Research
, Volume: 18( 1)Synthesis and Characterization of Biologically Important Organothallium Complexes with N4 and N6 Macrocyclic Ligands
- *Correspondence:
- VP Shukla
Department of Chemistry, Bipin Bihari College, Jhansi, UP 284001, India
E-mail: drvedshukla@gmail.com
Received: May 14, 2023, Manuscript No. TSIC-23-98758; Editor assigned: May 17, 2023, PreQC No. TSIC-23-98758 (PQ); Reviewed: May 31, 2023, QC No. TSIC-23-98758; Revised: July 27, 2023, Manuscript No. TSIC-23-98758 (R); Published: August 03, 2023, DOI: 10.37532/0974-746X.2023.18 (1).002.
Citation: VP Shukla, et al. Synthesis and Characterization of Biologically Important Organothallium Complexes with N4 and N6 Macrocyclic Ligands. Inorg Chem Ind J. 2023; 18 (1).002.
Abstract
Metal macro cycles are widely studied due to their special physicochemical properties and their potential applications in various fields. The novel complexes of (PhTlCl2) and (Ph2TlCl) were explored with macrocyclic Tetradentate (N4) and Hexadentate (N6) ligands. The synthesized complexes were characterized by elemental analysis, molar conductance, IR, and XPS spectral studies. The ligands and their metal complexes were screened for photoelectron peaks and geometry of complexes. The result show that the activity of ligands towards chelating activity becomes more pronounced and significant when coordinated to the bioactive metal ion. The proposed structure of complexes with said ligands have been compared and discussed.
Keywords
Metal macrocycles, Physicochemical, Molar conductance, Spectral parameters, Binding energy
Introduction
The chelation chemistry of macromolecules predecessor is a charming area which has attracted the attention of inorganic and organic chemists. Macrocyclic complexes of bioactive metals have been of great interest due to their importance in view of their various applications in the production of metal carbonyls, antimicrobial, biochemistry, organometallic chemistry. Chelation activity of amino acid, peptides, Schiff bases and their derivatives with biologically active metal ions are of great significance as many complex metal macrocyclic ligands equilibria occurring in enzymatic process [1,2]. An enormous number of organ thallium (III) complexes with a macrocyclic ligand have been reported. Various macrocyclic bioactive metal complexes have been used as in anti-cancerous, antifungal, antitumor, metal ion separation, photosenstizer chelation therapy and as NMR shift and relaxation agents. Macrocyclic complexes are also advantageous in terms of selectivity, since they have stringent structures and can thus exact special coordination geometry to the metal ion. In this communication, we report here the synthesis of (PhTlCl2) and (Ph2TlCl) complexes with N4 and N6 macrocyclic ligands. These complexes were characterised on the basis of elemental analysis, molar conductance IR and XPS spectral studies. Materials and Methods All reagents used were of A.R. grade (Aldrich). Solvents were distilled from relevant drying agent immediately in earlier of used metal complexes prepared by published method. The elemental analysis like C and H were determined by CDRI Lucknow, India, nitrogen and halogen were determined by kjeldhal's and Volhard's methods respectively. The melting point was evaluated by using in sealed capillary tube on melting point apparatus. Molar conductivity of complex was measured on Digisum electronic conductivity bridge in DMF at room temperature [3]. The infrared spectra of complexes and ligands were recorded on Perkin-Elmer 457 spectrometer at room temperature in KBr/CsCl pellets. Kratos analytical axis supra ESCA i.e. X-ray photoelectron spectra (XPS) instrument equipped with monochromatised AlKα (1486.6 ev.) source is used. All the peaks were rectified for charging with reference to C1S peak 284.8 ev and countered with Shirley background and a union of Gaussian and Lorentz an line-shapes, using ESCAPe computer software [4].
Preparation of PhTlCl2 and Ph2TlCl macrocyclic complexes with L1, L2, L3, L4, L5, L6, L7: PhTlCl2 and Ph2TlCl (1 mmol ) was dissolved in 20 ml of C2H5OH and to this a solution of 3,4 hexanedione (2 mmol) was added dropwise with constant stirring. This was followed by dropwise addition of 1,3-diaminopropane (2 mmol) in C2H5OH (20 ml) with stirring which was continued for 5 hrs. A white solid appeared which was filtered, washed with C2H5OH and dried under vacuum (with L1 Ligand). A similar procedure was adopted for the synthesis of PhTlCl2 and Ph2TlCl complexes of macrocycles derived from 3,4-hexanedione with 1,4-diaminobutane (L2), 1,5-diaminopentane (L3), 1,7-diaminoheptane (L4), 1,8-diaminooctane (L5), 1,9-diaminononane (L6) and 1,10-diaminoecane (L7) [5].
