wastewater by Magnetic Core Titanium Dioxide Nanoparticles. ChemEng Transact. 2013;32:2269-74.
- Zhang Q, GaoL, Guo J. EffectsofCalcinationonthe PhotocatalyticProeprtiesof NanosizedTiO2 PowdersPreparedbyTiCl4 Hydrolysies. OrgMagnetResonance. 1982;19(2):98-101.