Review
, Volume: 19( 1) DOI: 10.4172/tsnp.2023.19(1).003Prenylated Chalcones: A Review of Antimicrobial Properties and Structural Diversity
- *Correspondence:
- Rajni Godara
Department of Agricultural Chemicals,
ICAR-Indian Agricultural Research Institute,
New Delhi,
India;
E-mail: rajniiari1@gmail.com
Received: April 18, 2023, Manuscript No. TSNP-23-96295; Editor assigned: April 21, 2023, PreQC No. TSNP-23-96295 (PQ); Reviewed: May 05, 2023, QC No. TSNP-23-96295; Revised: June 16, 2023, Manuscript No. TSNP-23-96295 (R); Published: June 23, 2023, DOI: 10.4172/0974-7508.2023.19(1).6
Citation:Godara R, Kaushik P, Rana VS, et al. Prenylated Chalcones: A Review of Antimicrobial Properties and Structural Diversity. Nat Prod
Ind J. 2023;19(1):6.
Abstract
Chalcones, precursors to open chain flavonoids and isoflavonoids, are commonly found in edible plants. The remarkable bioactivity displayed by chalcones and their prenylated derivatives has garnered significant attention due to the presence of active α,β-unsaturated keto groups. The vast structural diversity of chalcones has paved the way for the development of newer agrochemicals. Frequently, naturally occurring chalcones contain prenyl units that are critical for their biological properties. This review focuses on prenylated chalcones, both naturally occurring and newly synthesized, that exhibit significant antimicrobial properties.
Keywords
Prenylated chalcones, Flavonoids, Isoflavonoids; Bioactivity; Agrochemicals.
Introduction
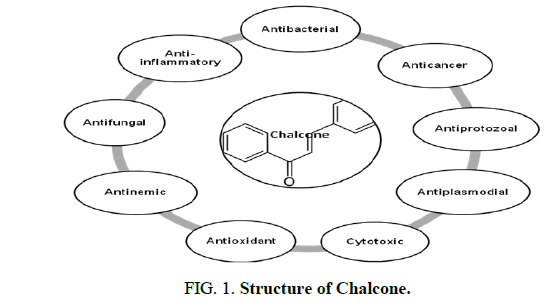
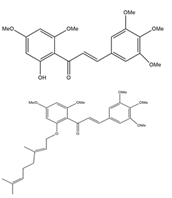
Chalcones are ubiquitous in edible and pharmacologic plants and are bio-precursors of plant flavonoids. They are secondary metabolites of the flavonoid (C6-C3-C6 system) family and their natural derivatives are important intermediates of the flavonoids biosynthetic pathway. These participate in interactions between plants and insects, operate as defensive chemicals and add to the therapeutic value of herbs. Due to their fascinating biological activity, phytochemicals, especially prenylated chalcones, are currently the subject of intense investigation for their potential health advantages. Structure-activity relationship (SAR) investigations revealed that the bioactivity of prenylated chalcones is significantly influenced by the presence of isoprenoid chains of various lengths and types. When compared to their non-prenylated analogues, these compounds exhibit exceptional features, including improved interaction with biological membranes and greater affinity for target proteins (Figure 1) [1].
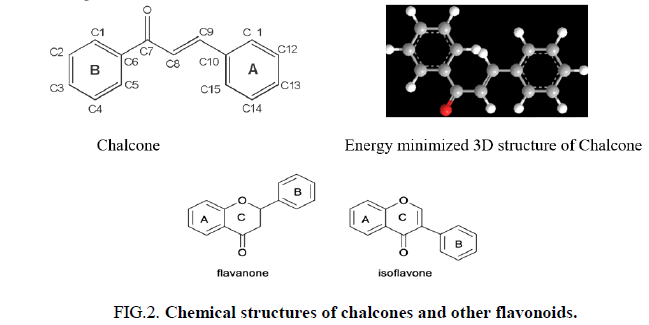
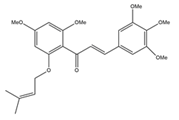
Growing interest in isoprenoid chemistry brought several prenylated chalcones to the forefront of research. According to extensive scientific research, chalcones exhibit additional fascinating biological characteristics such as antioxidant, cytotoxic, anticancer, antibacterial, antiprotozoal, antiulcer, antihistaminic and anti-inflammatory effects. The antimicrobial, antiviral, anti-invasive and anti-insecticidal properties of prenylated chalcones and their cyclic analogues were determined to be considerable (Figure 2).
