Original Article
, Volume: 12( 1)Synthesis and Characterization of Novel Dialdehydes based on SN2 Reaction of Aromatic Aldehyde
- *Correspondence:
- Ali A , M.S. Department of Organic Chemistry, Al-Baath University Homs, Syria, Tel: 00963031; Fax: 426716; E-mail: aliasmi.chemic@hotmail.com
Received: March 17, 2017; Accepted: May 22, 2017; Published: May 29, 2017
Citation: Ali A, Merza J. Synthesis and Characterization of Novel Dialdehydes based on SN2 Reaction of Aromatic Aldehyde. Inorg Chem Ind J. 2017;12(1):111.
Abstract
Four new dialdehydes compound (I, II, III, IV) containing oxygen donor atoms have been synthesized in good yields (about 80%) from the condensation reactions of Salicylaldehyde with dibromoethane and bis(2-chloroethyl) ether, And from the condensation reactions of Vanillin with di-bromoethane and bis(2-chloroethyl)ether], respectively. The reaction followed by using thin layer chromatography (T.L.C). Four series of dialdehydes, namely 2,2'-(ethane-1,2-diylbis(oxy)) dibenzaldehyde(I), 2,2'-((oxybis(ethane-2,1-diyl)) bis(oxy)) dibenzaldehyde (II), 4,4'-(ethane-1,2-diylbis (oxy))bis (3-methoxybenzaldehyde)(III), 4,4'-((oxybis (ethane-2,1-diyl))bis(oxy)) bis(3-methoxybenzaldehyde)(IV) were characterized by elemental analyses, FT-IR, UV-Vis, HPLC, 1H,13C-NMR
Keywords
Salicylaldehyde; Vanillin; Oxygen donor atoms; Dialdehydes
Introduction
Bimolecular nucleophilic substitution (SN2) reactions have been It is of extreme importance to the theory and practice in the field of organic chemistry and inorganic chemistry (SN2). Aromatic aldehydes are commercially significant compounds with applications in the fragrance, flavour and pharmaceutical industries. Biotransformation’s to produce these compounds are of interest as the products may be described as natural and exist in a relatively pure form, because of the high specificity of enzyme reactions [1]. The production of aromatic aldehydes is limited by the toxicity of the products, therefore the rapid removal or sequestration of the aldehyde by in situ product removal is desirable [2]. Such techniques previously used for aldehyde removal include gas stripping [3], pervaporation [4], aqueous-organic two-phase systems [5] and condensation reactions [6]. The production of aromatic aldehydes is limited by the toxicity of the products, therefore the rapid removal or sequestration of the aldehyde by in situ product removal is desirable [2].
Salicylaldehyde organic compound important in nature it is used in many chemical, industrial and pharmaceutical fields as dyes and medical supplies, also enters in some chemical reactions as a mediator, and others, [7] fragrances, essential oils in various biological applications [8]. Antibacterial her work is powerful enough to allow its use in very low concentrations. Also get in formulation of perfumes and fragrances.
Also, vanillin is very important and which is classified flavoring compound due to its antioxidant ability. Essential oil of vanillin was and is sometimes used in aromatherapy. may be used for the protection of foods against oxidation [9-11]. In old medicinal literature and used in folk medicine vanilla was described as a remedy for fevers [9]. Some vanillin derivatives potential as antibacterial agents against some Gram positive and Gram negative bacterial strains.
Experimental Methods
Spectrum NMR proton and carbon device 400 MHz model Bruker by Switzerland company, UV/Vis spectroscopy (model: Optizen 2120 UV), Infrared spectra of compounds were recorded in KBr pellets from the Japanese company Jasco spectrophotometer in the range 4000 cm-1 to 400 cm-1, thin layer chromatographic of aluminum coated by Silica Gel 60F254 measuring 20 X 20 from the German company Merck.
HPLC model KNAUER (Eurochrom HPLC Software. Version 3.05 P5) and HPLC methods (Methanol, acetonitrile, Water). ODS, C18.
