Review
, Volume: 14( 2)Synthesis and Characterization of Grafted SH-Functionalized Tripodal Ti-POSS Complex in Gold Nanoparticles as a Selective Epoxidation Catalyst
- *Correspondence:
- Emad Aish H Faculty of Science, King Faisal University, Al-Ahsa 31982, Saudi Arabia, Tel: 920002366-8003030308; E-mail: eaish@kfu.edu.sa
Received: March 26, 2018; Accepted: April 28, 2018; Published: May 23, 2018
Citation: Emad Aish H, Waleed Boraie E, Abdelwahed Sayed R. Synthesis and Characterization of Grafted SH-Functionalized Tripodal Ti-POSS Complex in Gold Nanoparticles as a Selective Epoxidation Catalyst. Org Chem Ind J. 2018;14(2):124
Abstract
Herein we described an easy and efficient method for preparation and grafting of SH-functionalized tripodal Ti-POSS complex in gold nanoparticles supported in SiO2 and obtained a hybrid material that possess two important features. The gold nanoparticles is of good particle size and capable of in situ generating H2O2 as the oxidant in propene epoxidation and SH-functionalized tripodal Ti-POSS catalyst possess an excellent epoxidation activity which will utilize the peroxide to form the propene oxide selectively. The hybrid catalyst showed very good PO selectivity up to 99%, and the conversion was good relative to Au/TiO2 catalyst. Also the catalyst was recycled four times with retained selectivity to PO and with same conversion.
Keywords
Nanoparticles; Titanium; Epoxidation; Ti-POSS; Graffting
Introduction
Supported gold catalysts have attracted much attention due to their wide applications in oxidation reactions [1-6]. Yet finding a highly active and selective oxidation catalyst is of a crucial need. Propylene oxide is an important intermediate in organic synthesis. This product is commercially produced using the chlorohydrin or the hydroperoxide routes, the production of undesired chlorinated byproducts or expensive H2O2 are the main drawbacks. Haruta et al. used small gold nanoparticles for oxidation propene by an O2/H2 mixture [7]. This catalyst, converts between 1%–10% propene with selectivity about 90%. However study the reaction mechanism of the catalyst is very significant. Catalysts consisting of gold nanoparticles on titanium containing supports offer a highly attractive alternative possibility in epoxidation of propene [8-11]. Gold–titania catalysts produce propene oxide out of propene, in a single reactor [12-18]. However, the conversion levels are very low as well as selectivity and stability of the catalysts are usually low. Studying the mechanism of the catalyst is of great need to tune the activity as well as the selectivity of the catalyst in the epoxidation reactions.
Herein we described an easy and efficient synthetic method for SH-functionalized tripodal Ti-POSS complex and then anchored this catalyst in Au nanoparticles supported in SiO2 and obtained a hybrid material that possess two important features. The gold nanoparticles is of good particle size (2-5 nm) for epoxidation of alkene and capable of in situ generating H2O2 as the oxidant in propene epoxidation and SH-functionalized tripodal Ti-POSS catalyst possess an excellent epoxidation activity which will utilize the peroxide to form the propene oxide selectively. Based on our preliminary epoxidation experiments using the hybrid catalyst 4 to form propene oxide (PO) from propene, the catalyst showed very good PO selectivity up to 99%, and the conversion was comparable to Au/TiO2 catalyst.
Experimental
Materials and characterization methods
All experiments were performed under a dry Argon or nitrogen atmosphere using standard Schlenk techniques or in a glovebox. Most reagents were purchased from Sigma-Aldrich. Isobutyl-POSS-SH was purchased from Hybrid Plastics Inc. and dried overnight under vacuum at 50°C before use. All solvents were stored in a glovebox over 4-A°molecular sieves that had been dried in a vacuum oven at 150°C for at least two days before use. CDCl3 and C6D5CD3 were degassed by repeated freeze-pump-thaw cycles. UV-vis spectra were recorded in the absorption mode on an Agilent spectrophotometer using quartz cuvettes. The 1H, 13C, and 29Si NMR spectra were recorded on a Varian Gemini-200 spectrometer or a Varian VXR-400 spectrometer at room temperature. All chemical shifts are reported in units of δ (downfield from tetramethylsilane) and 1H and 13C chemical shifts were referenced to residual solvent peaks. To ensure accurate integrated intensities, [Cr (acetylacetonate)3] (0.05 M) was added to 13C and 29Si NMR samples as a shiftless relaxation agent and a delay of at least 5 s was used between observation pulses for 13C measurements and 10 s for 29Si measurements. 29Si NMR spectra were recorded with inverse-gated proton decoupling in order to increase resolution and minimize nuclear Overhauser enhancement effects.