Synthesis of macrocyclic PhTlCl2 and Ph2TlCl complexes with L8 and L9: PhTlCl2 and Ph2TlCl (1 mmol) was dissolved in 20 ml C2H5OH with stiring and (2 mmol) of 2,3-hexanedione in 15 ml of (C2H5OH) was added. A solution of 1,9-diaminononane or 1,10-diaminodecane (2 mmol) in 15 ml C2H5OH was added dropwise with constant stirring (5 hrs). The solid product was filtered, washed with C2H5OH and dried under reduced pressure.
Synthesis of ligand L10: In aqueous (50 ml) solution of semicarbazide hydrochloride (0.02 mol, 1.5 g) and 2,3-pentanedione (0.02 mol, 2.08 ml) were added slowly with constant stirring for 15-20 minutes. Both diamine and diketone were mixed in 1:1 molar concentration ratio. Mixture was cooled upto 5°C and kept undisturbed for 12 hrs [6]. On cooling white precipitate was filtered, washed with distilled water and dried under vaccum over P4O10. M.p. 240°C, Found C=51.7, H=6.4, N=30.0, calculated C=51.79, H=6.47, N=30.12.
Synthesis of ligand L11: Hot ethanolic solution (50 ml) thiosemicabazide (0.02 mol, 1.8 g) and 2,3-pentanedione (0.02 mol, 2.08 ml)were mixed slowly with constant stirring. The mixture was refluxed at 80°C for 6-8 hrs. on cooling upto 5°C cream coloured was filtered washed with cold C2H5OH and dried over P4O10, m.p. 220°C, found C=46.4, H=5.8, 27.0, calculated C=46.48, H=5.82, N=27.2.
Synthesis of ligand L12: Semicarbazide (0.02 mol, 3.82 g), hot ethanolic solution (50 ml) furil (0.02 mol, 3.82 g) and hot aqueous solution (50 ml) were added slowly with constant stirring. The mixture was refluxed at 80°C for 6-8 hrs [7,8]. On cooling upto 5°C, cream coloured precipitate was filtered, washed with cold C2H5OH and dried under vaccum over P4O10. m.p. 220°C, found= 57.5, H=3.0, N=18.12, calculated C=57.64, H=3.05, N=18.34).
Synthesis of ligand L13: Thiosemicabazide (0.02 mol, 1.83 g), hot ethanolic solution of furil (0.02 mol, 3.82 g) and hot ethanolic solution (50 ml) were mixed slowly with constant stirring. The mixture was refluxed at 80°C for 6-8 hrs. On cooling upto 5°C, coloured precipitate was filtered, washed with cold C2H5OH and dried under vaccum over P4O10.m.p. 236°C. Found C=54.12, H=2.78, N=17.14; calculated C=54.32, H=2.88, N=17.28.
Preparation of (PhTlCl2) and (Ph2Tl.L) complexes (where L=L10 to L13): (PhTlCl2) or (Ph2TlCl) (1 mmol) was dissolved in 20 ml C2H5OH with constant stirring and 1 mmol of prepared macrocyclic ligand (L10 or L11 or L12 or L13) in 15 ml of C2H5OH was added. The stirring was continued for 5 hrs. The solid product was filtered, washed with C2H5OH and dried over vaccum.
Preparation of complexes with L14, L15, L16, L17, L18 macrocyclic ligands: All the complexes were prepared by template method. Hot ethanolic solution (10 ml) of (PhTlCl2) or (Ph2TlCl) (0.001 mol), hot ethanolic solution (10 ml) of diamine i.e. diethylene triamine (L14 or L15 or L16 or L17) (0.002 mol) and ethanolic solution of carbonyl compounds i.e. benzaldehyde or salicylaldehyde or cyclohexanone or glutaric anhydride (0.002 mol) are mixed together and the mixture was refluxed for 4-5 hrs. On cooling coloured precipitate filtered, washed with ethanol and dried over P4O10 under vaccum . A hot ethanolic solution of glutaric acid (0.01 mol) is added to an etanolic solution of 2,6-diaminopyridine (0.01 mol) L18 and the resulting solution was refluxed for one hour. A solution of PhTlCl2 or Ph2TlCl (0.005 mol) was then added to the above solution and refluxed for 4-6 hrs. On cooling the solution a crystalline compound separates out [9-12]. It is then filtered, washed with ethanol and dried under vaccum over P4O10.