Reagents and materials
Salicylaldehyde, Vanillin, bis(2-chloroethyl) ether 98% and 1,2-dibromoethane were obtained from (sigma-Aldrich), anhydrous potassium carbonate 97.88% (by CHEMLAB), absolute ethanol, ether and dimethylformamide (DMF), (by CHEMLAB).
Synthesis of 2,2'-(ethane-1,2-diylbis(oxy)) dibenzaldehyde (EDOD)
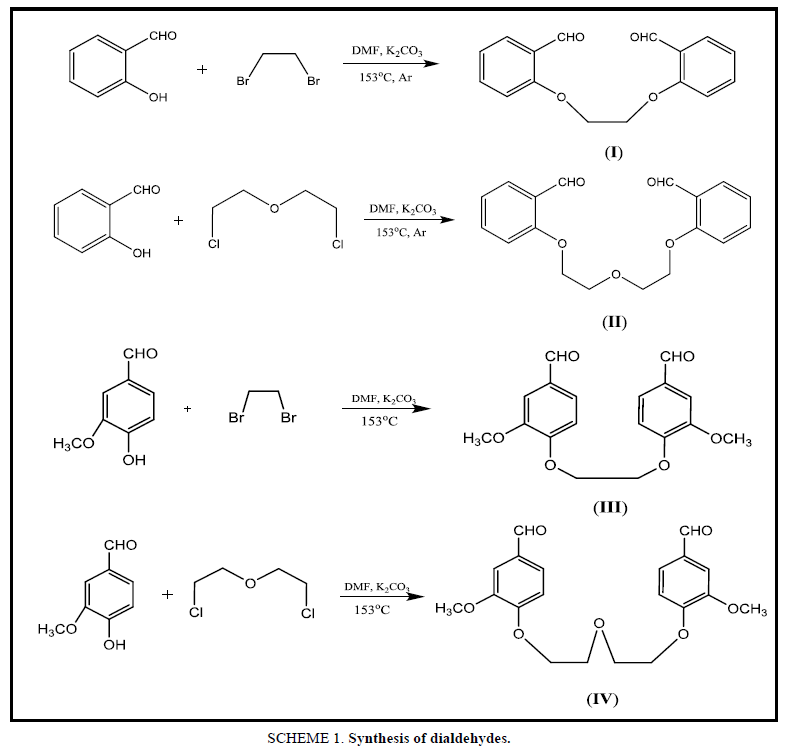
Salicylaldehyde (0.732 g, 0.006 mol) was dissolved in 10 mL of DMF in a 50 mL Tow-necked flask equipped with a condenser and magnetic stir bar. Anhydrous potassium carbonate (0.414 g, 0.003 mol) was added to the flask. Dibromoethane (0.588 g, 0.003 mol) was dissolved in 5 mL DMF was added dropwise into the reaction mixture under argon atmosphere. The mixture was heated for 12 h at 153°C under continuous stirring and then for 4h at room temperature. After cooling, the product was poured into 100 mL cold distilled water (approximately 5°C to 10°C). The precipitate was washed with 3 × 250 mL distilled water for separating mineral salts. Then EDOD was recrystallized from ethanol, filtered and dry with ether to get a solid Colour: Bright brown, M.P: 122°C, (yield 78%).
Synthesis of 2,2'-([oxybis(ethane-2,1-diyl]) bis(oxy) dibenzaldehyde (OEBOD)
Salicylaldehyde (1.22 g, 0.01 mol) was dissolved in 25 mL of DMF in a 100 mL Tow-necked flask equipped with a condenser and magnetic stir bar. Anhydrous potassium carbonate (1.38 g, 0.01 mol) was added to the flask. Bis (2-chloroethyl) ether (1.43 g, 0.01 mol) was dissolved in 15 mL DMF was added dropwise into the reaction mixture under argon atmosphere. The mixture was heated for 10 h at 153°C under continuous stirring and then for 4h at room temperature. We cooling product and then we washed with distilled water to remove salt. Then OEBOD was recrystallized from ethanol, filtered and dry with ether to get a solid Color: Bright orange, M.P: 75°C, (yield 80%).