Synthesis of [Bui6 (n-Propyl-SH)-Si7O9(OH)3] (2): A solution of (n-Propyl-SH)-Bui7-Si8O12 (1) (409 mg, 0.46 mmol) and 35% aqueous Et4NOH (0.2 mL, 0.49 mmol) was refluxed in THF (5 mL) for 4 h then neutralized with dilute aqueous HCl. Evaporation of the volatiles afforded a resinous white material, which was dissolved in Et2O and dried over anhydrous MgSO4. Filtration and evaporation of the solvent afforded crude 2 as a tacky white solid. Recrystallization from toluene-acetonitrile gave pure 2 as colorless crystals. Yield 142 mg (34%). Selected characterization data: 29SiNMR (200 MHz, CDCl3, 25°C) δ, -58.7 (s, SiOH), δ -67.2, -67.6, -68.4, -69.7 (s, Si of the POSS cage) ; 1HNMR (200 MHz, CDCl3, 25°C) δ 6.40 (br s, 3 H, OH), 1.83 (m, 7 H, –CH–), 1.26 (m,1 H, SH), 0.92 (d, 36H, CH3), 0.56 (d, 12 H, CH2), 2.56 (q, 2 H, CH2), 0.54 (m, 2 H, CH2); 13C NMR (200 MHz, CDCl3, 25°C) δ 26.0 (s, CH3), 25.9 (s, CH3), 25.9 (s, CH3), 24.1, 24.0 (s, CH2), 23.4, 23.0, 22.7 (s, CH), 22.6 (s, CH2); IR, ν cm-1 2609 (SH), 3336(Si-OH).
Synthesis of [Ti(NMe2){(Bui)6 (CH2)3SH-Si7O12}] (3): The compound [Ti(NMe2)4] (0.20 g, 0.89 mmol) was added via syringe to a stirred solution of 2 (0.695 g, 0.86 mmol) in ether 15 mL, afforded a deep yellow solution, which was stirred overnight, filtered and reduced to dryness under vacuum. The residue was dissolved in toluene and acetonitrile added dropwise to give a precipitate of 3. The yellow microcrystalline solid was isolated by filtration, washed with acetonitrile (3 × 5 mL) and dried under vacuum afforded 3 as a yellow powder (0.75 g, 97%). 1H NMR (CDCl3, 200.1 MHz): δ 3.23 (s, 6 H, NMe2), 1.82 (m, 6H, CH), 1.82(m,2H, CH2, (CH2)3SH), 1.19(t, 1H, SH), 0.94 (d, 36H, CH3), 0.56 (d, 14H, CH2); 13C NMR (CDCl3, 200 MHz): δ 39.3 (s, NMe2), 26.03, 25.97, 25.91 (s, CH3), 24.16, 24.08 (s, CH2), 22.73 (s, CH), 23.04(s, CH2), 29.91 (s, CH2SH). 29Si NMR (CDCl3, 200MHz): δ -67.9, -68.1 and -68.3 (s, 3:1:3). Elemental analysis found: C, 40.17%; H, 7.76%; N, 1.61%. Calc. for C29H67NSO12Si7Ti: C, 40.20%; H, 7.78%; N, 1.62%. IR, ν/cm-1 2624.6 (SH), 948.8 (Ti-O-Si). UV-vis: λmax/nm 230, corresponding to Ti silicate in tetrahedral environment.