Chemically, chalcones possess versatile and adaptable structures, making them ideal for the synthesis of a wide range of compounds with different structures. This property makes chalcones highly desirable as basic building blocks for the design of targeted molecules. The ability to modify and synthesize chalcones into various compounds offers vast possibilities for the creation of agents with specific molecular targets, providing a valuable tool for the development of new active ingredients and treatments [2].
Literature Review
Natural occurrence of prenylated chalcones
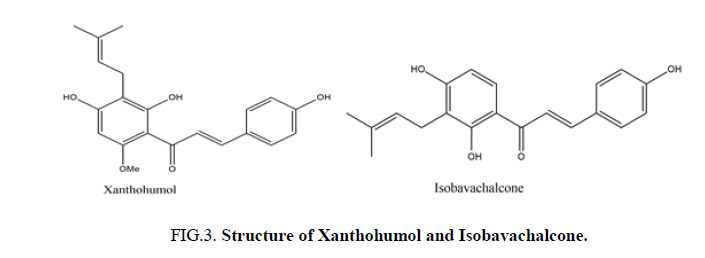
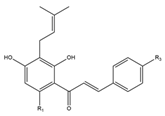
The presence of a prenylated side chain on the chalcone skeleton distinguishes a special class of flavonoids known as prenylated chalcones. These compounds have garnered significant research interest due to their unique biological and pharmacological properties. Chalcones with modified or unmodified prenyl moieties on rings A and B, as well as α and β-carbons, have been discovered in plants of the families Fabaceae, Moraceae, Zingiberaceae, and Cannabaceae. The prenyl moieties may consist of up to three C5-, C10- and C15- units. Notable examples include Xanthohumol and Isobavachalcone, which have been extensively studied for their diverse biological activities (Figure 3) [3].
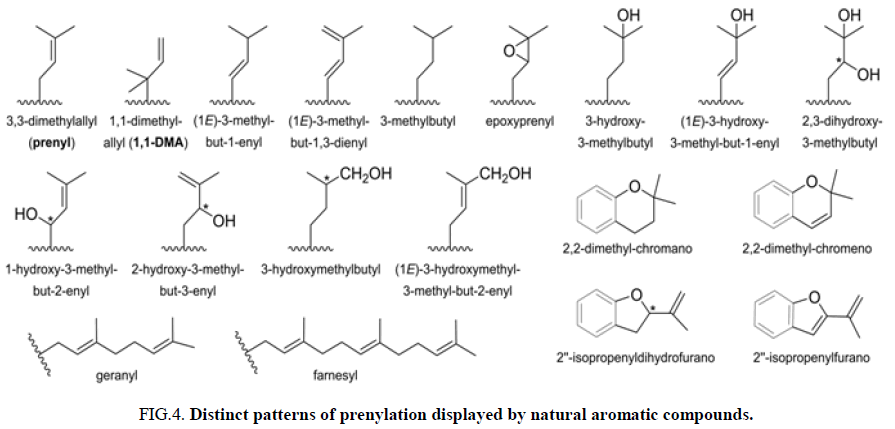
Prenylation refers to the addition of one or more C5 isoprene units to proteins and small molecules. This process leads to the formation of 3,3-dimethylallyl groups, which can undergo further chemical modifications such as cyclization, oxidation, dehydration, or reduction. This diversification of prenylation patterns in nature has attracted significant attention in the field of organic chemistry. The incorporation of prenyl fragments into the chalcone scaffold is limited to the aromatic rings, thus leading to increased interest in the synthesis and design of these prenylated chalcone conjugates (Figure 4) [4].