Synthesis of 4,4'-(ethane-1,2-diylbis [oxy]) bis (3-methoxybenzaldehyde) (EDOMB)
Vanillin (0.302 g, 0.002 mol) was dissolved in 20 mL of DMF in a 100 mL three-necked flask equipped with a condenser and magnetic stir bar (Scheme. 1). Anhydrous potassium carbonate (0.138 g, 0.001 mol) was added to the flask. 1.2-dibromoethane (1.265 g, 0.0048 mol) was dissolved in 10 mL DMF and added into the reaction mixture. The mixture was heated for 6 h at 153°C under continuous stirring. After cooling, the product was poured into 100 mL cold distilled water (approximately 5°C t o 10°C). The precipitate was washed with 3 × 250 mL distilled water for separating mineral salts. purified by crystallization from ethanol, washed several times with diethyl ether to get a solid Color: Light brown, M.P: 171°C (yield 82%).
Synthesis of 4,4'-([oxybis(ethane -2,1-diyl])bis(oxy)) bis(3-methoxy benzaldehyde) (OEDMB)
Vanillin (0.608 g, 0.004 mol) was dissolved in 30 mL of DMF in a 100 mL three-necked flask equipped with a condenser and magnetic stir bar. Anhydrous potassium carbonate (0.276 g, 0.002 mol) was added to the flask. bis(2-chloroethyl) ether (0.286 g, 0.002 mol) was dissolved in 15 mL DMF and added into the reaction mixture. The mixture was heated for 6 h at 153°C under continuous stirring. After cooling, the precipitate was formed, washed with 3 × 100 mL water for separating mineral salts. purified by crystallization from ethanol, washed several times with diethyl ether to get a solid Color: Light brown, M.P: 175°C, (yield 80%).
Results and Discussion
The new Dialdehyde compounds have been obtained from the reaction of Aromatic aldehyde with various Dihalide alkyl, as the molar ratio 2:1 (Ar-Aldehyde: Dihalide) as shown in the Table 1, analytical data given below:
| Entry | Products | ElementalAnalysis %Found(%Calc.) |
|
|---|---|---|---|
| C | O | ||
| 1 | (EDOD)(I) | 70.69(71.10) | 23.42(23.68) |
| 2 | (OEBOD)(II) | 66.89(68.78) | 25.13(25.45) |
| 3 | (EDOMB)(III) | 64.30(65.45) | 28.64(29.06) |
| 4 | (OEDMB)(IV) | 63.60(64.17) | 29.01(29.91) |
Table 1: Analytical data for dialdehydes.
The analytical data are given in Table 2 and are good agreement with general molecular formula proposed.
| Compound | ?(C=O)cm-1 | ?(OCH3)cm-1 | ?(Ar-O)cm-1 | ?(C-O)cm0-1 |
|---|---|---|---|---|
| I | 1713 | ?????? | 1283 | 1050 |
| II | 1711 | ?????? | 1280 | 1045 |
| III | 1716 | 1516 | 1288 | 1065 |
| IV | 1709 | 1512 | 1290 | 1065 |
Table 2: . IR spectral data for dialdehydes compounds.
