Original Article
, Volume: 12( 10)Removal of Rhodamine B Dye from Wastewater by Ultrasound-Assisted Fenton Process: A Comparison between Bath and Probe Type Sonicators
- *Corresponding Author:
- Chakrabarti S, Department of Chemical Engineering, University of Calcutta, Kolkata-700009, India, Tel: +91-9831384628; E-mail: sampac.2008@gmail.com
Received Date: October 05, 2016; Accepted Date: October 20, 2016; Published Date: October 25, 2016
Citation: Akram M, Chowdhury A, Chakrabarti S. Removal of Rhodamine B Dye from Wastewater by Ultrasound-Assisted Fenton Process: A Comparison between Bath and Probe Type Sonicators. Environ Sci Ind J. 2016;12(10):115.
Abstract
Sono-Fenton process is effective in treatment of turbid or dark wastewater. There are two ways of dispersing ultrasound in a solution - direct by probe and indirect by bath. In this work, a comparative study between the performances of the two methods of sonication has been reported in case of sono-Fenton degradation of Rhodamine B in water. Study of the influences of other process parameters viz pH, initial dye concentration, initial H2O2 concentration and initial Fe2+ concentration on the decolorisation efficiency were carried out for both types of sonicators. Blank experiments without ultrasound and without Fenton’s reagent were also performed for reference. Without ultrasound, the decolorisation by Fenton’s reagent only was 73.68% whereas with ultrasound it was 92.39% for bath-type and 93.85% for probe-type sonicator under high initial dye concentration (0.5 kg/m3). However, under low catalyst [Fe2+] dosage, decolorisation was 94% with probe sonicator and 55% with sonicator bath. Mineralization of the dye in terms of COD removal was studied. COD of 30.66% and 32.91% respectively were removed by bath-type and probe-type sonicators after 15 min of reaction whereas decolorisation was more than 99% under identical conditions. The results indicate that sono-Fenton process can be an effective treatment process for wastewaters containing organic dyes.
Keywords
Rhodamine B; Sono-Fenton process; Bath type sonicator; Probe type sonicator; Kinetic studies, Chemical oxygen demand
Introduction
Many industrial processes and agricultural and domestic activities lead to wastewater streams containing recalcitrant or refractory organic pollutants which need to be disposed of in a safe way. The concentration of chemicals in the treated effluent stream should be kept at a certain minimum level to comply with the environmental laws, which are becoming more stringent these days [1]. Refractory organic dyes present in wastewater can be a source of environmental pollution [2].
Rhodamine B (RhB) is a reddish violet coloured basic dye which belongs to the xanthene group of compounds. It is widely used as a coloring agent in jute, leather and textile industries and as a tracer in biological applications. It causes irritation to skins, eyes, respiratory and gastrointestinal tracts, and is a proven carcinogen and neurotoxin [3]. These dyes are discharged into natural water bodies and impart high chemical oxygen demand (COD) as well as toxicity to it. Due to the colour, they interfere with photosynthesis of aquatic plants, thereby affecting the aquatic ecosystem [4]. Coagulation, floatation, filtration and adsorption transfer the dye from one phase to another. Chemical based processes must incur the cost of chemicals as well as to face the problem of disposing the sludge generated. Moreover, these dyes are generally stable to chemical oxidizing agents [3]. Other technologies such as photocatalysis and fungi based bioreactors are also being explored [5-7]. Though sometimes their removal efficiencies are high with some dyes, most often they are not suitable for non-biodegradable refractory dyes, especially for azo dyes. Even if they work, the time taken for degradation is long. Alternative technologies such as advanced oxidation processes (AOP) are now being extensively explored as a viable means of treating these wastewaters. Fenton’s oxidation is an AOP, which is effective for degrading such pollutants by oxidation with hydroxyl radical (•OH) generated from Fenton’s reagent (ferrous iron and hydrogen peroxide, Fe2+/H2O2). The generated •OH radicals (Eq. 1) attack the unsaturated dye molecule and the chromophore of the dye molecule is destroyed and decolorised [8].
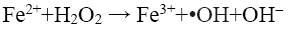 (1)
(1)
In presence of light or ultrasound, efficacy of Fenton’s reaction is enhanced. Ultrasound-assisted Fenton’s reaction or sono-Fenton process is especially effective for coloured or turbid effluent where penetration of light is too poor for photo-Fenton reaction. Ultrasound (US) coupled with Fenton’s reagent utilizes the advantages of these two methods to generate more •OH radicals and can effectively improve the degradation rate of organic pollutants. Mass transfer resistances associated with the Fenton based processes can also be eliminated due to turbulence caused by sonication [9].
In the sono-Fenton process free radicals are generated by transient collapse of cavitation bubbles which are formed due to the propagation of ultrasonic waves through water. The cavitation bubbles grow during compression-rarefaction cycles of the ultrasonic wave until they reach a critical size. Further compression leads to implosion of the bubbles which generates local temperatures around 5000 K and pressures of about 105 kPa. These high local temperature and pressures cause thermal dissociation of water vapour into hydroxyl radicals and hydrogen atoms [2,3]. The mechanism proposed for the generation of •OH by sonication is shown in Eq. 2 and 3:
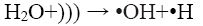 (2)
(2)
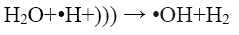 (3)
(3)
The degradation of organic pollutants by the reactive radical species can be explained by two mechanisms: a) thermal decomposition of the volatile organic molecules trapped inside the cavitation bubbles and b) reaction of •OH radicals with the pollutant molecules adsorbed on the bubble surface or present in the bulk solution [10].
Sono-Fenton process has already been used to decolorize solutions containing Reactive Blue 181 [4], Reactive Blue 19 [8], C.I. Reactive Orange 127 [10], C.I. Acid Orange 7 [11], Rhodamine B [3]. All these studies concluded that acidic pH was ideal for decolorization of dye solutions using sono-Fenton process. Decolorization rates were found to increase with decreasing initial dye concentration and increasing Fe2+ loading. This process was very effective for degradation of the dye solutions as evident from the percent decolorization reported by these researchers which were more than 90% at the optimum reaction conditions. Ultrasound can be dispersed in a solution by two ways — using indirect sonication bath or direct ultrasonic probe. It has been observed that direct ultrasonication requires less time but the indirect one is more uniform [12].