Structural classification of naturally occurring prenylated chalcones
Natural plants have a history of being used to cure a variety of human illnesses and also demonstrate antimicrobial properties. This is due to the presence of different classes of chemical constituents. Prenylated chalcones are categorized in this review based on the quantity, length and alterations of the prenyl moieties (Table 1) [5].
| Structural classification | Name | Plant | Activity |
|---|---|---|---|
| Chalcones with one C5-prenyl moiety Chalcones with one intact C5-prenyl moiety 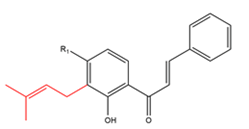 R1= OH R1 = OCH3 |
Isocordoin Derricin |
Leguminosae neuroscapha and L. nitidus | Antiviral |
Chalcones with one simply modified C5-prenyl moiety 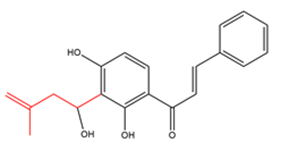 |
Sanjuanolide | Dalea frutescens | Antifungal |
Chalcones with one C5-prenyl moiety incorporated into a chromane, chromene or dihydrobenzofuran ring 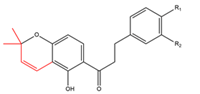 R1=OH R2=H R1=OCH3 R2=H R1=R2=OH |
Crotaramosmin Crotaramin Crotin |
Crotalaria ramosissima | Pesticidal |
Chalcones with a five-membered ring derived from dimethylallyl and β-carbon 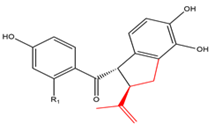 R1=OH R1=OCH3 |
Renifolins Renifolin E |
Desmodium renifolium Desmodium podocarpum | Antiviral Cytotoxic Antifungal |
| Chalcones with two C5-prenyl moieties Chalcones with two intact C5-prenyl moieties 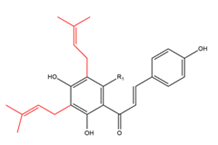 R1=OCH3 R1=H |
9(5’ -prenylxanthohumol) 10 Medicagenin | Humulus lupulus Crotalaria medicagenia | Antimalarial |
Chalcones with modified C5-prenyl moieties 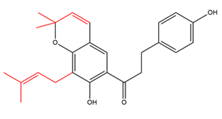 |
Elastichalcone A | Carpus elasticus | - |
Chalcones with two C5-prenyl moieties at both rings A and B 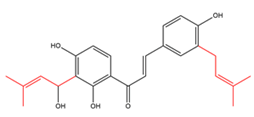 |
kanzonol C derivatives | Hedysarum gmelinii | Anti-influenza |
Chalcones with three C5-prenyl moieties 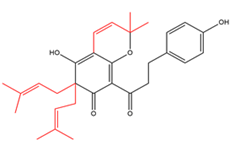 |
Flemiphilippinone C | Flemingia philippinensis | - |
Chalcones with C10-prenyl moieties 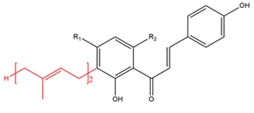 R1=H R2=OH R1=H R2=OCH3 |
Xanthoangelol Xanthoangelol F | Angelica keiskei Humulus lupulus | Antibacterial |
Chalcones with simply modified C10-prenyl moieties. 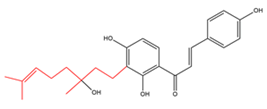 |
Derivatives of C30 -geranylated isoliquirtigenin | Angelica keiskei | Pesticidal |
Chalcones with C10-prenyl moieties as chromane, chromene, dihydrobenzofuran or cyclohexene rings 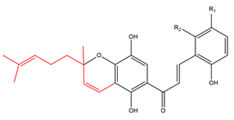 R1=H R2=H R1=H R2=OH R1=OH R2=H |
Flemingins A Flemingins B Flemingins C |
Flemingia grahamiana | Radical scavenging |
Chalcones with ring formation between the geranyl moiety and α,β-carbons 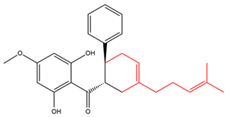 |
Nicolaioidesin C | Renealmia nicolaioides | Cytotoxic |
Chalcones with C15-prenyl moieties 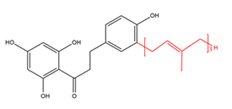 |
Bipinnatone A | Boronia bipinnata | Anti-inflammatory |
Chalcones with C5- and C10-prenyl moieties 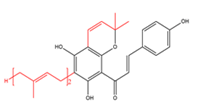 |
Mallotophilippen F | Mallotus philippensis | - |
Bichalcones with C5-prenyl moieties 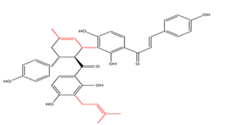 |
Dorstenone | Humulus lupulus | - |
TABLE 1. Prenylated chalcones structural classification along with name, plant and activity.
