Original Article
, Volume: 18( 1)Ninhydrin Based Chemo-Sensor for Simultaneous Detection of Co (II) and Cu (II) in Aqueous Medium
- *Correspondence:
- Gururaja GN, School of Chemical Sciences, Central University of Gujarat, Gandhinagar, India, Tel: 079 2397 7407; E-mail: gururajagn@gmail.com
Received: February 13, 2017; Accepted: February 21, 2018; Published: February 26, 2018
Citation: Prabhu SP, Kukalkar TS, Gururaja GN, et al. Ninhydrin Based Chemo-Sensor for Simultaneous Detection of Co (II) and Cu (II) in Aqueous Medium. Anal Chem Ind J. 2018;18(1):131
Abstract
Colorimetric sensor derived from ninhydrin is presented for the selective visual detection of heavy (toxic) metals Co (II) and Cu (II). The colour complex formed is stable at a wide range of pH. The detection limit of this colourimetric sensor in water is comparatively lower and was exploited to prepare optical solid sensor. Filter paper dip sticks developed was employed as a sensor to detect the presence and to distinguish between Co (II) and Cu (II).
Keywords
Intramolecular charge transfer (ICT); Millimolar; Dip sticks; Optical; Detection; Metals
Introduction
‘Heavy metals’ natural component of earth crust which in trace quantity are essential for many biological activities. However, presence of these above critical level poses significant environmental and occupational hazards [1]. The Heavy metal accumulation in living things can lead to heavy metal poisoning and may cause various types of acute and chronic disorders [2]. Among the essential heavy metals, cobalt (Co) and copper (Cu) represents class of essential elements required for various biochemical and physiological functions [3]. Deficiency of these may lead to a variety of deficiency diseases or syndromes [3]. Cobalt is the major constituent of vitamin B-12 [4]. However above a critical level, cobalt is toxic and lead to decreased cardiac output, thyroid enlargement, asthma and lung disease [5,6]. Copper is a constituent of several enzymes. Enzymes make use of Cu (II) oxidised state and reduced state Cu (I) in various biological redox reactions [3]. Similarly, these two oxidation states are also responsible for the generation of toxic superoxide and hydroxyl radicals in biological process [7-10]. Prolonged exposure of Cu (II) leads to irritation of nose and eyes, headache, stomachache, dizziness, diarrhea and neurodegenerative diseases [11]. Increased health concern over heavy metal contamination adds up to the studies concerning detection and subsequent removal of heavy metals. Various sophisticated instruments and methods have been developed for the detection of ions with selectivity and sensitivity [12-19]. Operational burden remains the main hurdle for extensive use. In this contest use of organic colorimetric receptors has gained considerable attention, which allows visual detection without any operational constraint [20-25].
Chromogenic reagent ninhydrin and its derivatives attracted our attention due to their analytical significance [26]. Ninhydrin based colorimetric sensors have been known for visual detection of heavy metal ions in aqueous medium. These colorimetric sensors are Schiff base derivative which involves more than one synthetic procedure [27]. Moreover, development of colorimetric sensor by a simple procedure, which displays distinct colour change to multiple ions, is highly desirable. Herein we report hydroxylamine derivative of ninhydrin as a sensor for selective visual detection of Co (II), Cu (II) in aqueous medium and development of dipstick device (paper strips) for visual detection and analysis of Co (II) and Cu (II) in an aqueous medium.