The aim of the present research is to compare the efficacy of two types of ultrasonic sonicators — probe and bath type — under identical experimental conditions for decolorization of Rhodamine B dye in aqueous solution using Fenton’s reagent. The effects of process parameters like pH, initial dye concentration, initial H2O2 and Fe2+ dosing on the decolorization efficiency were also studied to determine optimum values. Unassisted Fenton’s reaction and sonolysis experiments were done as blank experiments for comparison purpose. Sono-Fenton processes are generally very rapid and can be successfully applied to difficult wastewaters compared to the abovementioned other processes. Bio-based processes are generally slow and sono-Fenton reaction degrades pollutants within a few minutes. Bath type sonicator indirectly passes ultrasound to the solution whereas probe type sonicator sends ultrasound directly. Performance of the sono-Fenton process depends on the type of sonicator and here we compare their performances. Such a comparison has never been reported before and this is the novelty of this work.
Materials and Methods
Materials
Rhodamine B (C.I. No. 45170, mol wt. 479.01), was procured from Loba Chemie, Mumbai, India. Its chemical structure is illustrated in Figure 1. Hydrogen peroxide (H2O2, 30% w/v) was from Merck Specialities Private Limited, India. Crystalline ferrous sulphate heptahydrate (FeSO4.7H2O) was from Sisco Research Laboratories Pvt. Ltd, India. Sodium hydroxide (NaOH), sulphuric acid (H2SO4) were of analytical grade and purchased from Merck (Germany). Sodium bisulphite (NaHSO3) was from Loba Chemie. To stop the reaction at a desired point, 5N sodium bisulphite was used. The chemicals were used without further purification. The stock Rhodamine B solution of 1 kg/m3 was prepared by dissolving the Rhodamine B powder in distilled water and then stored in a dark place.
Experimental procedure
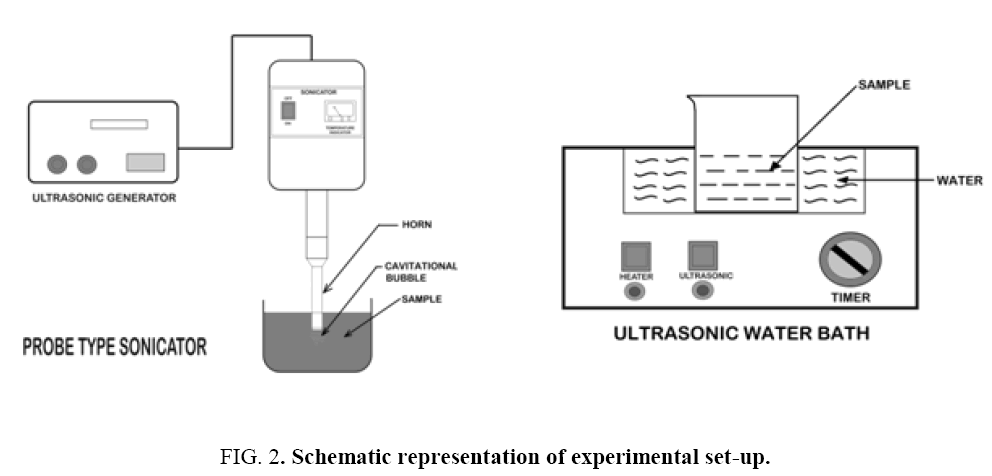
The sono-Fenton process (Fe2+/H2O2/US), was performed by using 30 kHz ± 3 kHz frequency and 120 W power probe type sonicator (Trans-O-Sonic, Model: D-120/P) and 20 kHz frequency bath type sonicator (Oscar Ultrasonic cleaner, model Microclean 101 with 20 kHz frequency and heating arrangement). Ultrasonic cleaning baths are most widely used as they provide a convenient and non-expensive source of ultrasound. On the other hand, an ultrasonic probe (horn) can be directly immersed in the reaction solution and it is preferred over an ultrasonic bath, as it is more energy efficient. Figure 2 shows the schematics of the experimental set-ups. Synthetic dye solution of 2.5 × 10-4 m3 of specific concentration was taken in a beaker and was placed in a bath-type and as well as probe-type sonicator. All the batch experiments were performed at room temperature (25°C). Required volumes of 0.5% FeSO4 solution and H2O2 (30% w/v) solution were added and the reaction mixture was exposed to ultrasonication. pH of the reaction mixture was measured using a calibrated pH test 20 instrument. The pH was adjusted by adding either 0.1N sulphuric acid or sodium hydroxide. During reaction, samples were withdrawn at fixed time intervals. The reaction was stopped using 10-8 m3 of 5N sodium bisulphite (NaHSO3) solution and the samples were analyzed spectrophotometrically for residual colour measurement. All the experiments were repeated at least thrice and the standard deviation was less than 5%.
Analytical methods
The residual hydrogen peroxide in the samples was quenched by using sodium bisulphite (NaHSO3) to stop the oxidation reaction [13]. The maximum absorbance wavelength (λmax) of Rhodamine B was determined as 554 nm using a UV-Vis spectrophotometer. Concentration of the residual dye in the simulated wastewater was calculated by the dye absorbance at λmax using calibration curve. Decolorization of Rhodamine B was calculated as shown in Eq. 4.
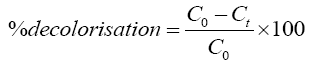 (4)
(4)
Where C0 is the initial concentration of Rhodamine B and Ct is the concentration of Rhodamine B at any time t. COD of the initial and treated sample was determined by standard open reflux method as described in APHA handbook [14].