FT-IR Results
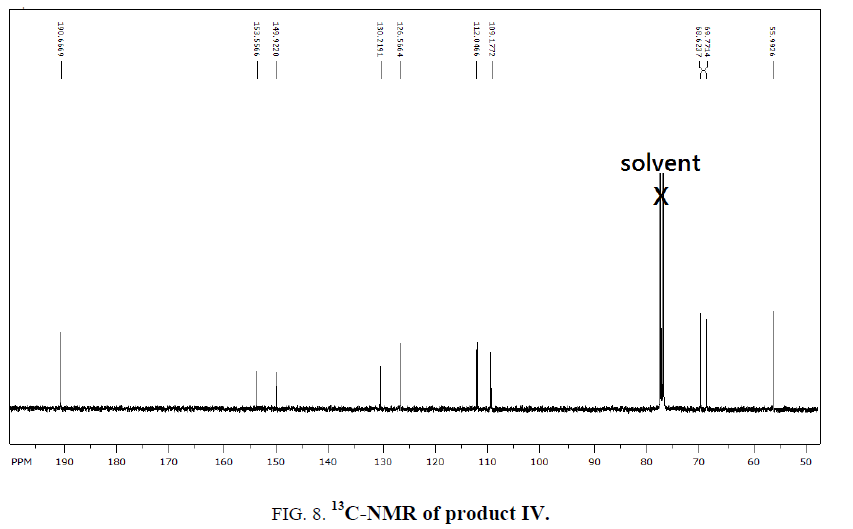
The Dialdehydes were further confirmed by IR spectroscopy, as shown the I, II, III, IV spectra, an obvious absorption peak at 1713, 1711, 1716, 1709 cm-1 respectively, belonged to carbonyl group. Also, there were two absorption peaks 1280 cm-1 and 1050 cm-1 which were the characteristic absorption band of the Ar-O and C-O-C stretching, respectively. The characteristic absorption between 2940 cm-1 and 1450 cm-1 indicate to (OCH3) methylene groups in the III and IV Compounds. whereas the bands occurring near 1594 cm-1and 3069 cm-1 regions are assigned to benzene ring vibration so the I, II, III, IV Compounds was successfully synthesized (Figure. 1-12).
Electronic spectral data
The data of the electronic spectra of the products in Chloroform solution are given in Table 3. All bands in the UV interval assigned to (π→π*) and (n→π*) transitions.
| Compounds | ElectronicSpectra(nm) | Assignments |
|---|---|---|
| I | 314 | π→π*,n→π* |
| II | 318 | |
| III | 270,308 | |
| IV | 274,309 |
Table 3: Characteristic infrared absorption frequencies (cm-1) of the dialdehyde.
Elemental analysis and characterization for the products
The Products was prepared by the condensation of aldehyde and alkyl halide in their 2:1 molar ratio, respectively. The results of elemental analyses, colors, yields and the melting points, are presented in Table 4.
| Compounds | Mol.Weight | Color | Yield(%) | M.P(°C) | Elementalanalysis %Found(%Calc.) |
|
|---|---|---|---|---|---|---|
| C | O | |||||
| I | 270 | Brightbrown | 81 | 120-122 | 70.69(71.10) | 23.42(23.68) |
| II | 314 | Brightbrown | 78 | 75 | 66.89(68.78) | 25.13(25.45) |
| III | 330 | Lightbrown | 82 | 171 | 64.30(65.45) | 28.64(29.06) |
| IV | 374 | Lightbrown | 79 | 130 | 63.60(64.17) | 29.01(29.91) |
Table 4: Physical data of synthesized compound.
Determination by high performance liquid chromatography (HPLC)
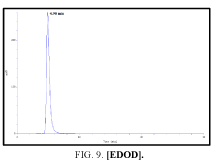
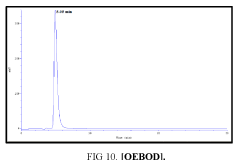
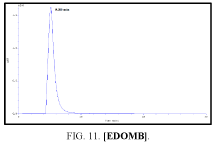
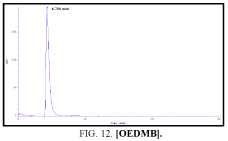
The high-performance liquid chromatograph consisted of two pumps (KNAUER). Sample solutions were injected with a Eurochrom HPLC Software, Version 3.05 P5. The analytical column was an inertsil ODS (150 mm × 4.6 ram). The excitation and emission wavelengths were fixed at 314, 318, 308 and 274 nm, respectively. The column was maintained at room temperature; and the flow-rate of the eluent was 1.0 ml.min-1. In Table 5 The results HPLC data of Products with (UV. λmax nm) and Retention Time.
| Sampleno | Product | UV,lmaxnm | Ret.Time (min) |
|---|---|---|---|
| I | [EDOD] | 314 | 4.900 |
| II | [OEBOD] | 318 | 4.950 |
| III | [EDOMB] | 308 | 4.767 |
| IV | [OEDMB] | 274 | 4.317 |
Table 5: HPLC separation of product (I, II, III, IV).
