Original Article
, Volume: 15( 1)Recycling of the Waste Cooking Oils as Non-Ionic Surfactants
- *Correspondence:
- Abdel Hameed RS Department of Chemistry, Faculty of Science, Hail University, Hail, 1560, Kingdom of Saudi Arabia
Tel: 00201095501017; E-mail: mredars2@yahoo.com; rsabdelhameed@yahoo.com
Received Date: January 03, 2017 Accepted Date: February 17, 2017 Published Date: February 20, 2017
Citation: Abdel Hameed RS. Recycling of the Waste Cooking Oils as Non-ionic Surfactants. Mater Sci Ind J. 2016;15(1):114.
Abstract
Efficient green synthesis of non-ionic surfactants from waste cooking oil, the used glyceryl trioleate (triolein) as waste cooking oil was collected, purified, and reacted with hydroxyl amine (ethanol amine) and ethylene glycol in the presence of CH3COONa and (CH3COO)2Mn as a catalyst to give octadec-9-enoic acid (2-hydroxy-ethyl)-amide, (OA) and Octadec-9-enoic acid 2-hydroxy-ethyl ester (OE) respectively as non-ionic surfactant. The produced compounds were separated with high yield, purified, and characterized by FT-IR, 1HNMR. The surface properties were studied at different temperatures. the surface parameters such as surface excess concentration (rmax), area per molecule at interface (Amin) and the effectiveness of surface tension reduction (πCMC) were determined from adsorption isotherm. Thermodynamic parameters for micellization and adsorption were calculated and discussed.
Keywords
Green synthesis; Surfactant; Oil recycling; CMC; Thermodynamics
Introduction
Waste management has evolved from simple to complex integrated systems by which wastes are not only transported and disposed of but also reused and recycled to produce valuable by-product, in recent decades. Recycling of oil is increasingly carried out for oil fuel production. Waste cooking oil collection is governed by environmental agency in the UK. The collection of WCO is the responsibility of the company registered as a waste carrier according to environmental agency [1]. Waste cooking oil (WCO) is a domestic waste generated every day by food industries, restaurants, and households. It is reported that in Europe 5 kg from WCO are formed per inhabitant, totaling 2.5 million metric tons per year. Recovering WCO for the production of biodiesel offers away of minimizing and avoiding this waste and related pollution [2]. WCO was collected for animal food producers but this came to an end after the introduction of animal by-product Regulations EC/1774/2002, which restricted the use of WCO in animal feed [3,4]. In recent years, water-soluble synthetic oligomers as commercial materials have been produced and scientifically studied at an acceleration pace. Many water soluble glycolic polymers, because of their amphipathic structure and surface activity, are used as surface active agents. The study of the surface and thermodynamic properties are important both for basic research and for industrial applications [5-8]. Surfactants or amphiphilic monomers are used together with polymers in a wide range of applications. In areas, as diverse as detergents, organic coating food and pharmaceutical industries, formulation contain a mixing a low molecular weight surfactant and polymer which may or may not be highly surface active [9]. There is no reported work on the use of expired or used cooking oils as surfactants. The aim of the present work is to prepare non-ionic surfactant from the waste cooking oil via reaction between the purified triolein (WCO) and ethanolamine in the presence of sodium acetate as a catalyst to give the corresponding amide as non-ionic surfactant and via the reaction of the purified WCO with ethylene glycol (EG) in the presence of manganese acetate as a catalyst, to obtained the corresponding ester, octadec-9-enoic acid (2-hydroxy-ethyl)-ester, the surface property of the prepared surfactants were determined at different temperature, thermodynamic parameters were calculated and discussed. The present work deals with green synthesis of water soluble non-ionic surfactants from waste cooking oil, WCO after recovery and purification of the WCO. The thermodynamics and surface properties of the synthesized surfactants are another objective of this work.
Experimental
Materials
Used waste cooking oil (WCO) was collected from the fried food restaurants. Ethanolamine (EA), ethylene glycol, sodium acetate, and manganese acetate were purchased from Aldrich Chemical Co., Ltd. (UK), were used for recycling of WCO.
Purification of the waste cooking oil (WCO)
The waste cooking oil (WCO) is treated through the following steps, settling the fine solid particles suspended with the oil, then boiling the oil with saturated sodium chloride water solution (oil/saline water ratio is 1:1) for 15 min, followed by phase separation using separating funnel, then the water residue in oil layer is removed by heating for 20 min at 100°C to gives the purified cooking oil as in the following flow diagram (Figure. 1).