Materials and Methods
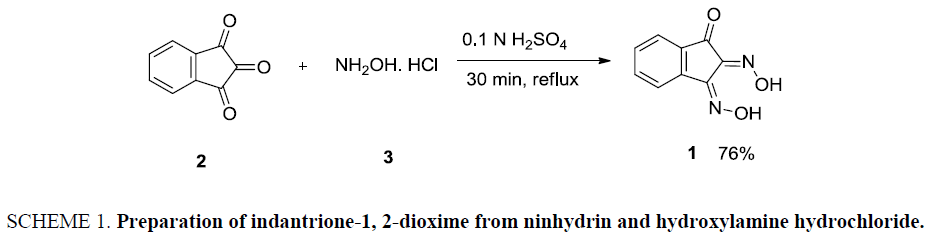
Colorimetric sensor 1 was synthesized from ninhydrin 2. Ninhydrin 2 reacted with hydroxylamine hydrochloride 3 under an acidic condition to afford hydroxyl amine derivative of ninhydrin, indantrione-1, 2-dioxime 1 with 76% yields. Indantrione-1, 2-dioxime 1 has been recrystallized from hot methanol which melts with decomposition at 172°C. Spectral studies of these compounds are consistent with the expected product (Scheme 1) [28].
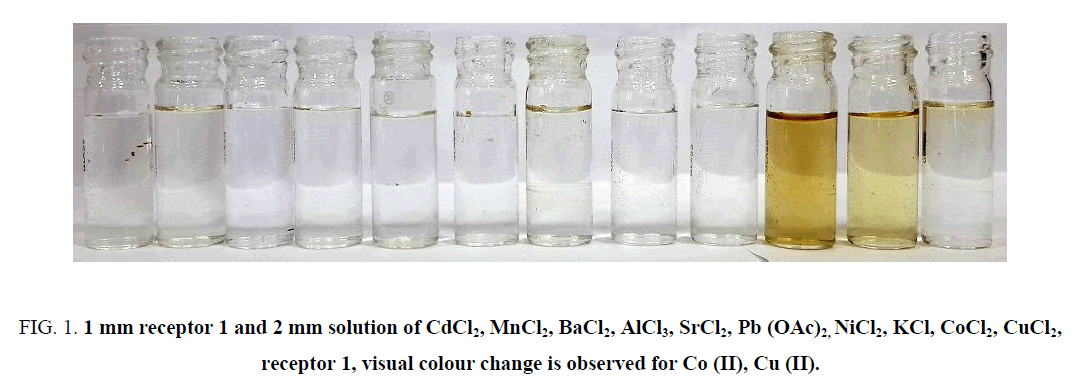
Colorimetric sensing ability of 1 was investigated using 1 mm solution in ethanol with 2 equivalents of various metal ion solutions (as chlorides and acetate) such as CoCl2, CuCl2, MnCl2, BaCl2, NiCl2, KCl, SrCl2, AlCl3, CdCl2, LiCl, Pb (OAc)2. Upon mixing receptor 1 and metal ion solutions in proper proportion, Co (II) and Cu (II) showed distinct colour change to yellow and other metal ions showed no visible colour change. However at higher concentration Co (II) showed orangeyellow, whereas Cu (II) showed olive green colour change respectively. Distinct colour change for Co (II) and Cu (II) signify the potential of receptor 1 as a sensor for visual detection of these metal ions in aqueous solution. Notably for the same concentration of receptor 1, visual colour change for Co (II) can be distinguished with Cu (II) (Figure 1). Origin of colour change may be attributed to intramolecular charge transfer of receptor 1 upon complexation of Co (II) or Cu (II) with nitrogen and hydroxyl group of receptor 1. Although complex of Ni (II) with indantrione-1, 2-dioxime, receptor 1 are known [29], with 0.1 mm concentration of receptor 1 no detectable colour change were observed for Ni (II).
Figure 1: 1 mm receptor 1 and 2 mm solution of CdCl2, MnCl2, BaCl2, AlCl3, SrCl2, Pb (OAc)2, NiCl2, KCl, CoCl2, CuCl2, receptor 1, visual colour change is observed for Co (II), Cu (II).
General
All reagents used in this study were of reagent grade and were used as received without any purification. The melting points were recorded on Thermionic melting point apparatus. IR spectra were recorded on a Shimadzu (IR Prestige-21) FT-IR spectrometer from 4000-400 cm-1 at a resolution of 4 cm-1. UV-visible spectra were recorded using Shimaddzu UV-2450 double beam spectrometer using quartz cell. NMR spectra (1H and 13C) were recorded on an AV 400 (Bruker) TMS as the internal standard. The coupling constants (J values) are given in Hz. Elemental analysis (C, H and N) was done on an Elementar vario Micro cube CHNS analyzer Figure 2.