Synthesis of Gold-nanoparticles (Au/SiO2): Gold was deposited on silica of type Davisil 645 by means of a deposition precipitation method using ammonia as in the literature method.19 Where (10 g) of silica was dispersed in 100 mL of demineralized water using a magnetic stirrer. The pH was raised to 9.5 using 2.5% ammonia solution. HAuCl4, 49 wt%, 100 mg was diluted in 40 mL of demineralized water and added gradually over a 15 min to the stirred SiO2, The pH of the solution is kept between 9.4 and 9.6 using NH3 solution. The mixture is stirred for additional hour, then filtered and washed with demineralized water (3 × 200 mL), dried overnight in air at 353 K and then calcinated. Where it is heated to 393 K (5 K/min heating) for 2 h followed by 4 h at 673 K (5 K/min heating and cooling). The resulting Au/SiO2 catalyst had a deep purple color. The resulting Au/SiO2 was analyzed by transmission electron microscopy (TEM), where at least 100 nanoparticles were measured for calculations.
Synthesis of target Ti-POSS-SH-Au/SiO2 hybrid material (4): The Ti-POSS-SH catalyst 3 (0.3 g, 1wt%) was dissolved in 10 mL diethyl ether and (3 g, 10 wt%) of Au/SiO2 nanoparticles was added to the previous solution in the glove-box and stir overnight, the solvent was removed under reduced pressure, the product was dried overnight to afford the hybrid material 4 which is light purple in color.
Epoxidation efficiency of catalyst 4
In order to test a catalyst, we used a reactor where a glass wool was lightly packed in the bottom of the column until the thermocouple came in contact with the glass wool. This was performed so that the thermocouple would reside in the catalysts bed yielding a more accurate temperature reading. 0.4 g of catalyst was added followed by glass wool lightly packed to keep catalyst in place. Oven was set to desired temperature and allowed to stabilize. The heat tape along output lines were set at 100°C. After the oven and heat tape were stable, the gas mixture was allowed to flow through reaction column.
Results and Discussion
Synthesis of gold nanoparticles deposited on SiO2
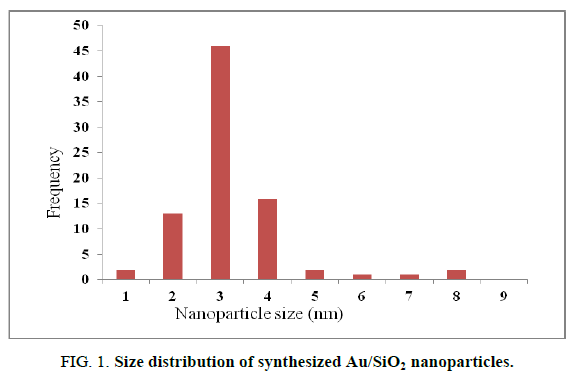
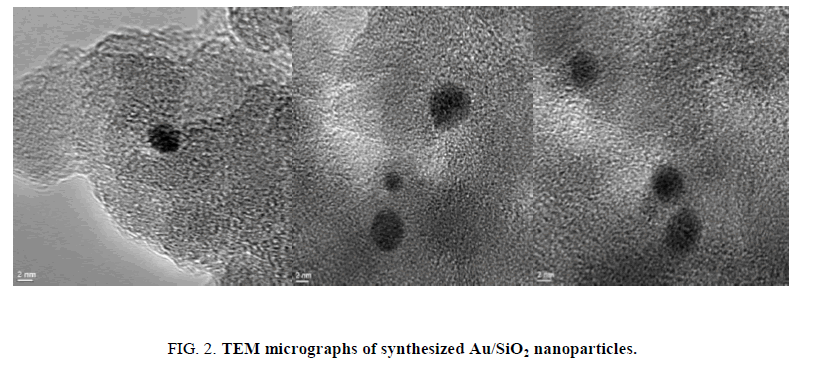
Using deposition precipitation route, 19 the Au nanoparticles were deposited on SiO2. The SiO2 is dispersed in water and the pH is raised to 9.5 using 2.5% ammonia solution, the pH value was kept between 9.4 and 9.6. Then, Au was slowly added over 15 min. The mixture was stirred for 1 h, filtered, washed, calcinated and then air cooled. The obtained Au/SiO2 was characterized by TEM. A (2-5 nm) size range was observed for Au/SiO2 (FIG. 1). TEM micrographs (FIG. 2) showed that the isolated gold nanoparticles did not overlap with each other. It has been reported that Au nanoparticles in the 2-5 nm range are the most active for the epoxidation of propene, so that Au/SiO2 nanoparticles should display catalytic activity for the formation of H2O2 from H2 and O2.