General insights into the synthesis of prenylated chalcones
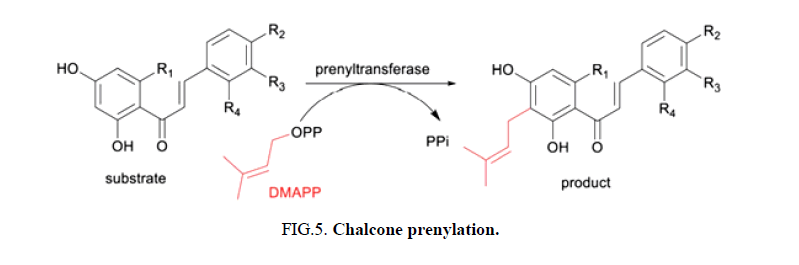
Biosynthesis: Prenylated chalcones in plants are derived from chalcones through prenylation by membrane bound prenyl transferases. Chalcones are produced by the condensation of cinnamoyl-CoA or 4-coumaroyl-CoA with three molecules of malonyl-CoA, catalyzed by Chalcone Synthases (CHS), the key enzyme of the flavonoid/isoflavonoid biosynthetic pathway [6-8]. CHS is a ubiquitous type III Polyketide Synthase (PKS) superfamily enzyme found in higher plants and is responsible for the biosynthesis of different chalcones. Chalcones are then cyclized by Chalcone Isomerases (CHIs) to form flavanones, which are oxidized to flavones by Flavone Synthases (FSs). Only two chalcone specific dimethylallyl (prenyl) transferases, SfiLDT and GuILDT, have been characterized to date. Synthetic prenylated chalcones can be produced by hybridizing simple chalcones with moieties such as prenyl, geranyl, and farnesyl (Figure 5) [9].
Chemical synthesis
The uncomplicated structure and convenient preparation of chalcones make them an appealing framework for creating a broad range of derivatives [10]. This, in turn, allows for the assessment of the impact of diverse functional groups on biological activities. Prenylation means the chemical modification of proteins and small molecules by introducing one or more C5 isoprene units. Rani et al., prepared prenyloxy chalcones 1 by reacting 4-prenyloxy 2-hydroxy acetophenone and corresponding aldehydes in EtOH and piperidine by refluxing (Table 2). After the completion of the reaction, which was monitored by TLC, ethanol was distilled off and the residue was poured on ice water. It was kept overnight in the refrigerator. The resulting solid was collected by filtration, washed with distilled water and crystallized from methanol to give corresponding prenyloxy chalcones 1 [11]. Osorio et al., developed a one-step synthesis of prenylated chalcones using ZnCl2 as a Lewis acid catalyst. The required acetophenone was added to an ethyl acetate solution in a round bottomed flask. Under vigorous stirring, a solution containing 3-methyl-2-buten-1-ol in ethyl acetate (10 mL) was slowly added drop by drop over an hour at 40°C. Afterward, the resulting mixture was heated and a pH 1 aqueous HCl solution was employed to break down the Zn complex [12]. Then, the organic layer was separated, pooled and were dried over anhydrous sodium sulphate and filtered. The products were obtained by silica gel flash column chromatography (ethyl acetate/hexanes) to yield the products. In another study by Passalacqua et al., prenylated chalcones 2-5 were prepared by refulxing a mixture of hydroxylated chalcones dissolved in anhydrous tetrahydrofuran and potassium carbonate. Subsequently, reulxed for 24 hr under inert atmosphere after adding isoprenyl, geranyl or farnesyl bromide [13]. Finally, ethyl acetate was used to extract the product, which was then dried over sodium sulphate and concentrated under reduced pressure. Flash chromatography was used to purify compounds 2-5 in increasing order of polarity using hexanes and ethyl acetate as solvents [14]. In a study where Neves et al., on base catalyzed aldol condensation of suitable acetophenone the desired chalcone 6 was obtained in 90% yield. Compound 6 was then used as precursor for the synthesis of the O-prenylated