Results and Discussion
These newly synthesized (PhTlCl2) and (Ph2TlCl) complexes were while solid and stable at room temperature. The elemental analysis and molar conductance data are listed in Table 1 [13,14]. The low molar conductance in DMF 20-30 ohm-1cm2mol-1 of these complexes indicates that all these complexes indicate that all these are nonelectrolyte.
| Sn. No. | Complexes | Found (Calc%) | Molar conductance ohm-1cm2mol-1 | ||
|---|---|---|---|---|---|
| C | H | N | |||
| 1 | (Ph2TlCl.L1) | 48.6 (48.7) | 5.4 (5.6) | 7.4 (7.5) | 18 |
| 2 | (Ph2TlCl.L2) | 52.8 (52.9) | 6.2 (6.3) | 7.4 (7.7) | 16 |
| 3 | (Ph2TlCl.L3) | 54.0 (54.1) | 6.4 (6.6) | 7.2 (7.4) | 20 |
| 4 | (Ph2TlCl.L4) | 55.0 (55.2) | 6.8 (6.9) | 7.0 (7.1) | 22 |
| 5 | (Ph2TlCl.L5) | 57.0 (57.2) | 7.2 (7.4) | 6.4 (6.6) | 18 |
| 6 | (Ph2TlCl.L6) | 58.0 (58.2) | 7.4 (7.6) | 6.2 (6.4) | 14 |
| 7 | (Ph2TlCl.L7) | 59.0 (59.0) | 7.6 (7.8) | 6.0 (6.2) | 16 |
| 8 | (Ph2TlCl.L8) | 63.2 (63.3) | 6.4 (6.6) | 5.4 (5.6) | 20 |
| 9 | (Ph2TlCl.L9) | 59.2 (59.0) | 7.6 (7.8) | 6.0 (6.2) | 18 |
| 10 | (Ph2TlCl.L10) | 44.0 (44.5) | 4.4 (4.6) | 4.8 (4.9) | 20 |
| 11 | (Ph2TlCl.L11) | 40.6 (40.8) | 4.1 (4.2) | 11.8 (11.9) | 18 |
| 12 | (Ph2TlCl.L12) | 47.6 (47.8) | 2.6 (2.8) | 9.6 (9.8) | 20 |
| 13 | (Ph2TlCl.L13) | 46.0 (46.1) | 2.6 (2.7) | 9.4 (9.5) | 23 |
| 14 | (Ph2TlCl.L14) | 60.4 (60.5) | 5.2 (5.3) | 8.4 (8.8) | 28 |
| 15 | (Ph2TlCl.L15) | 56.6 (56.7) | 5.0 (5.0) | 8.0 (8.2) | 18 |
| 16 | (Ph2TlCl.L16) | 59.2 (59.0) | 7.4 (7.6) | 8.4 (8.6) | 15 |
| 17 | (Ph2TlCl.L17) | 46.0 (46.2) | 4.4 (4.6) | 11.4 (11.5) | 20 |
| 18 | (Ph2TlCl.L18) | 48.4 (48.6) | 3.0 (3.2) | 11.2 (11.3) | 12 |
| 19 | (PhTlCl2.L1) | 43.4 (43.8) | 5.4 (5.6) | 8.4 (8.5) | 20 |
| 20 | (PhTlCl2.L2) | 44.8 (44.9) | 5.8 (5.9) | 8.0 (8.0) | 12 |
| 21 | (PhTlCl2.L3) | 47.0 (47.1) | 6.2 (6.3) | 7.4 (7.8) | 22 |
| 22 | (PhTlCl2.L4) | 75.4 (75.6) | 6.4 (6.6) | 7.4 (7.5) | 16 |
| 23 | (PhTlCl2.L5) | 51.0 (51.2) | 7.0 (7.1) | 7.0 (7.0) | 18 |
| 24 | (PhTlCl2.L6) | 52.2 (52.3) | 7.2 (7.4) | 6.4 (6.7) | 24 |
| 25 | (PhTlCl2.L7) | 53.4 (53.5) | 7.4 (7.6) | 6.4 (6.5) | 12 |
| 26 | (PhTlCl2.L8) | 58.2 (58.4) | 6.2 (6.4) | 5.8 (5.9) | 14 |
| 27 | (PhTlCl2.L9) | 53.4 (53.5) | 7.4 (7.6) | 6.4 (6.5) | 18 |
| 28 | (PhTlCl2.L10) | 35.4 (35.7) | 4.0 (4.1) | 9.0 (9.2) | 24 |
| 29 | (PhTlCl2.L11) | 32.4 (32.5) | 3.6 (3.7) | 12.4 (12.6) | 22 |
| 30 | (PhTlCl2.L12) | 41.0 (41.4) | 2.2 (2.3) | 10.2 (10.3) | 16 |
| 31 | (PhTlCl2.L13) | 39.4 (39.8) | 2.0 (2.2) | 9.8 (9.9) | 18 |
| 32 | (PhTlCl2.L14) | 55.0 (55.4) | 5.0 (5.0) | 9.0 (9.2) | 12 |
| 33 | (PhTlCl2.L15) | 51.6 (51.7) | 4.6 (4.7) | 8.4 (8.6) | 14 |
| 34 | (PhTlCl2.L16) | 54.0 (54.0) | 7.4 (7.5) | 9.0 (9.0) | 18 |
| 35 | (PhTlCl2.L17) | 38.4 (38.5) | 4.0 (4.2) | 12.0 (12.2 | 16 |
| 36 | (PhTlCl2.L18) | 41.0 (41.2) | 2.6 (2.7) | 12.0 (12.0) | 12 |
TABLE 1. Elemental analysis and molar conductance of (Ph2TlCl.L) and (PhTlCl2.L) complexes.