Synthesis
Spectroscopic analysis was carried out in micro analytical center of Cairo university. The fine chemicals were purchased from Aldrich Co. The green recycling process was monitored by TLC the product compound was extra purified using column chromatography technique. The general diagram for the green synthesis procedure as presented in Figure. 2.
Synthesis of Octadec-9-enoic acid (2-hydroxy-ethyl)-ester, (Octa Dec Hydroxyl ester, OE)
Triolein (I) (0.1 mol) and tri molecular ratio of ethylene glycol (0.3 mol) were refluxed in the presence of manganese acetate (0.5% wt.%) as a catalyst for 6 h at 120°C. The reaction mixture was cooled to room temperature, water was added to remove glycerol the product residue is a white viscous product, (II). This compound was extra purified using column chromatography.
II: (Y: 65%); Mol. F.: C20H39O3; Mol. Wt.: 327.53; m/z (FABMS): 326.3; Elemental analysis: (Calculated %) C: 73.79, H: 12.08, O: 14.6 (Found %) C: 73.83, H: 13.00, O: 14.6; 1HNMR (400 MHz, DMSO-d6):0.89 (t, 3H-CH3), 1.11-1.37 (m, 20H-10CH2), 1.54 (p, 2H-CH2), 1.91 (q, 4H-2CH2), 2.08 (t, 2H-CH2-CO), 2.6 (t, 2H-CH2-O), 3.76 (t, 2H-CH2-CO), 4.16 (s, OH), 5.42 (q, 2H-CH=). Figure. 3 show the chemical structure of the product and scheme of the synthesis.
Figure 3: Chemical structure of (ODHE) Compound (II) and (OA) compound (III) and the scheme of the synthesis.
Synthesis of Octadec-9-enoic acid (2-hydroxy-ethyl)-amide, (Octa Dec Hydroxyl amide, OA)
Triolein (I) (0.1 mol) and tri molecular ratio (0.3 mol) of ethanol amine were refluxed in the presence of manganese acetate as a catalyst for 5 h at 120°C. The reaction mixture was cooled to room temperature, water was added to remove glycerol and unreacted materials the product residue is a white yellow viscous product, (III). This compound was extra purified using column chromatography. Silica gel, eluting with ethyl acetate/hexanes (20/80).
III: (Y: 69%); Mol. F.: C20H39NO2; Mol. Wt.: 325.53; m/z (FABMS): 326.3; Elemental analysis: (Calculated %) C: 73.79, H: 12.08, N: 4.30, O: 9.83 (Found %) C: 73.83, H: 13.00, N: 4.25, O: 9.81; 1HNMR (400 MHz, DMSO-d6):0.89 (t, 3H-CH3), 1.11-1.37 (m, 20H-10CH2), 1.54 (p, 2H-CH2), 1.91 (q, 4H-2CH2), 2.08 (t, 2H-CH2-CO), 3.29 (t, 2H-CH2-NH), 3.76 (t, 2H-CH2-CO), 4.16 (s, OH), 5.42 (q, 2H-CH=), 6.93 (s, NH). 13CNMR (400 MHz, DMSO): 11.90 (CH3), 22.19 (CH2), 25.63 (CH2), 28.40 (CH2), 29.45 (CH2), 30.14 (6CH2), 31.80 (CH2), 33.70 (2CH2-CH=), 36.44 (CH2-CO), 41.32 (CH2-NH), 61.14 (CH2OH), 130.50 (2CH=), 172.66 (C=O). The chemical structure of the product as shown in the Figure. 3.
Surface property characterizations
Cloud point of 2% of the prepared surfactants aqueous solutions were determined visually by testing the temperature at which turbidity was observed, and temperature at which turbidity disappeared by cooling. The cloud point of the system was taken as average of the tow points. Surface tension measurements were carried out at different surfactant concentrations and temperatures, (303 k, 313 k, 323 k, and 333 k) by using platinum tensiometer. The surface tension was determined using a Kruss K-12 tensiometer.
Results and Discussion
Surface Activity of the prepared surfactants results from the hydrophobic effect. When the surfactants adsorbed at interface even at low concentration, the adsorption desorption balance (result from thermal motion), this balance need some time to establish the interfacial conditions. This difference is due to the difference in hydrophilic-lipophilic balance (HLB) of the surfactants. The HLB values were calculated by using the general formula for non-ionic surfactants [10-15].