Procedure for the preparation of (2E, 3Z)-2, 3-bis (hydroxylamino)-2, 3-dihydro-1H-inde-1-one1
Ninhydrin (2.84 g, 0.0159 mmol) and hydroxylamine hydrochloride (0.0318 mmol) were dissolved in 80 mL of 0.1 N H2SO4. The reaction mixture was gently boiled on the hot plate for 15-20 minutes followed by cooling it in the ice bath Figure 3 and Figure 4. The product precipitated was filtered through Buckner funnel and washed with warm dilute acidic solution followed by repeated washing with cold water Figure 5. The products were recrystallised with hot methanol Figure 6 and Figure 7.
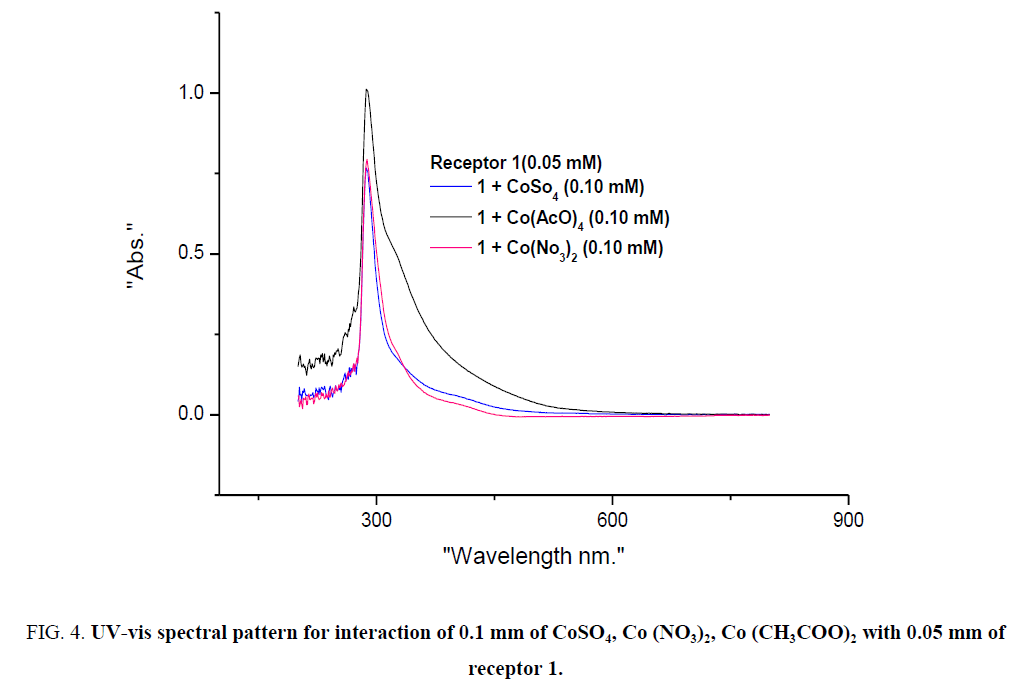
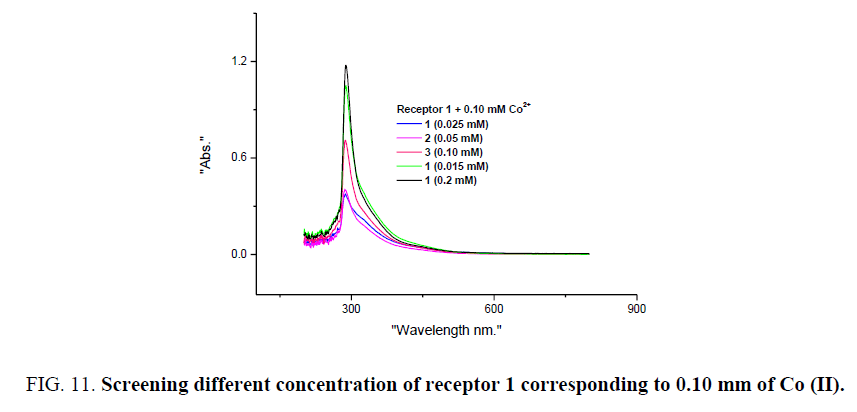
Figure 4: UV-vis spectral pattern for interaction of 0.1 mm of CoSO4, Co (NO3)2, Co (CH3COO)2 with 0.05 mm of receptor 1.