Characterisation of (PhTlCl2.L) and (Ph2TlCl.L) complexes , Where, L=L1 or L2 or L3 or L4 or L5 or L6 or L7 or L8 or L9 : In the IR spectra of the macrocyclic complexes, no absorption band was observed at 1700 cm-1 and 3200-3400 cm-1 indicating the absence of unreacted >C=O or –NH2 group. Thus>C=O or –NH2 groups have condensed to give C=N linkage. All the complexes synthesised during present investigations show one peak and one strong absorption bands in the region 1520-1540 cm-1 and 1580-1630 cm-1 respectively which can be attributed to the uncoordinated >C=N group and coordinated >C=N group. The bands at 409-413 cm-1 are due to coordinated chloro coordinated group behaving as a monodentate ligand. 1HNMR spectrum of one representative (PhTlCl2) and (Ph2TlCl) complexes have been recorded. The α-CH2 protons of the amine residue give a triplet at δ2.63 ppm due to coupling with the β-CH2 protons. The β-CH2 protons of the amine residue give a broad peak at δ1.50 ppm. The remaining metylene protons (γ and other) of the amine residue give rise to a broad peak at δ1.32 ppm. In macrocyclic precursor, 1,2,8,9-tetraphenyl-3,7-diazaduohepta-2,7-diene 1,9-diene 1,9-dione (KIM) the α-CH2 protons have been reported to appear as a triplet at δ3.63 ppm and β-CH2 protons as a quintet at δ2.11 ppm. The free macrocycle has not been isolated during the present investigation but it is expected to exhibit the α and β-CH2 protons almost at the same position as reported for KIM. As compared to KIM, in the (PhTlCl2) and (Ph2TlCl) complex of the macrocycle these peaks observed at higher field. The high field shifting of these protons confirms the coordination of the nitrogen atom of the macrocycle to thallium ion. The CH3a protons of the ketone residue give rise to a triplet at δ0.99 ppm due to coupling with the CH2b protons. The peaks due to CH2b protons of the ketone residue merge with the peaks of the CH2 (β and others) protons of the amine residue [15].
Characteriation of (PhTlCl2.L10) and (Ph2TlCl.L10) complexes: The infrared spectrum of ligand L10 does not exhibit any characteristic for free –NH and OH groups indicating the absence of free primary diamine and hydroxyl group, which suggest the complete condensation of keto group with amino group. In this spectrum, appearance of new bands characterstic of thioamide group at 1690ϑ (C=O) amide I, 1579ϑ (CO-NH), 1438ϑ (C-N) + δ (NH) amide II, 1262 δ (N-H) amide III and 728 φ cm-1 amide IV, which support the macrocyclic species. A sharp band observed in the region 3346 cm-1 and 3490 cm-1 may be assigned to ϑ (N-H) of secondary amino group. IR spectra of all (PhTlCl2.L10) and (Ph2TlCl.L10) complexes have shown the shifting in ϑC=N, to lower side than ligand, an uncoordinated peak appear on same position as in ligand and there is no change in ϑ(C=O) and ϑ(NH) absorption bands in all (PhTlCl2.L10) and (Ph2TlCl.L10) complexes than ligand confirms that coordination takes place through the nitrogen of ϑC=N group, one C=N is uncoordinated but not through ϑC=O and –NH groups.