HLB=[MH/(MH+ML)] × 20, Where MH is the formula weight of the hydrophilic portion of the surfactant molecule and ML is the formula weight of the hydrophobic portion. HLB and molecular weight values of non-ionic surfactants prepared from WCO were calculated and listed in Table 1. It is obvious that the OE surfactants have lower HLB values than OA which can be attributed to the structure of OE surfactants is more hydrophobic surfactants. Classical equations derived by Griffin and Davies were used to calculate the HLB number of surfactants [10,11]. The HLB equations consider only the chemical compositions, and the effect of position isomerism is not considered. The cloud point of the synthesized surfactant was used to represent HLB as its difficult to determine HLB experimentally. The cloud point is defined as the temperature at the surfactant loses sufficient water solubility and a cloudy dispersion observed [12-18]. At the cloud point, the surfactant ceases to perform some or all of its normal functions. So, that cloud point used to limit the choice of for different applications. the cloud point in solution of non-ionic surfactant was regarded as a pseudo phase inversion. The cloud point and the phase inversion temperature (PIT) are directly correlated when surfactant alone is dispersed in water. For polyoxyethylene-based surfactant, phase inversion temperature can be defined as the temperature at which the HLB of the surfactant occurs only at interface [13,18]. A study on the effect of structural changes in the surfactant molecule on its cloud point shows that, at constant oxyethylene content the cloud point is lowered due to decreased molecular weight of the surfactant and increasing length of the hydrophobic group [14,15]. In this respect, the cloud points of the prepared surfactants were determined and listed in Table 1. Careful inspection of data indicate that the cloud point of OA adduct surfactants was higher than that determined for OE surfactants which correlated to high HLB values of OA surfactants. Accordingly, the prepared OA surfactants were highly water soluble surfactants than OE surfactants. By measuring the surface or interfacial tension versus time for a freshly formed surface the surface activity can be determined. The surfactant micellization and adsorption depend on the critical micelle concentrations (CMC), which was determined by the surface balance method. The CMC values of the prepared surfactants were determined at 303 K, 313 K, 323 K, and 333 K from the slope change of the plotted data of surface tension (γ) versus the natural logarithm of the concentration and listed in Table 1. Adsorption isotherms are illustrated in Figure. 4. The isotherms are used for estimating surface activity and confirming the purity of the studied surfactants. It is of interest to mention that all obtained isotherms showed one phase, which is considered as an indication on the purity of the prepared surfactants. Recorded data show that the CMC decreases with temperature for the prepared non-ionic monomeric surfactants. The reduction in CMC values is due to the decrease in the solubility of the surfactants. The direct determination of the amount of surfactant adsorbed per unit area of liquid-gas or liquid-liquid interface, although possible, is not generally considered as the difficulty of isolating the interfacial region from the bulk phase for purpose of analysis when the interfacial region is small, and of measuring the interfacial area when it is large. The amount of material adsorbed per unit area of interface is calculated indirectly from surface tension measurements.
Figure 4: The adsorption isotherms of the prepared surfactants from waste cooking oil.
| Compound | Molecular weight (g/mol) | Cloud point °C | HLB | Temperature (K) | Surface property | Surface activity | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| CMC X104 mold dm-3 | γCMC mNm-1 | Γ max.1010 mol cm-2 | Amin. (nm2/molecule) | Π CMC (mNm-1) | PC20 | |||||
| OE | 327.53 | 78 | 16.8 | 303 | 3.3 | 40 | 0.79 | 0.2 | 33.9 | 6.4 |
| 313 | 1.7 | 38 | 0.77 | 0.21 | 35.8 | 6.9 | ||||
| 323 | 0.9 | 35 | 0.76 | 0.22 | 35.6 | 7.2 | ||||
| 333 | 0.5 | 33 | 0.73 | 0.23 | 36.4 | 8.4 | ||||
| OA | 325.53 | 80 | 18.2 | 303 | 9 | 41 | 0.65 | 0.26 | 33.6 | 5.2 |
| 313 | 6.3 | 39 | 0.59 | 0.29 | 34.5 | 5.6 | ||||
| 323 | 2.4 | 37 | 0.54 | 0.34 | 35.1 | 5.9 | ||||
| 333 | 1.1 | 34 | 0.47 | 0.42 | 35.6 | 6.7 | ||||
Table 1. CMC data and surface properties at different temperature of the surfactant prepared from waste cooking oil.
As a result, a plot of surface (or interfacial) tension as a function of equilibrium, concentration of surfactant in one of the liquid phases, rather than an adsorption isotherm, is generally used to describe adsorption of this interface can readily be calculated as surface excess concentration Γmax.