derivatives 7 and 8. This synthetic approach was based on the O-alkylation of 6 with prenyl or geranyl bromide in presence of Tetrabutylammonium Hydroxide (TBAOH; Bu4NOH) at room temperature, leading to (prenyloxy) chalcone 7 (35% yield) and (geranyloxy) chalcone 8 (62% yield), respectively. Jin et al., describes a practical and additive free method for the prenylation of chalcones using prenylzinc. The suggested methodology is appealing because it allows access to a new class of prenylated chalcone produced from two classes of natural chemicals in a straightforward, high yielding, and region selective synthetic step. Dong et al., using Support Vector Machine (SVM) classification model, prepared five prenylated chalcones [15]. Demethoxymethylation of the initially prepared chalcones lead to the products 9-14.1H-NMR and ESI-MS were used to determine the structures of the produced compounds [16]. These are some of the commonlyused methods to synthesize prenylated chalcones.
| Structure of prenylated chalcones | Number |
|---|---|
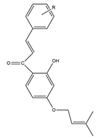 R-p-OCH3 p-CH3 p-Cl p-N(CH3)2 o-Cl |
1 |
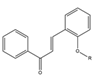 2R-  3R- 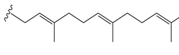 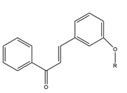 4R- 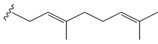 5R- 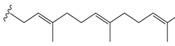 |
2-3 |
  |
6 7 8 |
 9 R1=OH R3=OH 10 R1=OH R3=Ome 11 R1=H R3=OH 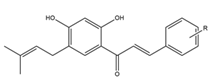 12 R= 3’-OH 13 R=2’,4’-diOH 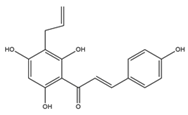 |
9-11 12-13 14 |
TABLE 2. The penylated chalcone compounds synthesized by different researchers.
Discussion
Bioactivity of prenylated chalcones
Antibacterial activity: Chalcones have been reported to have antibacterial activity against various bacterial species in scientific reports. In vitro studies have shown that chalcones have bactericidal and bacteriostatic effects against a range of gram-positive and gram-negative bacteria, including drug resistant strains [17]. There is some evidence to support the idea that prenylation enhances lipophilicity, which increases biological activity by facilitating molecules rapid passage through macrophage and parasite cell membrane barriers. Sasaki’s group discovered that prenylated C-methyl flavonoids had a Minimum Inhibitory Concentration (MIC) value of 1.95 mg/ml, which was substantially lower than that of flavonoids without a prenyl group (7.8 mg/ml) and were therefore more efficient at inhibiting Trichophyton sp. Five prenyl flavonoids, isolated from five distinct medicinal plants, demonstrated substantial antibacterial activity with MIC values of 5 mg/ml-30 mg/ml, according to a similar finding by Sohn et al. It was proposed that the prenyl group's presence and location were crucial for antibacterial activity. The fused pyran ring system's prenyl group mostly controlled this activity. On the other hand, the presence of the prenyl group on the aryl substitution was not required as reported by Madan et al. Chalcones with prenyl or geranyl groups from Angelica keiskei, including deoxyxanthoangelol H, xanthoangelol, xanthoangelol F, xanthoangelol H, 4-hydroxyderricin and deoxydihydroxanthoangelol H, and their derivatives, were synthesized [18]. Gram-positive bacteria were found to be inhibited by the synthetic chalcones 4-hydroxyderricin, isobavachalcone, bavachalcone, xanthoangelol F, thoangelol and broussochalcone B.