Characterisation of (PhTlCl2.L11) and (Ph2TlCl.L11) complexes: The infrared spectrum of ligand L11 does not exhibit any band around 3400 cm-1 characteristic for free –NH groups, indicating the absence of free primary amine and hydroxyl group which suggest complete condensation of keto group with amino group. In this spectrum, appearance of new band characteristic of thioamide groups at 1607 ϑ (CS-NH), 1516 ϑ (C-N) + δ(N-H), 1263 δ(N-H), which support the macrocyclic species. A broad band observed in the region 3168 cm-1 due to ϑ (N-H) of secondary amino group. IR spectra of all (PhTlCl2.L11) and (Ph2TlCl.L11) complexes have shown the shifting in ϑC=N to lower side than ligand but one ϑC=N peak on same position as in ligand and there is no change in ϑC=S and ϑN-H absorption bands in all (PhTlCl2.L11) and (Ph2TlCl.L11) complexes than ligand, confirms that coordination takes place through the nitrogen of ϑC=N group, but not through –C=S and –NH groups [16,17].
Characterisation of (PhTlCl2.L12) and (Ph2TlCl.L12) complexes: The infrared spectrum of ligand L12 does not exhibit any characteristic bands for free –NH groups and the appearance of new bands characteristic of amide group at 1685ϑ (C=O) amideI, 1603 ϑ (CONH), 1507ϑ (CN) + δ (NH) amide II, 669φ(C=O) cm-1 and a bandat 2921 cm-1 shows C-H stretching, which support the macrocyclic species. Bands observed in the region 3293-3049 cm-1 due to ϑ (NH) of secondary amino group. IR spectra of all (PhTlCl2.L12) and (Ph2TlCl.L12) complexes have shown the shifting in ϑC=N, to lower side than ligand but one ϑC=N on same position as in ligand and there is no change in ϑ(C=O) and ϑ(NH) absorption bands in all (PhTlCl2.L12) and (Ph2TlCl.L12) complexes than ligand, confirms that coordination takes place through the nitrogen of ϑC=N group but not through –C=O and –NH groups.
Characterisation of (PhTlCl2.L13) and (Ph2TlCl.L13) complexes: The infrared spectrum of ligand L13 does not exhibit any characteristics band for free –NH group, and the appearance of new bands characteristics to thioamide group at 1644 ϑ (C=S) amide I, 1619 ϑ (CS-NH), 1531 ϑ(C-N) + δ(N-H), 1284 δ(N-H) and 646 φ(C=S)35-36 , which support macrocyclic. A sharp band observed in the region 3262-3177 cm-1 due to ϑ (N-H) of secondary amino group37. IR spectra of all (PhTlCl2.L) and (Ph2TlCl.L) complexes have shown the shifting in ϑC=S to lower side than ligand but one ϑC=N peak on same position as ligand and there is no change in ϑC=S and ϑN-H in all (PhTlCl2.L) and (Ph2TlCl.L) complexes than ligand, confirms that coordination takes place through the nitrogen of ϑC=N group but not through –C=S and –NH groups [18,19].
Characterisation of (PhTlCl2.L13) and (Ph2TlCl.L13) complexes (where L=L14, L15, L16, L17 and L18): An examination of the IR spectra of (PhTlCl2.L) and (Ph2TlCl.L) (where L=L14, L15, L16, L17 and L18) have shown the absence of absorption around 3400 cm-1. This shows the absence of a free amino group [20]. The presence and the shifting of the ϑC=N bands 1620 cm-1 towards lowerside in all these metal complexes and one ϑC=N peak present on same position as in ligand indicates that coordination takes placethrough the nitrogen of the ϑC=N group and one ϑC=N is uncoordinated. The IR spectra of (PhTlCl2.L) and (Ph2TlCl.L) have shownabsorption band at 403-413 cm-1.