Surface excess concentration of surfactant at the interface can be calculated from surface or interfacial tension data from the following equation:
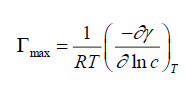 (1)
(1)
Where (-∂γ/∂ln c)T is the slope of the plot of γ versus ln c at a constant temperature (T), and R is the gas constant in J mol-1K- 1. Surface excess concentration at saturation is a useful measure of the effectiveness of adsorption of surfactant at the liquidliquid or liquid-gas interface, since it is the maximum value which adsorption can attain. The Γmax values were used for calculating the minimum area Amin at the aqueous-air interface. Area per molecule at the interface (Amin) gives information on the degree of packing and orientation of the adsorbed surfactant molecules, when compared with the dimensions of the molecule as obtained by use of models. From the surface excess concentration, the area per molecule at interface is calculated using the following equation:
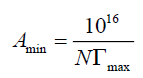 (2)
(2)
Where N is AvoGadro’s number. Surface tension values at CMC were used to calculate values of surface pressure (effectiveness). the effectiveness of surface tension reduction, πCMC=γ0 – γCMC, where γ0 is the surface tension of water and γCMC is the surface tension of solution at CMC was determined at different temperatures. The values of πCMC show that, the most efficient one is that gives the greater lowering in surface tension at the critical micelle concentration. The effectiveness increases with increasing the length of carbon chain in the hydrophobic moiety. Efficiency PC20 is determined by the concentration (mol/L) capable to suppress the surface tension by 20 dyne/cm. The efficiency of the prepared surfactants, appeared in Table 1, increases with increasing temperature. Careful inspection of data showed that the prepared surfactants based on waste cooking oil have high Amin values. This observation was referred to the presence of hydrophilic ends on the side of the prepared surfactant which reflect on the packing of the surfactants at the air-water interface. This can be attributed to the behavior of surfactants with hydrophilic groups at opposite ends of the molecule which show large area per molecule at the interface and are probably lying flat at the interface with both hydrophilic groups in contact with the aqueous phase. It was also observed that the Amin of the ester based surfactants, OE is higher than that of amide based surfactant, OA. This can be attributed to the fact that, the ester based non-ionic surfactants have low solubility than amide based. which reflects the easier for packing at the interface; then the molecule occupies a smaller area, that is, OE surfactant possess higher adsorption at interface than OA surfactant. Careful inspection of data, indicates that, Amin of the surfactants have two opposite relations with the temperature. The Amin may be increased or decreased with increasing the temperature. For polyoxyethylated nonionic surfactants, the lack of significant temperature effect may be resulted from two compensating effects [15,16]. Decrease in Amin at the surface due to increased dehydration of the hydrophilic group at higher temperature; and increase in Amin as a result of enhanced molecular motion at higher temperature. In the present system, it was found that the Amin increases with increase in temperature, as would be expected from the increased thermal agitation of the molecules in the surface film. Inspection of data also show that the effectiveness of surface tension reduction, πCMC, of OE surfactant more than OA. If the area of hydrophilic group is greater than that of the hydrophobic chain, the larger the hydrophilic group, the smaller of the amount adsorbed at saturation surface [15-17].
Finally, it was concluded that, the OE surfactant increase the surface excess of molecule and decreases Amin of molecule at air-water interface. This behavior can be attributed to increment of hydrophobic interaction at interface, which increase with ester than amide, which reflects on increasing of surfactant concentration and consequently decreases area per molecule. It is evident that, the minimum area per molecule at air-water interface can be contributed to the molecular area. Accordingly, the adsorption of the surfactant molecules at air-water interface increase in case of ester based surfactant, OE than amide based surfactant, OA which favors adsorption at solid liquid interface.
Thermodynamic parameters of micellization of prepared surfactants
The micelles formation in aqueous solutions can be viewed as a compromise between the tendency for alkyl chains to avoid energetically unfavorable contacts with water and the desire for the polar parts to maintain contact with the aqueous environment [18,19]. The ability for micellization processes depends on the change on thermodynamic parameters, (enthalpy ΔH, entropy ΔS, and free energy ΔG) of micellization. Thermodynamic parameters of micellization of the prepared non-ionic surfactants were calculated and listed in Table 2. The thermodynamic functions of micellization are the free energies ΔGmic, enthalpies ΔHmic, and entropies ΔHmic, of micellization for non-ionic surfactants.