Shows the chromatographic separation of four aromatic aldehydes (I,II,III,IV) derivatization from salicylaldehyde and Vanillin. The resulting derivatives were completely determination of purity by a reversed-phase ODS column with simple linear gradient elution with methanol absolute (99.9% for 15 min).
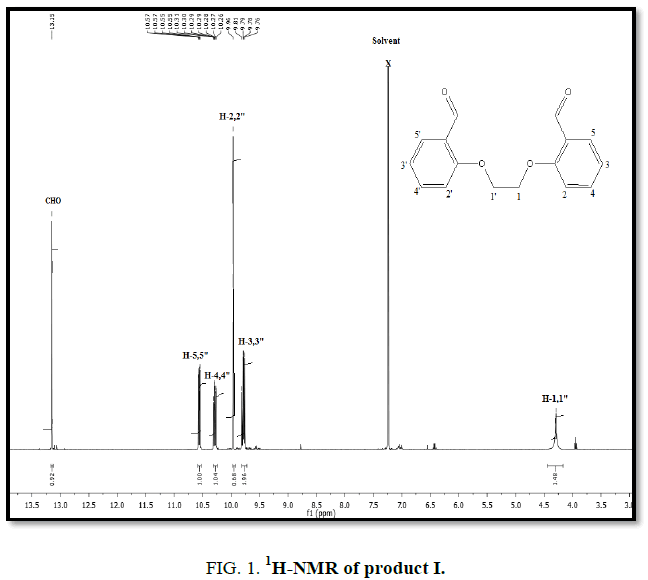
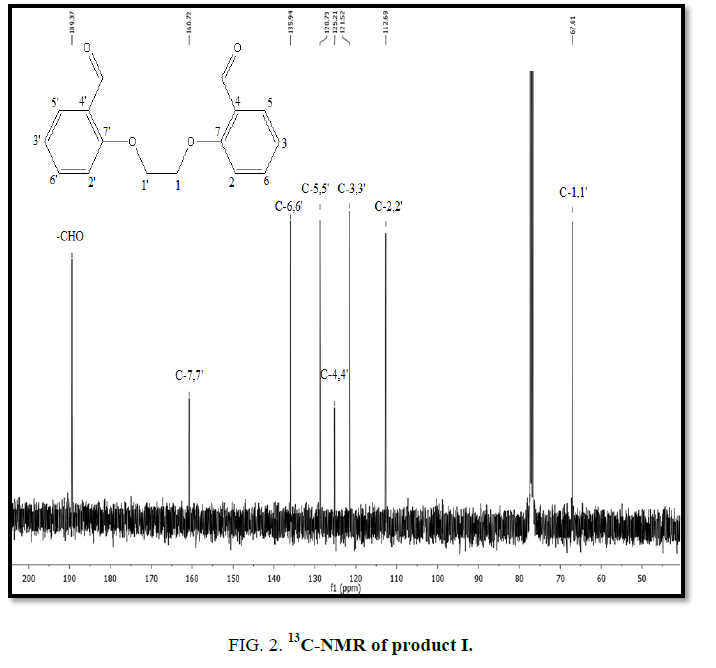
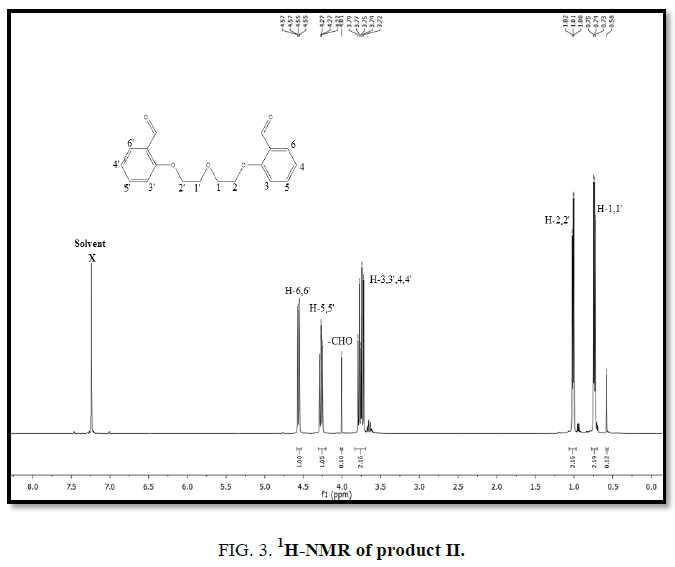
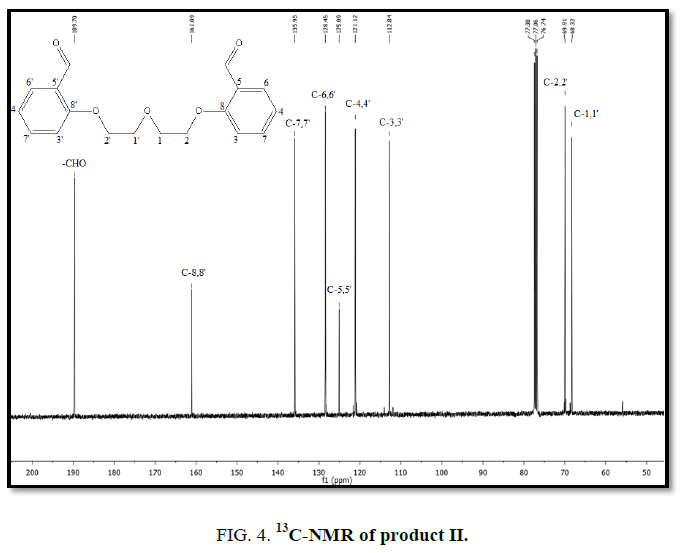