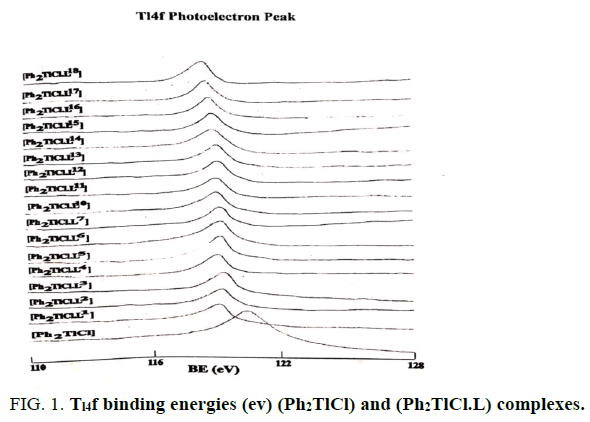
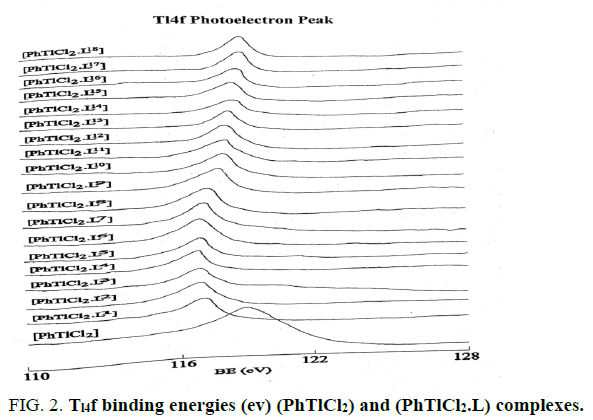
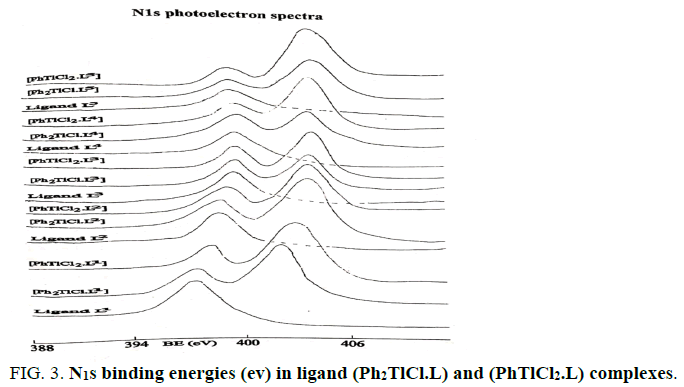
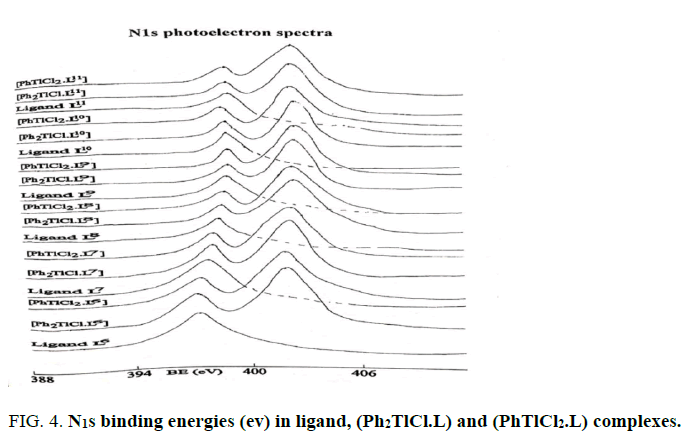
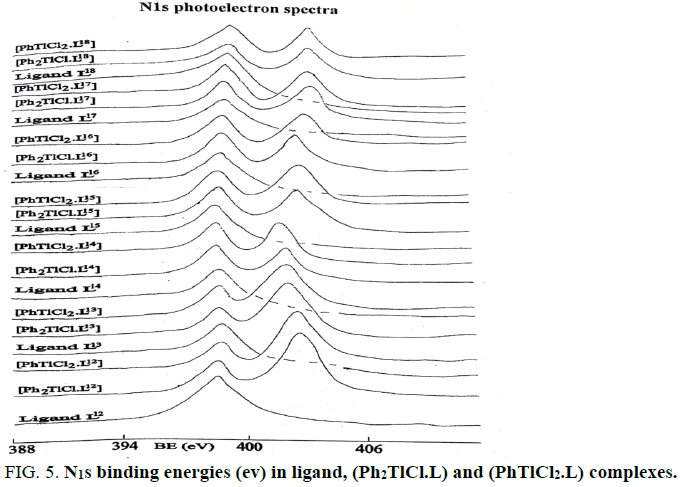
The Tl4f and N1S binding energies (ev) data of (PhTlCl2) or ( Ph2TlCl) and (PhTlCl2.L) or (Ph2TlCl.L) where L= L1, L2, L3, L4, L5,L6, L7, L8, L9, L10, L11, L12, L13, L14, L15, L16, L17 and L18 are listed in Tables 2 and 3. It may be seem that Tl4f photoelectron peaks binding energy values were observed more in metal salts than in metal complexes [21,22]. It suggested that thallium ions have more electron density in metal complexes than (Ph2TlCl) or (PhTlCl2) due to involvement of metal ion in coordination. Further, N1S have shown two symmetrical peaks one with higher binding energy in (PhTlCl2.L) or (Ph2TlCl.L) complexes than free nitrogen atom N1S photoelectron peak as in free ligand other on same BE as in ligand in 3:1 intensity ratio, while in complexes intensity ratio 3:3 (Figures 1-6).