Herein we described an easy and efficient method for preparation and grafting of SH-functionalized tripodal Ti-POSS complex in gold nanoparticles supported in SiO2 and obtained a hybrid material that possess two important features. The gold nanoparticles is of good particle size and capable of in situ generating H2O2 as the oxidant in propene epoxidation and SH-functionalized tripodal Ti-POSS catalyst possess an excellent epoxidation activity which will utilize the peroxide to form the propene oxide selectively. This hybrid material has potential application in the epoxidation of alkenes. The hybrid catalyst 4 showed very good PO selectivity up to 99%, and the conversion was good relative to Au/TiO2 catalyst. Also the catalyst 4 was recycled four times with retained selectivity to PO and with same conversion.
Synthesis of SH-functionalized Ti-POSS complexes
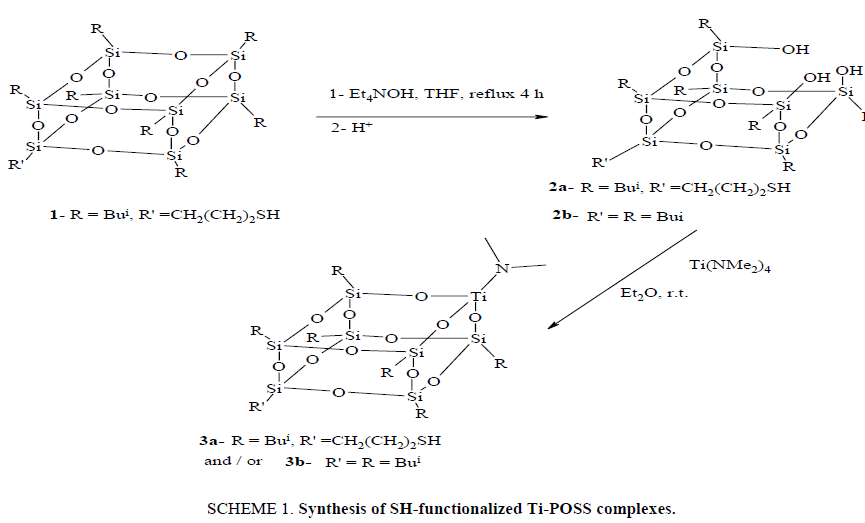
The synthesis of 2 (SCHEME 1) was accomplished by one silicon corner excision from POSS-SH 1 using Et4NOH to produce (HS(CH2)3(Bui)6Si7O9(OH)3 (2), yield (34%). The structure of 2 was characterized by (1H, 13C, and 29Si) NMR and IR. 29Si NMR showed Si-OH at -58.7 and overlapping Si atoms for POSS between δ -67.2 and - 69.7. The 1H NMR SH group at δ 1.2 and SiOH protons at δ 6.40 ppm. The IR for 2 showed OH at (3336 cm-1) and SH at (2609 cm-1). Ti was inserted into 2 via protonolysis of Ti(NMe2)4 with one equivalent of 2 afforded 3 in excellent yield (SCHEME 1). The structure of 3 was characterized by (1H, 13C and 29Si) NMR, UV-vis, IR, and elemental analysis. 1H NMR showed the loss of SiOH at (6.40 ppm) while the SH remained at 1.2 in 3. The 29Si NMR showed no SiOH resonances at -58.7 ppm and showed the Si atoms of POSS cadge at -67.9, -68.1 and -68.3 ppm. IR spectra of 3 showed the loss of the OH at (3336 cm-1) while the SH at (2624 cm-1) remained. UV-vis spectra showed λmax at (230 nm) corresponding to Ti silicate in tetrahedral environment.