Result & Discussions
Decolorization of RhB with sono-Fenton processes with both types of sonicators, Fenton’s process and sonolysis
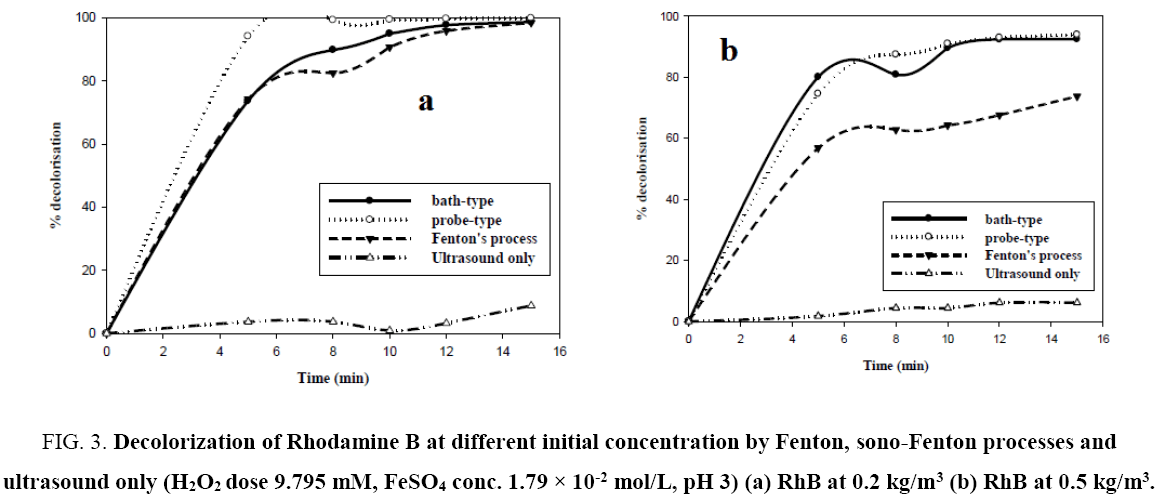
Figure 3a and 3b shows the degree of decolorization of RhB versus time for experiments carried out using only ultrasound, ultrasound with Fenton’s reagent and only Fenton’s process with 0.2 kg/m3 mgL-1 and 0.5 kg/m3 Rhodamine B dye solution. From the figures, for both the concentrations, only sonolysis led to negligible dye decolorization. Fenton’s process and sono- Fenton process have almost an equal efficacy in decolorization of dye at low initial dye concentration. However, there is a significant increase of decolorization by sono-Fenton process over Fenton process in case of higher initial dye concentration. This can be explained by the fact that for low initial dye concentration the amount of •OH radicals generated by Fenton’s process is sufficient to destroy the chromophores of the dye, hence there is no additional advantage by using sono-Fenton process. But for higher concentration of dye, the amount of •OH radicals generated by Fenton process alone is insufficient to destroy the higher numbers of chromophores. Sono-Fenton process, due to the synergistic effects of Fenton’s process and ultrasound, generates higher numbers of hydroxyl radicals which can destroy the pollutants present in a highly concentrated dye solution. However, probe type sonicator is advantageous in both ranges of concentration.
Figure 3:Decolorization of Rhodamine B at different initial concentration by Fenton, sono-Fenton processes and ultrasound only (H2O2 dose 9.795 mM, FeSO4 conc. 1.79 × 10-2 mol/L, pH 3) (a) RhB at 0.2 kg/m3 (b) RhB at 0.5 kg/m3.
Effect of pH
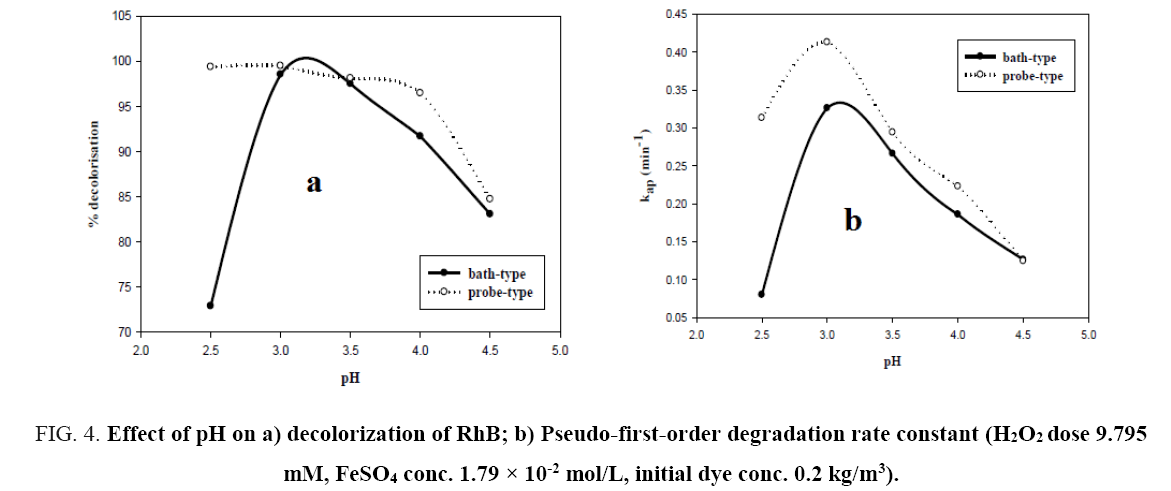
pH of the solution has a strong influence on the decolorization of dye by sono-Fenton process, as it controls the concentration of ferrous ions (Fe2+) and the production of •OH in the solution [10]. Effect of initial pH on dye decolorization was studied for both probe and bath type sonicators at a pH ranging from 2.5 to 4.5 at a fixed FeSO4 concentration of 1.79 × 10-2 mol/L, H2O2 dosage of 9.795 mM and RhB concentration of 0.2 kg/m3. It can be seen from Figure 4a that maximum decolorization of RhB was obtained at pH 3 for both probe and bath type sonicators. Under the acidic conditions (pH around 3), generation of hydroxyl radicals due to decomposition of hydrogen peroxide is favored i.e., recombination reaction between the free radicals is hampered thereby increasing the availability of the free radicals for the desired oxidation of the organic pollutants [9]. At low pH, a significant difference in percent decolorization of RhB can be observed in probe and bath-type sonicators. The reason may be that due to high concentration of H+ ions in the solution, they react with •OH radicals and hydrogen peroxide which is stabilized by forming H3O2 + as shown in Eq. 5 and 6 [9,10]. At low pH, due to this scavenging effect of excess H+ ions over the •OH there is a reduced generation of •OH. Hence decolorization of dye is also decreased in bath-type sonicator. But in probe-type, because of the more intense ultrasonic irradiation, excess numbers of •OH are generated and the scavenging effect of H+ ions becomes negligible. The decolorization of RhB decreases substantially at pH>4 in both types of sonicator because of Fe3+ precipitation which suppresses the generation of •OH radicals and lowers catalytic activity during the decomposition of H2O2.
Figure 4: Effect of pH on a) decolorization of RhB; b) Pseudo-first-order degradation rate constant (H2O2 dose 9.795 mM, FeSO4 conc. 1.79 × 10-2 mol/L, initial dye conc. 0.2 kg/m3).