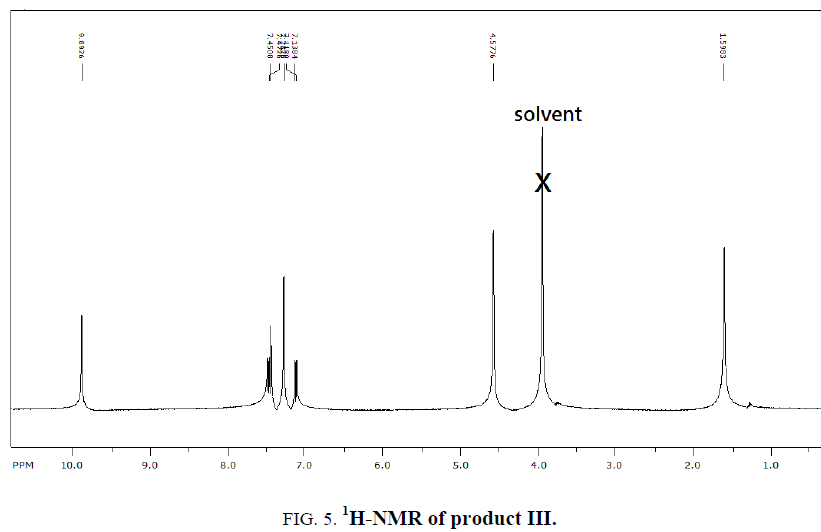
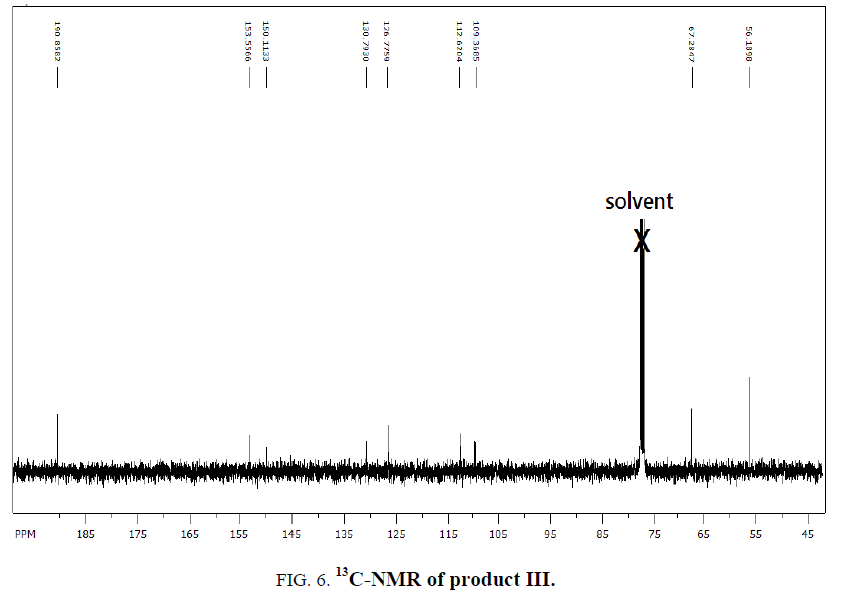
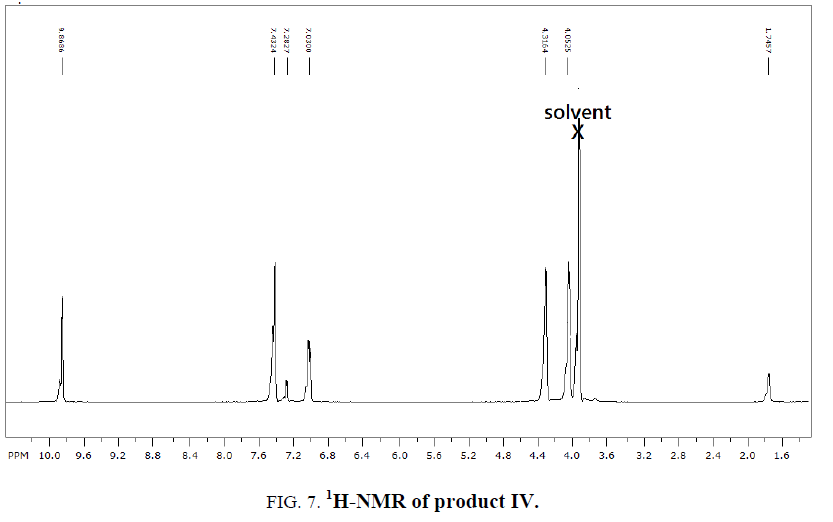
1H-NMR,13C-NMR spectra
The synthesized compound is further supported by the 1H,13C-NMR spectra.
The multiple observed in the region around δ 3.0 ppm to 10 ppm in all the listed compound have been assigned to the aromatic protons. The signal in the region 0.5 ppm to 4.5 ppm is due to methylene protons nearer to oxygen. As the methoxy group protons are attached to the phenyl ring the signal for these protons in the compounds (III, IV) appear in the upfield region δ 1.59 ppm and 1.74 ppm and the selected spectra are shown (Tables 6 and 7).
| Compound | Ar-H(ppm) | CH2(ppm) | CHO(ppm) | OCH3 |
|---|---|---|---|---|
| EDOD | 9.75-10.57 | 4.29 | 13.15 | ?????? |
| OEBOD | 3.7-4.6 | 0.5-1.1 | 4.0 | ?????? |
| EDOMB | 7.1-7.5 | 4.57 | 9.8 | 1.59 |
| OEDMB | 7.0-7.4 | 4.0-4.3 | 9.8 | 1.74 |
Table 6: 1H-NMR spectral data of products.