Antifungal activity: Many naturally occurring chalcones are poisonous to a wide range of plant pathogens that lead to considerable agronomic and financial losses around the globe. The creation of new antifungal drugs was prompted by the rapid evolution of antibacterial agent resistance. Prenylated chalcones can protect plants from diseases by significantly reducing fungal activity, according to research literature [19]. In a recent study, the effectiveness of hop essential oil and hydro-alcoholic crude extracts from various hop parts, including the leaves, stems, rhizomes, and female cones (also known as hops), against Zymoseptoria tritici, the most common and harmful pathogen on wheat crops, was evaluated using spotting bioassays on PDA medium. Only the crude extract and essential oil from hops effectively slowed fungal growth, according to dose-response curves [20]. Only desmethylxanthohumol and co-humulone among the isolated prenylated chalcones and acylphloroglucinol derivatives shown antifungal action against Z. tritici, with half-maximal inhibitory concentrations of 0.2 g L-1 and 0.11 g L-1, respectively. Chalcones and their derivatives may therefore be a contender to research as a secure antifungal agent because they may not have an impact on the host.
Antinematic activity: PPNs, or plant parasitic nematodes, are among the most significant soil borne parasites that harm practically all agricultural plant species. Due to their extensive host range, which includes vegetables, cereals and pulses, Root Knot Nematodes (RKNs) are the most devastating pathogens in terms of yield loss. One of the major RKNs that affect vegetables from solanaceous family, is Meloidogyne incognita. Several pesticides, including carbofuran, have been used to combat nematodes. Carbofuran, however, is surrounded by controversy because of environmental worries. To effectively manage nematodes, it is currently necessary to develop environmentally safe alternatives. Many organic and synthetic compounds have been shown to be efficient against M. incognita. With the ultimate goal of identifying new compounds active against root knot nematodes, chalcones and various acetophenones were synthesized and tested in vitro against Meloidogyne incognita. The two most potent acetophenones with EC50/24 h values of 12 ± 5 mg L−1 and 15 ± 4 mg L−1, were 4-nitroacetophenone and 4-iodoacetophenone, respectively. Based on their substitution pattern, the nematicidal activity varied when converted to the chalcones. The synthesis of the corresponding chalcone through the condensation of 4-nitroacetophenone with 2,4,6-trihydroxybenzaldehyde to give (E)-1-(4-nitrophenyl)-3-(2,4,6-trihydroxyphenyl)prop-2-en-1-one led to a slight reduction in activity (EC50/24 h value 25 ± 17 mg L−1). Moreover, (E)-3-(2-hydroxy-5-iodophenyl)-1-(4-methoxyphenyl)prop-2-en-1-one showed better activity than 4-methoxyacetophenone [21].