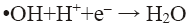 (5)
(5)
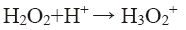 (6)
(6)
Many researchers have reported that acidic conditions favour the sono-degradation of phenol [15], p-nitrophenol [16], 2- chlorophenol [17]. Lu et al. [18] have reported a high rate constant for the degradation of dichlorvos at pH 3 using Fenton’s process. Li and Song [19] determined the optimum pH value as 3 for the decolorization of Acid Red 97 by sono-Fenton process. Similarly, Özdemir et al. [10] have also reported maximum colour and COD removal of CI Reactive Orange 127 at pH 3 by sono-Fenton process. The kinetics of the sonochemical degradation of RhB can be expressed as a pseudo-first order-reaction [20,21] as shown below:
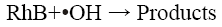 (7)
(7)
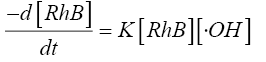 (8)
(8)
The concentration of •OH can be assumed to be constant during the reaction since it is extremely reactive and is consumed as soon as it is generated. Hence there is no accumulation of •OH in the solution. Considering this assumption Eq.8 can be integrated to the give the following form:
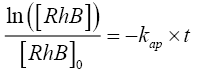 (9)
(9)
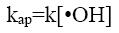 (10)
(10)
where [RhB]0 and [RhB] are the initial concentration and the concentration of RhB (kg/m3) at time t, kap and t are the pseudofirst- order rate constant (min-1) and sonication time respectively.
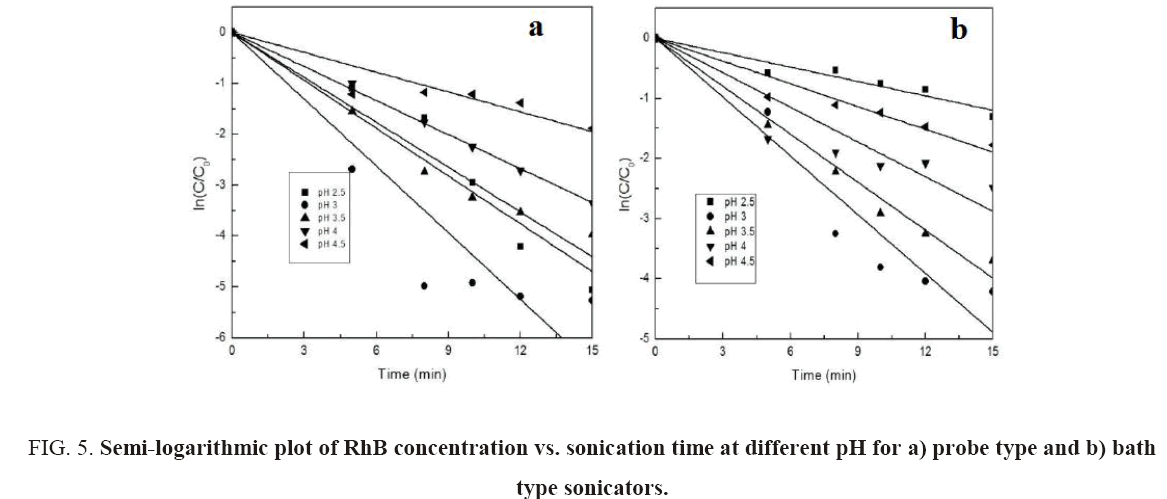
From Figure 5a and 5b, it can be seen that the semi-logarithmic graphs for the variation of RhB concentration with time at different pH are linear which confirms the proposed pseudo-first-order kinetics for decolorization of RhB. Figure 4b shows the variation of kap with pH. The graph further demonstrates that pH 3 is the optimum pH for the decolorization since the rate constants for both probe and bath-type sonicator are maximum at that pH. Behnajady et al. [20] and Siddique et al. [8] have reported similar variations of decolorization rate constant with pH.
Figure 5: Semi-logarithmic plot of RhB concentration vs. sonication time at different pH for a) probe type and b) bath type sonicators.
Effect of initial dye concentration
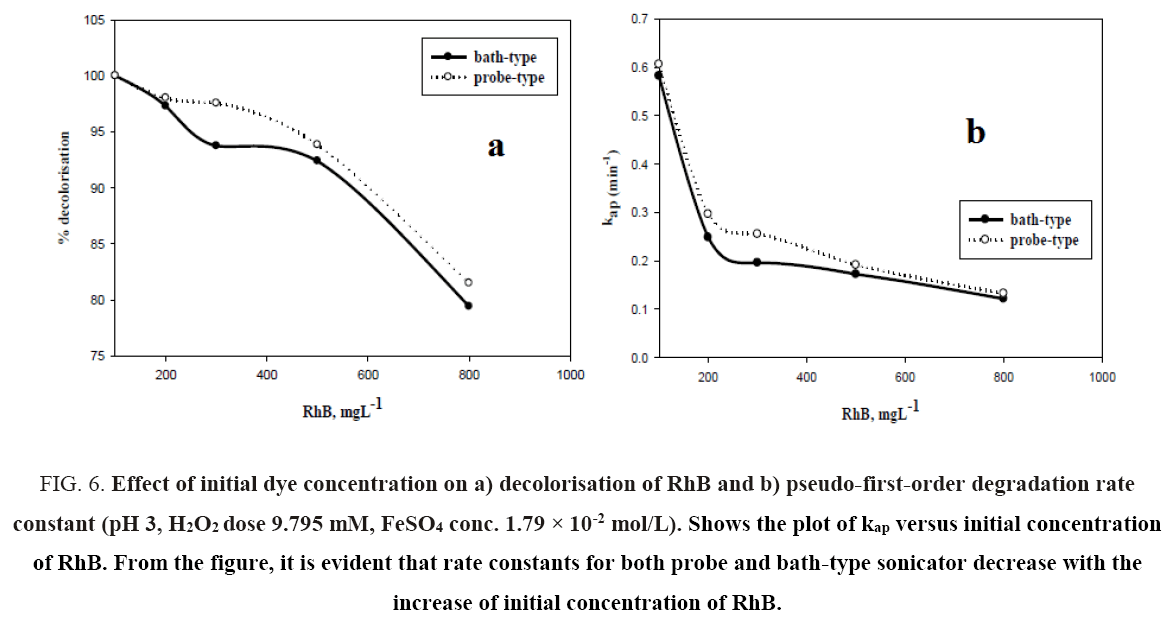
Initial dye concentration is an important parameter for the sono-Fenton degradation of Rhodamine B. The effect of the initial concentration of RhB on decolorization efficiency in probe and bath type sonicators was investigated with the dye concentration ranging from 0.1 kg/m3 to 0.8 kg/m3 at pH 3, FeSO4 concentration of 1.79 × 10-2 mol.L-1 and H2O2 dosage of 9.795 mM. The results are shown in Figure 6a. It can be seen from the figure that decolorization efficiency for both probe and bath type sonicators decrease with increasing initial dye concentration. In case of bath sonicator, the percent degradation decreased from 100% at 0.1 kg/m3 to 79.42% at 0.8 kg/m3. For probe type sonicator, the corresponding decrease was from 100% to 81.49%. This may be due to the decreased cavitational effects at higher pollutant load and also insufficient generation of hydroxyl radicals to destroy the organic pollutants completely [22]. The limited diffusion of hydroxyl radicals out of the interfacial regions of the collapsing cavitation bubble [23] may result in a decrease in the degradation rate with increasing pollutant concentration. Higher concentrations of dye require greater amounts of •OH radicals for decolorisation, hence other parameters remaining same, greater initial concentration of dye results in reduced decolorisation of dye. Neppolian et al. [23] have reported a decrease in the reaction rate constant with an increase in initial concentration of methyl tert-butyl ether (MTBE) over the range of 2.84 × 10-2 to 2.84 × 10-1 mM using ultrasound with fixed ultrasonic frequency of 20 kHz. Babuponnusami and Muthukumar [24] have reported similar decrease in the extent of phenol degradation with an increase in the initial phenol concentration over the range of 0.1 kg/m3 to 0.4 kg/m3 by Fenton, sono-Fenton and sono-photo-Fenton Methods.