| Sr. No. | Compound | T14f |
|---|---|---|
| 1 | (Ph2TlCl) | 119.8 |
| 2 | (Ph2TlCl.L1) | 116.4 |
| 3 | (Ph2TlCl.L2) | 116.4 |
| 4 | (Ph2TlCl.L3) | 116.4 |
| 5 | (Ph2TlCl.L4) | 116.4 |
| 6 | (Ph2TlCl.L5) | 116.4 |
| 7 | (Ph2TlCl.L6) | 116.4 |
| 8 | (Ph2TlCl.L7) | 116.4 |
| 9 | (Ph2TlCl.L10) | 116.4 |
| 10 | (Ph2TlCl.L11) | 116.4 |
| 11 | (Ph2TlCl.L12) | 116.4 |
| 12 | (Ph2TlCl.L13) | 116.4 |
| 13 | (Ph2TlCl.L14) | 116.4 |
| 14 | (Ph2TlCl.L15) | 116.4 |
| 15 | (Ph2TlCl.L16) | 116.4 |
| 16 | (Ph2TlCl.L17) | 116.4 |
| 17 | (Ph2TlCl.L18) | 116.4 |
TABLE 2. TL4f Binding energies (ev) in (Ph2TlCl) and (Ph2TlCl.L).
| Sr. No. | Compound | T14f |
|---|---|---|
| 1 | (PhTlCl2) | 119.6 |
| 2 | (PhTlCl2.L1) | 116.2 |
| 3 | (PhTlCl2.L2) | 116.2 |
| 4 | (PhTlCl2.L3) | 116.2 |
| 5 | (PhTlCl2.L4) | 116.2 |
| 6 | (PhTlCl2.L5) | 116.2 |
| 7 | (PhTlCl2.L6) | 116.2 |
| 8 | (PhTlCl2.L7) | 116.2 |
| 9 | (PhTlCl2.L8) | 116.2 |
| 10 | (PhTlCl2.L9) | 116.2 |
| 11 | (PhTlCl2.L10) | 116.2 |
| 12 | (PhTlCl2.L11) | 116.2 |
| 13 | (PhTlCl2.L12) | 116.2 |
| 14 | (PhTlCl2.L13) | 116.2 |
| 15 | (PhTlCl2.L14) | 116.2 |
| 16 | (PhTlCl2.L15) | 116.2 |
| 17 | (PhTlCl2.L16) | 116.2 |
| 18 | (PhTlCl2.L17) | 116.2 |
| 19 | (PhTlCl2.L18) | 116.2 |
TABLE 3. T14f Binding Energies (ev) in (PhTlCl2) and (PhTlCl2.L) complexes.
FIG 6: Octahedral Geometry of (Ph2TlCl.L) complexes, Where In (Ph2TlCl.L)= X=Cl; X1= Ph, In (PhTlCl2.L)= X= Cl; X1=Cl.
Conclusion
On the basis of elemental analysis, molar conductance measurement, IR and XPS data and the subsequent discussion for the complexes the geometry as shown below.
References
- Kumar R, Singh R. Coordination chemistry of copper (II) complexes with N4, N4S2, and N4O2 donor macrocyclic ligands: Biological aspects-antifungal, synthesis, spectral studies, and magnetic moments. Russ J Coord Chem. 2006;32:192-198.
- Chaudhary A, Bansal N, Gajraj A, et al. Antifertility, antibacterial, antifungal and percent disease incidence aspects of macrocyclic complexes of manganese (II). J Inorg Biochem. 2003;96(2-3):393-400.
[Crossref] [Google Scholar] [PubMed]
- Rasool R, Hasnain S, Nishat N. Metal based Schiff base polymers: Preparation, spectral, thermal and their in vitro biological investigation. Des Monomers Polym. 2014;17(3):217-226.
- Saranya J, Lakshmi SS. In vitro antioxidant, antimicrobial and larvicidal studies of schiff base transition metal complexes. J Chem Pharm Res. 2015;7(4):180-186.
- Zhong ZJ, You XZ, Mak TC, et al. Synthesis, crystal structure and properties of a linear trinuclear manganese (II) complex (Mn3 (CH3CO2)2 (bpc)2 (py)4 (H2O)2)· 0.5 H2O. Polyhedron. 1994;13(14):2157-2161.