Antiprotozoal activity: This activity is demonstrated against various protozoan parasites including Leishmania, Trypanosoma, and Plasmodium species, the causative agents of diseases such as leishmaniasis, African sleeping sickness, and malaria, respectively. These infections are caused by single celled parasites and current treatments are often inadequate or have significant side effects. The lack of licensed vaccines adds to the global burden of these diseases, making safe and effective drugs essential to their prevention and treatment. Unfortunately, where drugs are available, their effectiveness is being jeopardized by parasite drug resistance. A set of novel prenylated chalcones was produced via Claisen-Schmidt condensation, followed by evaluating their influence on the survival of Trypanosomatidae parasites such as Leishmania amazonensis, Leishmania infantum, and Trypanosoma cruzi. Most of the screened compounds showed good anti-leishmanial and anti-trypanosomal activities owing to the fact that the prenylated chalcones evaluated in this work contain O-isoprenyl and O-farnesyl at C-2 or O-farnesyl and O-geranyl at the C-3 positions; thus these prenyl moieties may result in an increased lipophilicity of the compounds that is responsible for the augmented leishmanicidal activity by facilitating the passage of the molecules through the cell membrane barriers of the macrophages and parasites. Malaria is a significant global health problem, and the search for new and effective treatments is ongoing. Prenylated chalcones have been shown to have promising anti-plasmodial activity in laboratory studies and animal models, leading to interest in their potential as a treatment for malaria. Frolich et al., assessed the in vitro anti-plasmodial activity of xanthohumol, the primary hop chalcone, and seven of its derivatives. The research also included an investigation into the impact of the compounds on the degradation of haemin, which depends on Glutathione (GSH), to determine the contribution of chalcones to their antimalarial effect. Out of the eight compounds tested, four demonstrated activity against at least one of the two Plasmodium falciparum strains, with IC50 values <25 μM. Xanthohumol displayed the most significant activity, with IC50 values of 8.2 ± 0.3 (poW) and 24.0 ± 0.8 μM (Dd2). In addition, three of these compounds exhibited activity in the haemin degradation assay. The mechanism of action of prenylated chalcones against these parasites is not fully understood but is thought to involve the disruption of cellular processes such as cell membrane integrity, energy metabolism, and DNA synthesis. The prenylated chalcones are of interest for their potential as new treatments for protozoan infections.
Conclusion
Chalcones, possessing a 1,3-diaryl-2-propen-1-one skeleton, are known for their simple structure yet high degree of diversity through various modifications. This review focuses on the prenylated chalcones, which have a wide range of biological properties and are frequently found in families such as Fabaceae (Leguminosae), Zingiberaceae, Cannabaceae, Moraceae, Apiaceae, Euphorbiaceae, Asteraceae, Piperaceae, Scrophulariaceae, and Annonaceae. These compounds are characterized by having one to two C5-prenyl moieties or one C10-prenyl moiety and are synthesized in plants through prenylation using membrane-bound prenyl transferases from chalcones. The study of nature-inspired hybridization has led to the experimentation of incorporating moieties like prenyl, geranyl, farnesyl, etc. into the chalcone scaffold. However, the incorporation of prenyl fragments is limited to aromatic rings. Ongoing research efforts are focused on expanding both the substrates and targets to broaden the scope of prenylated chalcones. In conclusion, these prenylated chalcones exhibit great potential as leads in the discovery of new compounds with properties such as nematicidal, antibacterial, antifungal, anti-plasmodial, anti-leishmanial, and anti-trypanosomal activities, and may have practical applications in crop management as active ingredients.
Competing Interests
There are no conflicts of interest to disclose.
References
- Magalhaes EP, Gomes ND, de Freitas TA, et al. Chloride substitution on 2-hydroxy-3,4,6-trimethoxyphenylchalcones improves in vitro selectivity on Trypanosoma cruzi strain Y. Chem Biol Interact. 2022;361:109920.
[Crossref] [Google Scholar] [PubMed]
- Yamazaki Y, Suh DY, Sitthithaworn W, et al. Diverse chalcone synthase superfamily enzymes from the most primitive vascular plant, Psilotum nudum. Planta. 2001;214:75-84.
[Crossref] [Google Scholar] [PubMed]
- Austin MB, Noel JP. The chalcone synthase superfamily of type III polyketide synthases. Nat Prod Rep. 2003;20(1):79-110.
[Crossref] [Google Scholar] [PubMed]
- Koes RE, Quattrocchio F, Mol JN. The flavonoid biosynthetic pathway in plants: Function and evolution. BioEssays. 1994;16(2):123-132.
- Ferrer JL, Jez JM, Bowman ME, et al. Structure of chalcone synthase and the molecular basis of plant polyketide biosynthesis. Nat Struct Biol. 1999;6(8):775-884.
[Crossref] [Google Scholar] [PubMed]
- Abad P, Gouzy J, Aury JM, et al. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat Biotechnol. 2008;26(8):909-915.