Synthesis of Ti-POSS-SH-Au/SiO2 hybrid material 4
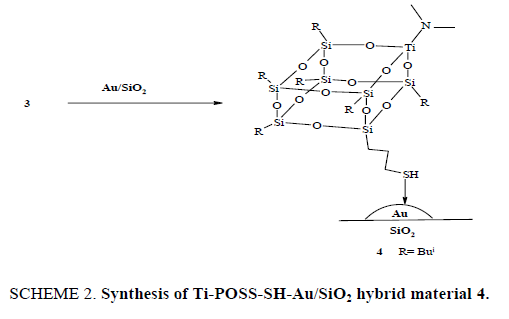
After preparing Au/SiO2 nanoparticles that possess a correct size range for epoxidation, a tripodal Ti-POSS-SH complex 3 was synthesized and was then grafted on Au nanoparticle. The Au-SH bond dissociation was determined to be 50 kcal/mol, so that Au-SH bonds display good strength [19-23]. SH-functionalized tripodal Ti-POSS 3 was grafted in the Au/SiO2 support (SCHEME 2) by adding Au/SiO2 support to the solution of 3 in Et2O. The mixture was allowed to stir overnight and then dried under reduced pressure. The resulting material was washed with acetonitrile and then the solvent was removed under reduced pressure to afford the hybrid material 4.
Herein we described an easy and efficient method for preparation and grafting of SH-functionalized tripodal Ti-POSS complex in gold nanoparticles supported in SiO2 and obtained a hybrid material that possess two important features. The gold nanoparticles is of good particle size and capable of in situ generating H2O2 as the oxidant in propene epoxidation and SH-functionalized tripodal Ti-POSS catalyst possess an excellent epoxidation activity which will utilize the peroxide to form the propene oxide selectively. This hybrid material has potential application in the epoxidation of alkenes. The hybrid catalyst 4 showed very good PO selectivity up to 99%, and the conversion was good relative to Au/TiO2 catalyst. Also the catalyst 4 was recycled four times with retained selectivity to PO and with same conversion.
Epoxidation efficiency of catalyst 4
In order to test a catalyst, we used reactor where a glass wool was lightly packed in the bottom of the column until the thermocouple came in contact with the glass wool. This was performed so that the thermocouple would reside in the catalysts bed yielding a more accurate temperature reading. 0.4 g of catalyst was added followed by glass wool lightly packed to keep catalyst in place. Oven was set to desired temperature and allowed to stabilize. After the oven was stable, the gas mixture was allowed to flow through reaction column.
Based on our preliminary epoxidation experiments using 4 to form propene oxide (PO) from propene (TABLE 1, ENTRY 3-5), the catalyst 4 showed very good PO selectivity up to 99%, and the conversion was good relative to Au/TiO2 catalyst. Also the hybrid catalysts were recycled for four times and still have the same selectivity to PO with retained conversion.
| Entry No. | Catalyst | Temp.°C | Selectivity for PO % | Yield % |
|---|---|---|---|---|
| 1 | Au/SiO2 | 70 | 0 | 0 |
| 2 | Au/TiO2 | 70 | 100 | 0.8 |
| 3 | 4 | 70 | 99 | 1.4 |
| 4 | 4* | 70 | 98 | 1.2 |
| 5 | 4** | 70 | 98 | 1.2 |
0.4 g catalysts (5% Ti loading for 4), 1:1:1 O2:H2:Propene, Feed flow=100 sccm, * recycled 4, ** 4 times recycled 4
Table 1: Epoxidation efficiency of catalyst 4.
Conclusion
The present study describes the synthesis and characterization of SH-functionalized tripodal Ti-POSS catalyst and it’s grafting in gold nanoparticles supported in SiO2 by an in situ method, the Ti-POSS-SH-Au/SiO2 hybrid material 4 has potential application in the epoxidation of alkenes using in situ generated H2O2 from H2 and O2 gases over the bifunctional hybrid catalyst. We have previously proven that tripodal Ti-POSS complexes have novel epoxidation properties where we immobilized those catalysts in PDMS membrane or in hyper-branched polymer.22, 23. The hybrid catalyst 4 showed very good PO selectivity up to 99%, and the conversion was good relative to Au/TiO2 catalyst. Also the hybrid catalyst 4 was recycled for four times and still has the same selectivity to PO with same conversion.
Acknowledgement
Thanks are expressed to the King Faisal University (the Deanship of Scientific Research: project 170041) for funding and support of this work. Some of the work and analyses were done at Menoufiya University; thanks are expressed to them for their support of this work.
Supporting Information
Full experimental detail, 1H and 13C NMR spectra. This material can be found via the “Supplementary Content” section of this article’s webpage.
References
- Green IX, Wenjie T, Matthew N, et al. Insights into catalytic oxidation at the Au/TiO2 dual perimeter sites. Acc Chem Res. 2014;47:805-15.