Figure 6: Effect of initial dye concentration on a) decolorisation of RhB and b) pseudo-first-order degradation rate constant (pH 3, H2O2 dose 9.795 mM, FeSO4 conc. 1.79 × 10-2 mol/L). Shows the plot of kap versus initial concentration of RhB. From the figure, it is evident that rate constants for both probe and bath-type sonicator decrease with the increase of initial concentration of RhB.
Effect of H2O2 dosing
Hydrogen peroxide plays an important role in the Fenton’s oxidation process, as it is the source of •OH that are required for oxidation. However, an excess amount of H2O2 not only reduces the treatment efficiency, but also raises the cost of treatment, as it is the main cost of the process [10]. It should be noted that H2O2 concentration has a significant effect because excess of H2O2 can react with the just formed hydroxyl radicals (Eq. 11) causing a reduction in the performance of process [3,4,8-10].
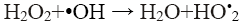 (11)
(11)
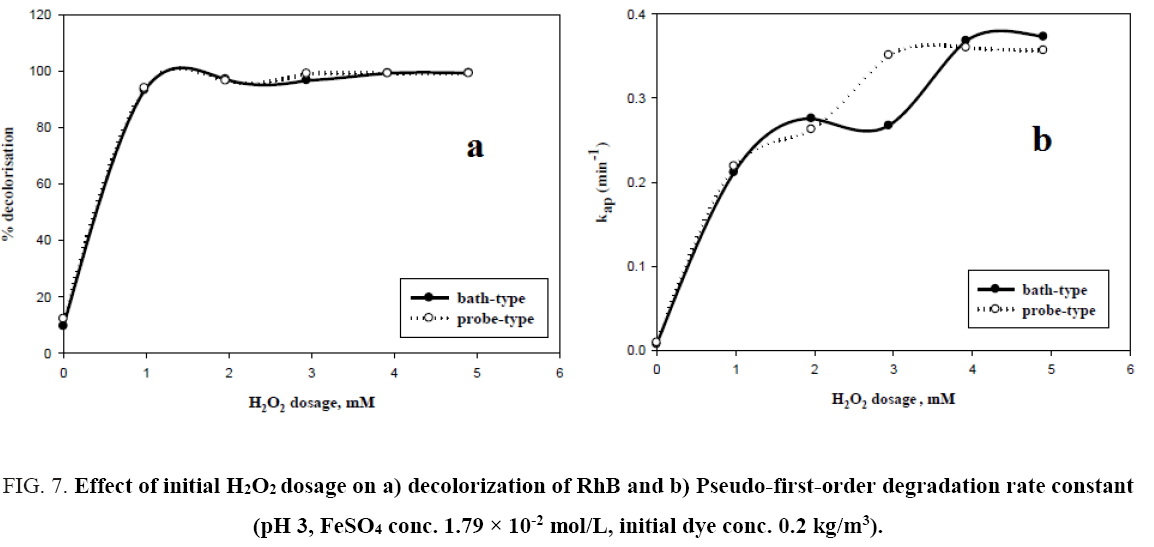
The decolorisation of RhB at different H2O2 concentration has been studied for both probe type and bath type sonicator keeping ferrous sulphate concentration fixed at 1.79 × 10-2 mol/L, initial pH at 3, and dye concentration at 0.2 kg/m3. With increasing H2O2 concentration, there is an increase in percent decolorization up to a certain concentration of H2O2 for both types of sonicators. On further increasing H2O2 dosage there was no change in the percent decolorization. For both bath and probe sonicators, this optimum value of hydrogen peroxide dosing is nearly 4 mM in 2.5 × 10-4 m3 of solution. It can also be seen from Figure 7a, that the two decolorisation profiles obtained by using probe and bath type sonicators under the otherwise same experimental conditions, nearly overlap. The reason may be that there is generation of enough hydroxyl radicals in bath-type sonicator and excess hydroxyl radicals in probe-type sonicator with respect to the initial dye concentration used (0.2 kg/m3). It can also be noted that the decolorisation of RhB in both cases was rapid in the initial period of the reaction and then it would slow down and level off. Since initially the concentration of H2O2 is high, a large amount of hydroxyl radicals generated and start reacting with the organic molecules. As the reaction goes on, H2O2 is gradually depleted and hence there is a fall in the generation of hydroxyl radicals, leading to a decrease in the decolorisation rate which attains a steady value after 8 min. Many researchers have reported [3,8,12] that an optimum concentration of H2O2 exists where maximum degradation of organic pollutants is observed and excessive amounts of H2O2 could reduce the degradation rate. Basturk and Karatas [4] investigated the degradation of Reactive Blue 181 using Fenton and sono-Fenton process and observed that the degradation efficiency increases with an increase in hydrogen peroxide loading till an optimum of 0.05 kg/m3 by Fenton process and 0.04 kg/m3 by sono-Fenton process. Özdemir et al. [10] studied the decolorisation of dye C.I. Reactive Orange 127 using Fenton and sono-Fenton process and reported the maximum decolorisation efficiency was obtained at 0.015 kg/m3 of H2O2 by Fenton process and 0.005 kg/m3 of H2O2 by sono-Fenton process.