| Temperature (K) | 303 k | 313 k | 323 k | 333 k | ΔSmic J k-1 mol-1 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | -ΔGmic KJ mol-1 |
-ΔHmic KJ mol-1 |
-ΔGmic KJ mol-1 |
-ΔHmic KJ mol-1 |
-ΔGmic KJ mol-1 |
-ΔHmic KJ mol-1 |
-ΔGmic KJ mol-1 |
-ΔHmic KJ mol-1 |
|
| OE | 19.4 | 39 | 21.8 | 38.7 | 24.5 | 38.1 | 27.1 | 37.5 | 0.21 |
| OA | 16.5 | 50.8 | 18.7 | 50.6 | 21.1 | 50.4 | 23 | 50.3 | 0.24 |
Table 2. Thermodynamic parameters of micellization of nonionic surfactants derived from waste cooking oil.
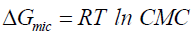 (3)
(3)
Values of ΔSmic were obtained from equation (4) by invoking the values of ΔGmic at 303 K, 313 K, 32 K, and 33 K.
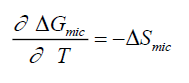 (4)
(4)
In addition, ΔHmic, was calculated from ΔGmic and ΔSmic by applying equation (5):
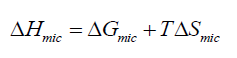 (5)
(5)
The thermodynamic parameters values of adsorption, ΔGad, ΔHad, and ΔSad were calculated via equations (6), (7), and (8), respectively [18,20].
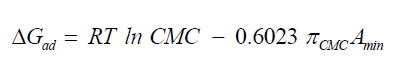 (6)
(6)
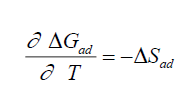 (7)
(7)
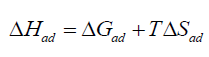 (8)
(8)
The values of ΔGmic, ΔHmic, and ΔSmic for the prepared surfactants are calculated and are listed in Table 2. Analyzing the thermodynamic parameters of micellization leads to the fact that micellization process is spontaneous (Δ
The values of ΔGad, ΔHad, and ΔSad for the prepared non-ionic surfactants are calculated and listed in Table 3. All ΔGadvalues are more negative than ΔGmic, indicating that adsorption at the interface is accompanied by a decrease in the system free energy. This may be due to the effect of steric factor on inhibition of the micellization more than its effect on the adsorption process.
| Temperature (K) | 303 k | 313 k | 323 k | 333 k | ?Sads. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | -?Gads KJ mol-1 | - ?Hads KJ mol-1 | -?Gads. KJ mol-1 |
-?Hads KJ mol-1 | -?Gads. KJ mol-1 |
-?Hads KJ mol-1 | -?Gads. KJ mol-1 |
-?Hads. KJ mol-1 |
|
| OE | 24.4 | 45.8 | 29.5 | 43.2 | 32.1 | 43.1 | 33.5 | 43.9 | 0.34 |
| OA | 26.5 | 90.6 | 28.7 | 92.4 | 31.4 | 92.9 | 35 | 94 | 0.4 |
Table 3. Thermodynamic parameters of adsorption of nonionic surfactants derived from Waste cooking oil at different temperatures.
On the other hand, it was observed that amide based surfactant, OA derived from waste cooking oil have more negative values of ΔGad than that ester based surfactant, OE. This observation indicates that the OA surfactants favor the adsorption at interface than OE surfactants. The values of ΔSad are all positive and have greater values than ΔSmic for non-ionic surfactants. This may reflect the greater freedom of motion of the hydrophobic chains at the planar air-aqueous solution interface compared to that in the relatively cramped interior beneath of the convex surface of the micelle. This shows that the steric factor inhibits micellization more than do adsorption for non-ionic surfactants. While the positive values of ΔHad are much lower than the corresponding values of ΔHmic which indicates that the dehydration-breaking of hydrogen bonds at adsorption is easier than at micellization [15,18,22].
Conclusions
The following conclusions can be extracted from the previous discussion:
1) Waste cooking oil can be easily recycled via solvent free green synthesis in the presence of catalyst.
2) The produced compounds were separated with high yield, purified, and characterized by FT-IR, 1HNMR.
3) The obtained compounds act as non-ionic surfactants of highly surface activities.
4) Ester based surfactant derived from waste cooking oil increase the surface excess of molecules at interface and consequently, decreases Amin of molecule at air-water interface.
5) The surface and thermodynamic parameters indicate that the prepared surfactants based on waste cooking oil WCO favor adsorption than micellization.