- Haruta M. Catalysis of gold nanoparticles deposited on metal oxides. Cattech. 2002;6:102-15.
- Huang JH, Haruta M. Gas-phase propene epoxidation over coinage metal catalysts. Res Chem Intermed. 2012;38:1-24.
- Sinha K, Seelan S, Tsubota, S, et al. Catalysis by gold nanoparticles: Epoxidation of propene. Top Catal. 2004;29:95-102.
- Meenakshisundaram S, Nowicka E, Miedziak PJ, et al. Oxidation of alcohols using supported gold and gold-palladium nanoparticles. Faraday Discuss. 2010;145:341-56.
- Gluhoi AC, Bakker JW, Nieuwenhuys BE, et al. Gold, still a surprising catalyst: Selective hydrogenation of acetylene to ethylene over Au nanoparticles. Catal Today. 2010;154:13-20.
- Hayashi T, Tanaka K, Haruta M. Selective vapor-phase epoxidation of propylene over Au/TiO2 catalysts in the presence of oxygen and hydrogen. J Catal. 1998;178:566-75.
- Hashmi ASK, Hutchings GJ, Angew. Gold catalysis. Chem Int Ed. 2006;45:7896-936.
- Sinha AK, Seelan S, Tsubota S, et al. Catalysis by gold nanoparticles: Epoxidation of propene. Catal. 2004;29:95-102.
- Nijhuis TA, Makkee M, Moulijn JA, et al. The production of propene oxide: Catalytic processes and recent developments. Ind Eng Chem Res. 2006;45:3447-459.
- Tullo AH, Short PL. Women in industry. Chem Eng News. 2006;84:22-3.
- Stangland EE, Stavens KB, Andres RP, et al. Characterization of Gold-Titania catalysts via oxidation of propylene to propylene oxide. J Catal. 2000;191:332-47.
- Sivadinarayana C, Choudhary TV, Daemen LL, et al. The nature of the surface species formed on Au/TiO2 during the reaction of H2 and O2: An inelastic neutron scattering study. J Am Chem Soc. 2004;126:38-9.
- Edwards J, Landon P, Carley AF, et al. Nanocrystalline gold and gold-palladium as effective catalysts for selective oxidation. J Mater Res. 2007;22:831-37.
- Landon P, Collier PJ, Papworth AJ, et al. Direct formation of hydrogen peroxide from H2/O2 using a gold catalyst. Chem Commun. 2002:2058-059.
- Chowdhury B, Bravo-Suarez JJ, Mimura N, et al. In situ UV-vis and EPR study on the formation of hydroperoxide species during direct gas phase propylene epoxidation over Au/Ti-SiO2 catalyst. J Phys Chem B. 2006;110:2995-999.
- Ishihara T, Ohura Y, Yoshida S, et al. Synthesis of hydrogen peroxide by direct oxidation of H2 with O2 on Au/SiO2 catalyst. Appl Catal A. 2005;291:215.
- Sinha AK, Seelan S, Tsubota S, et al. A three-dimensional mesoporous titanosilicate support for gold nanoparticles: Vapor-phase epoxidation of propene with high conversion. Angew Chem Int Ed. 2004;43:1546-548.
- Nijhuis TA. Spectroscopic evidence for the adsorption of propene on gold nanoparticles during the hydro-epoxidation of propene. J Catal. 2008;258:256-64.
- Xue Y, Li X, Li H, et al. Quantifying thiol-gold interactions towards the efficient strength control. Nature Communications. 2014;5:4348.
- Zhu Y, Wang, Q, Cornwall RG, et al. Organocatalytic asymmetric epoxidation and aziridination of Olefins and their synthetic applications. Chem Rev. 2014;114:8199.
- Aish EH, Crocker M, Ladipo F. Tripodal titanium silsesquioxane complexes immobilized in polydimethylsiloxane (PDMS) membrane: Selective catalysts for epoxidation of cyclohexene and 1-octene with aqueous hydrogen peroxide. J Catal. 2010;273:66-2.
- Aish EH. Synthesis and catalytic applications of chemically grafted SiH-functionalized tripodal Ti-POSS complexes in crosslinked hyperbranched poly (siloxysilane). Aust J of Chem. 2015;68:1091-101.