Figure 7: Effect of initial H2O2 dosage on a) decolorization of RhB and b) Pseudo-first-order degradation rate constant (pH 3, FeSO4 conc. 1.79 × 10-2 mol/L, initial dye conc. 0.2 kg/m3).
Effect of initial Fe2+ dosing
The influence of ferrous ion on sono-Fenton degradation of organic pollutants has been investigated by many researchers. Most studies have shown that degradation of pollutants by sono-Fenton process was significantly increased with increasing amount of iron salts [25]. The decolorisation of RhB at different FeSO4 dosing ranging from 0.054 mM to 0.126 mM in 2.5 × 10-4 m3 solution has been studied for both probe type and bath type sonicator keeping hydrogen peroxide dosage fixed at 3.92 mM in 2.5 × 10-4 m3 solution, initial pH at 3, and dye concentration at 0.2 kg/m3.
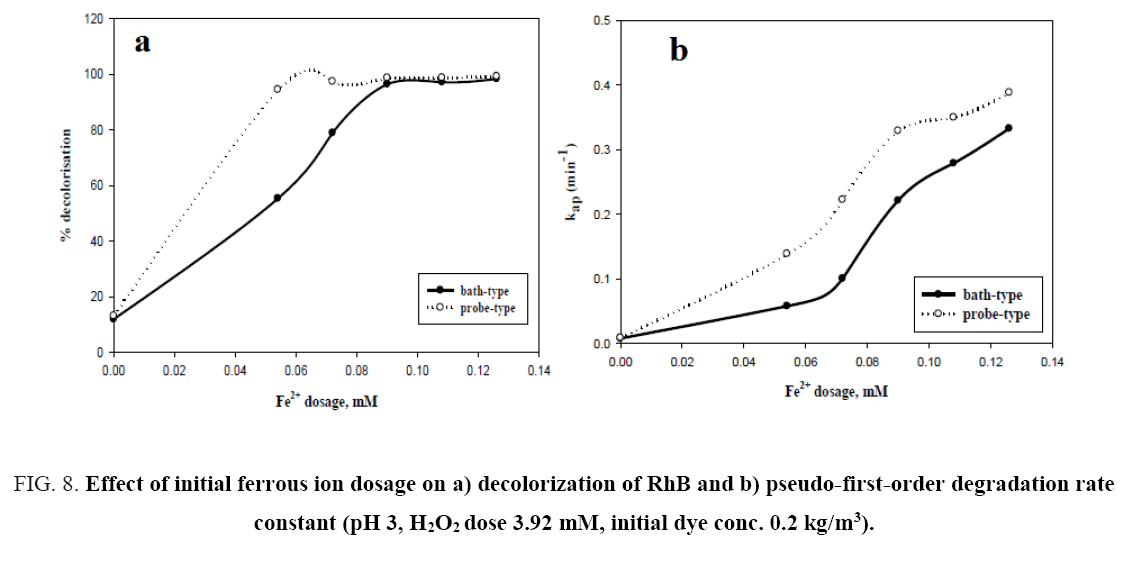
From Figure 8a, it can be observed that percent decolorization of RhB increases with the increase of ferrous ion in both probe type and bath type sonicators, which is in corroboration with the findings of previous researchers [3,4,8,10]. At the maximum Fe2+ dosing (0.126 mM) the percent decolorization obtained was 99.29% and 98.42% for probe and bath type sonicators respectively. Enhancement of degradation by Fe(II) or Fe(III) addition in a catalytic amount was because of H2O2 decomposition and •OH generation in Fenton’s (Fe(II)/H2O2) and Fenton’s like (Fe(III)/H2O2) processes (Eq. 12-16) [3].
Figure 8: Effect of initial ferrous ion dosage on a) decolorization of RhB and b) pseudo-first-order degradation rate constant (pH 3, H2O2 dose 3.92 mM, initial dye conc. 0.2 kg/m3).
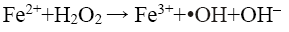 (12)
(12)
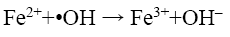 (13)
(13)
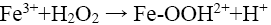 (14)
(14)
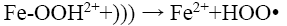 (15)
(15)
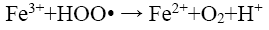 (16)
(16)
Özdemir et al. [10] have shown that there is an optimum value of Fe2+ dosing beyond which decolorisation of dye decreases due to radical scavenging by Fe2+ as shown in the following reaction:
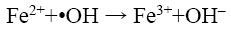 (17)
(17)
But in the present study a continuous increase in decolorisation has been observed with increasing Fe2+ dosing in the experimental range. This may be due to the relatively low concentration of Fe2+ with the respect to the dye concentration. From the figure, it can also be seen that there is a significant difference in the decolorisation percentage (94% vs 55.3%) between probe type and bath type sonicator at low Fe2+ dosing (0.054 mM). This is because the localized intensity of the ultrasonic wave in probe type sonicators is generally much higher than that in bath type [26]. This causes the formed Fe-OOH2 in probe type sonicators to be easily decomposed to Fe2+ and HOO∙ as shown in Eq. 15. The Fe2+ thus formed is again cycled in the Fenton reactions (Eq. 12 and 13), thereby increasing the decolorisation efficiency. However, at high Fe2+ dosing, the amount of Fe-OOH2 formed is itself very large and despite the slow decomposition of the intermediate in bath type sonicator, the amount of Fe2+ formed becomes quite high, and hence the performance of the bath type sonicator becomes almost identical to that of the probe type.