[Crossref] [Google Scholar] [PubMed]
- Bocquet L, Riviere C, Dermont C, et al. Antifungal activity of hop extracts and compounds against the wheat pathogen Zymoseptoria tritici. Indust Crops Prod. 2018;122:290-297.
- Caboni P, Aissani N, Demurtas M, et al. Nematicidal activity of acetophenones and chalcones against Meloidogyne incognita and structure–activity considerations. Pest Manag Sci. 2016;72(1):125-130.
[Crossref] [Google Scholar] [PubMed]
- Dong X, Chen J, Jiang C, et al. Design, synthesis, and biological evaluation of prenylated chalcones as vasorelaxant agents. Arch Pharm. 2009;342(7):428-432.
[Crossref] [Google Scholar] [PubMed]
- Frolich S, Schubert C, Bienzle U, et al. In vitro anti-plasmodial activity of prenylated chalcone derivatives of hops (Humulus lupulus) and their interaction with haemin. J Antimicrob Chemother. 2005;55(6):883-887.
[Crossref] [Google Scholar] [PubMed]
- Jin HS, Zhang SQ, Sun R, et al. Introduction of prenyl fragment into chalcones through α- regioselective 1,2-addition in THF. RSC Adv. 2014;4(42):21810-21814.
- Madan S, Singh GN, Kohli K, et al. Isoflavonoids from Flemingia strobilifera (L) R. Br. roots. Acta Pol Pharm. 2009;66(3):297-303.
[Google Scholar] [PubMed]
- Neves MP, Lima RT, Choosang K, et al. Synthesis of a natural chalcone and its prenyl analogs evaluation of tumor cell growth inhibitory activities, and effects on cell cycle and apoptosis. Chem Biodivers. 2012;9(6):1133-1143.
[Crossref] [Google Scholar] [PubMed]
- Osorio ME, Quiroz KA, Carvajal MA, et al. Synthesis, anti-phytopathogenic and DPPH radical scavenging activities of C-prenylated acetophenones and benzaldehydes. J Chil Chem Soc. 2016;61(3):3095-3101.
- Rani MS, Kalyani NC, Murthy C, et al. Piperidine mediated synthesis of prenylated chalcones and 8-substituted-2,5-dihydro-2-(4-tolybenzo)-5-(3-methylbut-2-enyloxy) phenol-1, 5-benzothiazepines and its derivatives as anticancer agents. Rasayan J Chem. 2019;12(2):796-802.
- Sasaki H, Kashiwada Y, Shibata H, et al. Prenylated flavonoids from Desmodium caudatum and evaluation of their anti-MRSA activity. Phytochem. 2012;82:136-142.
[Crossref] [Google Scholar] [PubMed]
- Shakil NA, Pankaj Kumar J, Pandey RK, et al. Nematicidal prenylated flavanones from Phyllanthus niruri. Phytochem. 2008;69(3):759-764.
[Crossref] [Google Scholar] [PubMed]
- Sohn HY, Son KH, Kwon CS, et al. Antimicrobial and cytotoxic activity of 18 prenylated flavonoids isolated from medicinal plants: Morus alba L., Morus mongolica Schneider, Broussnetia papyrifera (L.) Vent, Sophora flavescens Ait and Echinosophora koreensis Nakai. Phytomedicine. 2004;11(7-8):666-672.
[Crossref] [Google Scholar] [PubMed]
- Sugamoto K, Matsusita YI, Matsui K, et al. Synthesis and antibacterial activity of chalcones bearing prenyl or geranyl groups from Angelica keiskei. Tetrahedron. 2011;67(29):5346–5359.
- Yadav DK, Tripathi KP, Kaushik P, et al. Microwave assisted synthesis, characterization and biological activities of ferrocenyl chalcones and their QSAR analysis: Part II. J Environ Sci Health B. 2020;56(1):82-97.
[Crossref] [Google Scholar] [PubMed]
- Zhou K, Yang S, Li SM. Naturally occurring prenylated chalcones from plants: Structural diversity, distribution, activities and biosynthesis. Nat Prod Rep. 2021;38(12):2236–2260.
[Crossref] [Google Scholar] [PubMed]