6) Amide based surfactant, OA derived from waste cooking oil have more negative values of ΔGad than that ester based surfactant, OE.
7) This observation indicates that the OA surfactants favor the adsorption than OE surfactants.
References
- Peiro LT, Mendez GV, Durany XG. Exergy analysis of integrated waste management in the recovery and recycling of used cooking oils. Environ Sci Technol. 2008;42(13):4977-81.
- Ripa M, Buonaurio C, Mellino S, et al. Recycling waste cooking oil into biodiesel: A life cycle assessment. Int J Perform Engi. 2014;10(4):347-56.
- Caputo AC, Scacchia F, Pelagagge PM. Disposal of by-products in olive oil industry: Waste-to-energy solutions. Appl Therm Eng. 2003;23(2):197-214.
- Mittelbach M, Remschmidt C. Biodiesel: The Comprehensive Handbook; Martin Mittelbach: Graz, Austria; 2005. 170 p.
- Atta AM. Synthesis and surface activity of poly (maleic diester) surfactants. Polym Int. 1999;48(7):571-9.
- Atta AM, Arndt KF. Synthesis and characterization of anionic graft copolymers containing poly (ethylene oxide) grafts. J Appl Polym Sci. 2002;86(5):1138-48.
- Atta AM, El-Sockary MA, Abdel Salam S. Recycled poly (ethylene terephthalate) waste oligomers as corrosion inhibitors of steel. Prog Rubber Plast Re Technol. 2007;23(4):241.
- Atta AM. Surface and thermodynamic parameters of polymeric surfactants from recycled poly (ethylene terephthalate). Polym Int. 2007;56(8):984-95.
- Atta AM, Ramadan AM, Shaffei KA, et al. Synthesis and properties of non-ionic surfactants from rosin-imides maleic anhydride adduct. J Dispersion Sci Technol. 2009;30(7):1100-10.
- Kuperkar K, Modi J, Patel K. Surface-active properties and antimicrobial study of conventional cationic and synthesized symmetrical gemini surfactants. J Surfact Deterg. 2012;15(1):107-15.
- Aiad IA, Badawi AM, El-Sukkary MM, et al. Synthesis and biocidal activity of some naphthalene-based cationic surfactants. J Surfact Deterg. 2012;15(2):223-34.
- Negm NA, El Farargy AF, Mohammed DE, et al. Environmentally friendly non-ionic surfactants derived from tannic acid: synthesis, characterization and surface activity. J Surfact Deterg. 2012;15(4):433-43.
- Azzam EM, Negm NA, Gad EA. Surface and thermodynamic properties of diquaternary bola-form amphiphiles containing aromatic spacer. J Ads Sci Technol. 2004;22:663-72.
- Negm NA, Aiad IA. Synthesis and characterization of multifunctional surfactants in oil-field protection applications. J Surfact Deterg. 2007;10(2):87-92.
- Zaky MF, Badawi AM, El Sabbah I, et al. Synthesis, characterization and surface activities of cationic polysaccharide (Aloe) schiff base surfactants. J Surfact Deterg. 2015;18(3):455-61.
- Laska U, Wilk A, Maliszewska I, et al. Novel glucose-derived gemini surfactants with a 1, 1′-ethylenebisurea spacer: preparation, thermotropic behavior, and biological properties. J Surfact Deterg. 2006;9(2):115-24.
- Hafiz AA, Badawi AM, El-Deeb FI, et al. Ferrocene-based cationic surfactants: Surface and antimicrobial properties. J Surfact Deterg. 2010;13(2):165-72.
- Atta AM, El-Kafrawy AF, Abdel-Rauf ME, et al. Surface and thermodynamic properties of non-ionic surfactants based on rosin-maleic anhydride and acrylic acid adducts. J Dispersion Sci Technol. 2010;31(4):567-76.
- Schramm LL. Surfactants: Fundamentals and applications in the petroleum industry. Cambridge: Cambridge University Press; 2000. 17 p.
- Wu J, Xu Y, Dabros T, et al. Effect of EO and PO positions in non-ionic surfactants on surfactant properties and demulsification performance. Colloids Surf A. 2005;252(1):79-85.
- Norvais?as P, Petrauskas V, Matulis D. Thermodynamics of cationic and anionic surfactant interaction. J Phys Chem B. 2012;116(7):2138-44.
- Wertz DH. Relationship between the gas-phase entropies of molecules and their entropies of solvation in water and 1-octanol. JACS. 1980;102(16):5316-22.