The relationship between Fe2+ dosing and pseudo-first-order rate constant is shown in Figure 8b and indicates that the rate constant for both types of sonicator increases with increasing Fe2+ loading. A similar trend was reported by Siddique et al. [8]. The values of the rate constants for the two types of sonicators under varying process conditions are given in Table 1.
| Parameter | Value | Bath-type sonicator | Probe-type sonicator | ||
|---|---|---|---|---|---|
| K (min-1) | R2 | K (min-1) | R2 | ||
| pH | 2.5 | 0.0798 | 0.9264 | 0.3132 | 0.9309 |
| 3 | 0.326 | 0.9101 | 0.4128 | 0.9079 | |
| 3.5 | 0.266 | 0.9812 | 0.2939 | 0.9605 | |
| 4 | 0.1857 | 0.9079 | 0.2227 | 0.9979 | |
| 4.5 | 0.1262 | 0.9193 | 0.1243 | 0.9764 | |
| Initial Conc. (kg/m3) | 0.1 | 0.582 | 0.944 | 0.606 | 0.837 |
| 0.2 | 0.2483 | 0.9926 | 0.2963 | 0.9764 | |
| 0.3 | 0.1958 | 0.9762 | 0.2551 | 0.9783 | |
| 0.5 | 0.172 | 0.90 | 0.1911 | 0.9387 | |
| 0.8 | 0.1213 | 0.9037 | 0.1323 | 0.9024 | |
| Initial Fe2+ dosing(mM) | 0 | 0.008 | 0.919 | 0.009 | 0.966 |
| 0.054 | 0.058 | 0.8396 | 0.139 | 0.7684 | |
| 0.072 | 0.1005 | 0.977 | 0.2226 | 0.9348 | |
| 0.090 | 0.2214 | 0.9628 | 0.3293 | 0.952 | |
| 0.108 | 0.2791 | 0.8863 | 0.3501 | 0.8618 | |
| 0.126 | 0.333 | 0.8474 | 0.3887 | 0.8756 | |
| Initial H2O2 dosing(mM) | 0 | 0.007 | 0.891 | 0.009 | 0.897 |
| 0.98 | 0.2115 | 0.8911 | 0.2189 | 0.90 | |
| 1.96 | 0.2757 | 0.8983 | 0.2627 | 0.899 | |
| 2.94 | 0.2672 | 0.8978 | 0.351 | 0.933 | |
| 3.92 | 0.3682 | 0.9271 | 0.3601 | 0.9301 | |
| 4.9 | 0.373 | 0.9346 | 0.3567 | 0.94 |
Table 1: First order rate constants for different process parameters.
Reduction of chemical oxygen demand (COD) with time as measure of mineralization
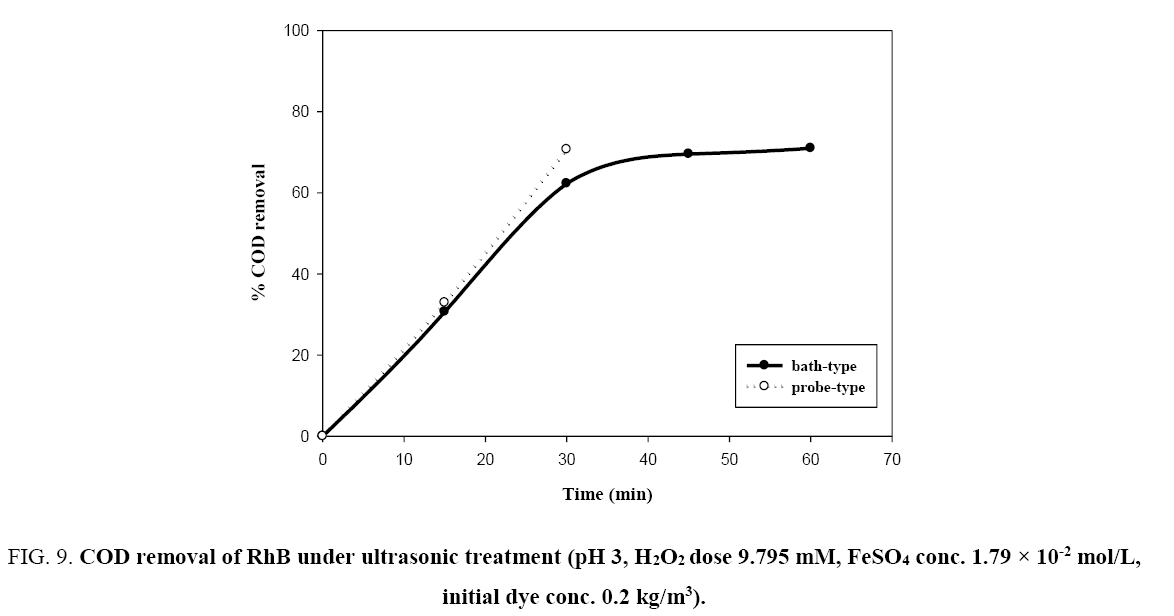
It is well known that complete decolorisation of dye does not mean complete mineralization [11]. The degradation of RhB in terms of COD removal was studied. From the Figure 9, only 30.66% and 32.91% COD respectively were removed by bath-type and probe-type sonicators at 15 min of reaction time when hydrogen peroxide concentration was 9.795 mM, initial concentration of RhB was 0.2 kg/m3 and the pH value was 3, while over 99% decolorisation efficiency was achieved at 15 min of the reaction ( Figure 4). COD was not completely removed under the ultrasonic action even with a long treatment period (60 min). This is since intermediate products of RhB are resistant towards oxidative degradation by sonochemical treatment and complete oxidation may proceed at a much slower rate [3,11]. From Figure 9, it can be observed that the rate of COD removal is faster in probe-type sonicator than bath-type sonicator under identical conditions. The reason may be that the localized intensity of the ultrasonic wave in probe type sonicators is generally much higher than that in bath type, which in turn produce more hydroxyl radicals (•OH) which is responsible for the degradation of organic pollutants [26].
Figure 9: COD removal of RhB under ultrasonic treatment (pH 3, H2O2 dose 9.795 mM, FeSO4 conc. 1.79 × 10-2 mol/L, initial dye conc. 0.2 kg/m3).