| Compound | Ar-C(ppm) | CH2(ppm) | CHO(ppm) | OCH3 |
|---|---|---|---|---|
| EDOD | 112.6-160.7 | 67.01 | 189.3 | ??????? |
| OEBOD | 112.8-161.0 | 68.3-69.9 | 189.7 | ??????? |
| EDOMB | 109.5-153.5 | 67.3 | 190.8 | 56.04 |
| OEDMB | 109.3-153.8 | 69.5-69.8 | 190.8 | 55.9 |
Table 7: 13C-NMR spectral data of products.
The absence of a resonance in the region δ 14.0 ppm to 15.0 ppm in the compounds indicate deprotonation of the phenolic (-OH) group of the Aromatic aldehydes and coordination to alkyl halide through the phenolic oxygen.
Conclusion
In summary, the Preparation of Dialdehydes compounds based on SN2 Bimolecular nucleophilic substitution (SN2) reactions was successfully studied by synthesis of all the products. Characterization of Salicylaldehyde and Vanillin derivatives have proven to successfully synthesized. The results can be summarized as follows:
I. The compounds were derived by the condensation of salicylaldehyde and vanillin with dibromoethane and bis (2-chloroethyl) ether in 2:1 molar ratio with high yields
II. All the products have been characterized by Available technologies (Elemental analysis, FT-IR, UV-vis, HPLC and 1H,13C-NMR).
Acknowledgement
The manuscript was based upon work supported by The Central Laboratory Department of Chemistry, Faculty of Science Al-Baath University.
References
- Ashraf G. Heterologous expression of stress-responsive DUF538 domain containing protein and its morpho-biochemical consequences. Plant J. 2011;30(5):351-8.
- Takahashi S, Yoshikawa M, Kamada A, et al. The photoconvertible water-soluble chlorophyll-binding protein of Chenopodium album is a member of DUF538, a superfamily that distributes in Embryophyta. J Plant Physiol. 2013;170(17):1549-52.
- Ashraf G, Kohnehrouz SB.Identification ofDUF538cDNA clone from Celosia cristata expressed sequences of nonstressed and stressed leaves. RussJPlant Physiol. 2010;57(2):247-52.
- Nakagami H, Sugiyama N, Mochida K, et al. Large-scale comparative phosphoproteomics identifies conserved phosphorylation sites in plants. Plant Physiol. 2010;153(3):1161-74.
- Ashraf G, Kohnehrouz SB. ProtJ.2013;32:163.
- Ashraf G.Prediction of tertiary structure homology between bactericidal/permeability increasing protein of innate immune system and hydrolase enzymes. IntJBiosci. 2014;5(2):1-6.
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seiolman JG, Smith JA, et al. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 1991.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402-8.
- Rabbani MA, Maruyama M, Abe H, et al. Monitoring expression profiles of rice genes under cold, drought, and high-salinity stresses and abscisic acid application using cDNA microarray and RNA gel-blot analyses. Plant physiol. 2003;133(4):1755-67.
- Shinozaki K, Yamaguchi SK, Seki M, et al. Regulatory network of gene expression in the drought and cold stress responses.CurrOpinPlant Biol. 2003;6(5):410-7.
- Shinozaki K, Yamaguchi SK. Gene networks involved in drought stress response and tolerance. JExpBot. 2007;58(2):221-7.
- Yamaguchi SK, Shinozaki K. Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci. 2005;10(2):88-94.
- Bartels D, Sunkars R. Drought and Salt Tolerance in Plants. CritRevPlant Sci. 2005;24(1):23-58.
- Seki M, Narusaka M, Abe H, et al. Monitoring the expression pattern of 1300 Arabidopsis genes under drought and cold stresses by using a full-length cDNA microarray. Plant Cell. 2001;13(1):61-72.
- Schimid M, Davison TS, Hens SR, et al. A gene expression map of Arabidopsis thaliana development. NatGenet. 2005;37(5):501-6.