- Anacona JR, Bastardo E, Camus J, et al. Manganese (II) and palladium (II) complexes containing a new macrocyclic Schiff base ligand: Antibacterial properties. Transit Met Chem. 1999;24(4):478-480.
- Nazmutdinov RR, Roznyatovskaya NV, Glukhov DV, et al. Medium and interfacial effects in the multistep reduction of binuclear complexes with Robson type ligand. Inorg Chem. 2008;47(15):6659-6673.
[Crossref] [Google Scholar] [PubMed]
- Saleh AA. Synthesis and spectroscopic studies of novel mononuclear complexes of cyclic and acyclic Schiff-base derivatives of tridentate and tetradentate coordination with some bivalent transition metal ions. J Coord Chem. 2005;58(3):255-270.
- Bénazeth S, Purans J, Chalbot MC, et al. Temperature and pH dependence XAFS study of Gd (DOTA) and Gd (DTPA) 2-complexes: Solid state and solution structures. Inorg Chem. 1998;37(15):3667-3674.
[Crossref] [Google Scholar] [PubMed]
- Henrick K, Matthews RW, Tasker PA, et al. Molecular structures of methyl-5,10,15,20-tetraphenylporphinatothallium (III) and chloro-5,10,15,20-tetraphenylporphinatothallium (III). Inorg Chem. 1977;16(12):3293-3298.
- Kawasaki Y, Kitano RI. Dimethylthallium complexes of dicyclohexyl-18-crown-6. Chem Lett. 1978;7(12):1427-1430.
- Bertolo E, Bastida R, De Blas A, et al. Lanthanide (III) nitrate complexes of two 17 membered N3O2 donor macrocycles. J Incl Phenom Macrocycl Chem. 1999;35:191-198.
- Kawasaki Y, Yokota W, Enomoto N. Preparation of monophenylthallium (iii) dibenzo-18-crown-6 complexes with a soft ligand: (c6h5tl (dbc)x) clo4. Chem Lett. 1982;11(6):941-942.
- El-Boraey HA, El-Gammal OA. New 15-membered tetraaza (N4) macrocyclic ligand and its transition metal complexes: Spectral, magnetic, thermal and anticancer activity. Spectrochim Acta A Mol Biomol Spectrosc. 2015;138:553-562.
[Crossref] [Google Scholar] [PubMed]
- Tyagi M, Chandra S, Akhtar J, et al. Modern spectroscopic technique in the characterization of biosensitive macrocyclic Schiff base ligand and its complexes: Inhibitory activity against plantpathogenic fungi. Spectrochim Acta A Mol Biomol Spectrosc. 2014;118:1056-1061.
[Crossref] [Google Scholar] [PubMed]
- Chaudhary A, Singh RV. Antitumour, antimicrobial and antifertility affects of potentially biodynamic sixteen to twenty four membered tetraazamacrocyclic ligands and their tin (II) complexes. Phosphorus Sulfur Silicon Relat Elem. 2007;182(11):2647-2665.
- Adam KR, Antolovich M, Baldwin DS, et al. Ligand design and metal-ion recognition. The interaction of copper (II) with a range of 16 to 19 membered macrocycles incorporating oxygen, nitrogen and sulfur donor atoms. J Chem Soc Dalton Trans 1993;1(7):1013-1017.
- Bonnett R. Photosensitizers of the porphyrin and phthalocyanine series for photodynamic therapy. Chem Soc Rev. 1995;24(1):19-33.
- Fernandes AS, Cabral MF, Costa J, et al. Two macrocyclic pentaaza compounds containing pyridine evaluated as novel chelating agents in copper (II) and nickel (II) overload. J Inorg Biochem. 2011;105(3):410-419.
- Andersen O, Aaseth J. Molecular mechanisms of in vivo metal chelation: Implications for clinical treatment of metal intoxications. Environ Health Perspect. 2002;110(5):887-890.
[Crossref] [Google Scholar] [PubMed]
- Gonçalves S, Fernandes AS, Oliveira NG, et al. Cytotoxic effects of cadmium in mammary epithelial cells: Protective role of the macrocycle (15) pyN5. Food Chem Toxicol. 2012;50(6):2180-2187.
[Crossref] [Google Scholar] [PubMed]
- Rajak A, Srivastava A, Shrivastava SC, et al. Synthesis and spectral studies of Ru (II) complexes with macrocyclic ligands. Orient J Chem. 2021;37(4).