Conclusion
The present investigation effectively proves that the synergistic action of ultrasound and Fenton’s process is very effective in decolorizing Rhodamine B dye solution, while only sonolysis has no effect. Fenton’s process without ultrasound is effective in decolorizing dilute dye solutions, but sono-Fenton process is effective for concentrated dye solutions. The percentage decolorisation by sono-Fenton process increased with decreasing initial concentration of dye. The decolorisation was favoured at acidic pH with maxima at pH 3. The optimum H2O2 dosage was found to be 3.92 mM. Increasing Fe2+ dosing was found to increase decolorisation efficiency in both types of sonicators. At low Fe2+ dosage, probe type sonicator was much more effective than bath type sonicator or ordinary Fenton’s process, but at high Fe2+ dosage, this difference became insignificant. Under optimum experimental conditions, the percent decolorization with sonicator bath was 96.55% and with probe sonicator was 98.75%. COD was removed 30.66% and 32.91% with respect to bath-type and probe-type sonicators after 15 min degradation at hydrogen peroxide concentration of 9.795 mM, and pH 3 for a 0.2 kg/m3 dye solution, when the decolorization was more than 99%. The study indicates that ultrasound assisted Fenton’s process is an effective option for the treatment of dye-containing simulated wastewaters. Further investigations on the application of sono-Fenton process for the treatment of real-life industrial wastewaters are under progress.
Acknowledgements
The authors gratefully acknowledge the University Grants Commission (UGC) for providing the financial support for the study through the Maulana Azad National Fellowship for Minority Students (Award letter no: F1-17.1/2014-15/MANF-2014-15-MUS-WES-36479/(SA-III/Website), dated February, 2015). The authors would also like to thank the laboratory staff members of the Department of Chemical Engineering, University of Calcutta for their invaluable assistance in carrying out the experimental work.
References
- Colonna GM, Caronna T, Marcandalli B. Oxidative degradation of dyes byultraviolet radiation in the presence of hydrogen peroxide.Dyes Pigm. 1999;41(3):211-20.
- Mishra KP, Gogate PR. Intensification of degradation of aqueous solutions of rhodamine B using sonochemical reactors at operating capacity of 7 L.J Environ Manage.2011;92(8):1972-7.
- Merouani S, Hamdaoui O, Saoudi F, et al.Sonochemical degradation of Rhodamine B in aqueous phase: Effects of additives.ChemEngJ.2010;158(3):550-7.
- Basturk E, Karatas M.Advanced oxidation of Reactive Blue 181 solution: A comparison between Fenton and sono-Fenton Process.Ultrason Sonochem. 2014;21(5):1881-5.
- Deveci E, Dizge N, Yatmatz H, et al. Degradation of recalcitrant textile dyes by coupling fungal and photocatalytic membrane reactors.Clean Soil Air Water.2016;44(10):1345-51.
- Jaiswal N, Pandey V, Dwivedi U. Immobilisation of Papyalaccase in chitosan led to improved multipronged stability and dye discoloration.Int J Biol Macromol. 2016;86:288-95.
- Bilal M, Iqbal H, Hu H, et al.Enhanced bio-catalytic performance and dye degradation potential of chitosan-encapsulated horseradish peroxidase in a packed bed reactor system. Sci Total Environ. 2016;S0048-9697(16)32145-3.
- Siddique M, Farooq R,Price GJ. Synergistic effects of combining ultrasound with the Fenton process in the degradation of Reactive Blue 19.Ultrason Sonochem.2014;21(3):1206-12.
- Bagal MV, Gogate PR. Wastewater treatment using hybrid treatment schemes based on cavitation and Fenton chemistry: A review. Ultrason Sonochem.2014;21(1):1-14.
- Özdemir C, Öden MK, Sahinkaya S, et al. The sonochemical decolorisation of textile azo dye CI Reactive Orange 127. Color Technol. 2011;127(4):268-73.
- Zhang H, Zhang J, Zhang C, et al.Degradation of C.I. Acid Orange 7 by the advanced Fenton process in combination with ultrasonic irradiation.Ultrason Sonochem.2009;16(3):325-30.
- Santos HM, Lodeiro C, Capelo-Martinez JL. The power of ultrasound, in ultrasound in chemistry: Analytical applications. Weinheim: Wiley-VCH Verlag GmBH& Co., Germany; 2008.
- Azbar N, Yonar T, Kestioglu K.Comparison of various advanced oxidation processes and chemical treatment methods for COD and color removal from a polyester and acetate fiber dyeing effluent.Chemosphere. 2004;55(1):35-43.
- Rice EW, Greenberg AE, Eaton AD, et al. Standard Methods for the Examination of Water and Wastewater. 21st ed.Denver: American Public Health Association, American Water Works Association, Water Environment Federation, USA; 2005.
- Currel DL, Wilheim G, Nagy S. Effect of certain variables on ultrasonic cleavage of phenol and of pyridine. J Am Chem Soc.1963;85(2):127-30.
- Kotronarou A, Mills G, Hoffmann MR. Ultrasonic irradiation of p-nitrophenol in aqueous solution. J Phys Chem.1991;95(9):3630-8.
- Ku Y, Chen KY, Lee KC. Ultrasonic destruction of 2-chlorophenol in aqueous solution. Water Res.1997;31(4):929-35.
- Lu MC, Chen JN, Chang CP. Oxidation of dichlorvos with hydrogen peroxide using ferrous ion as catalyst. J Hazard Mater.1999;65(3):277-88.
- Li JT, Song YL.Degradation of AR 97 aqueous solution by combination of ultrasound and Fenton reagent.Environ Prog Sustain Energy.2010;29(1):101-6.
- Behnajady MA, Modirshahla N, Molanee S. Ultrasonic degradation of Rhodamine B in aqueous solution: Influence of operational parameters.J Hazard Mater.2008;152(1):381-6.
- Mehrdad A, Hashemzadeh R. Ultrasonic degradation of Rhodamine B in the presence of hydrogen peroxide and some metal oxide. Ultrason Sonochem. 2010;17(1):168-72.
- Wang TH, Kang SF, Lin YH. Comparison among Fenton-related processes to remove 2,4-dinitrophenol. J Environ Sci Health A.1999;34(6):1267-81.
- Neppolian B, Jung H, Choi H, et al. Sonolytic degradation of methyl tert-butyl ether: the role of coupled Fenton process and persulphate ion. Water Res.2002;36(19):4699-708.
- Babuponnusami A, Muthukuma K. Research article degradation of phenol in aqueous solution by Fenton, Sono-Fenton and Sono-photo-Fenton methods. Clean SoilAirWater.2011;39(2):142-7.
- Ma YS. Short review: Current trends and future challenges in the application of sono-Fenton oxidation for wastewater treatment.Sustain Environ Res.2012;22(5):271-8.
- Dhanalakshmi NP, Nagarajan R. Ultrasonic intensification of the chemical degradation of methyl violet: An experimental study.IntJChemMolNuclMaterMetallurgEng. 2011;5(11):1019-24.