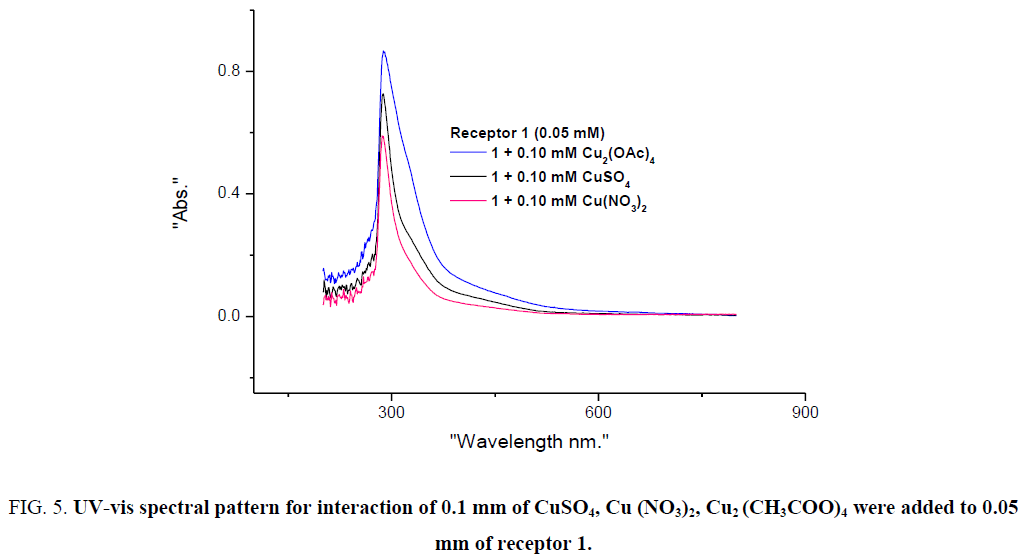
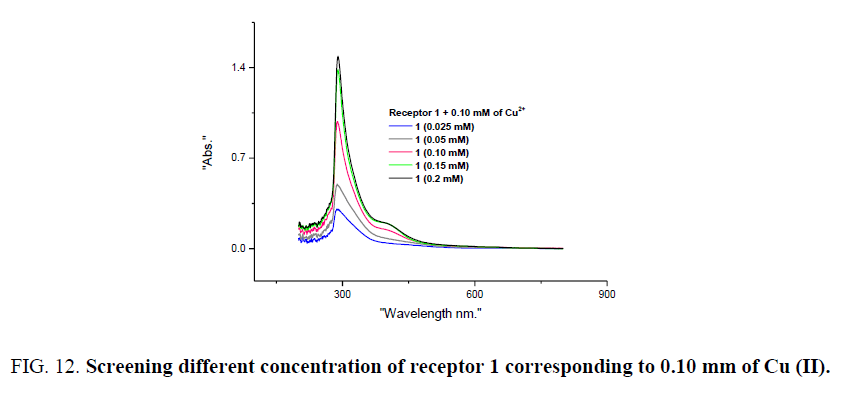
Figure 5: UV-vis spectral pattern for interaction of 0.1 mm of CuSO4, Cu (NO3)2, Cu2 (CH3COO)4 were added to 0.05 mm of receptor 1.
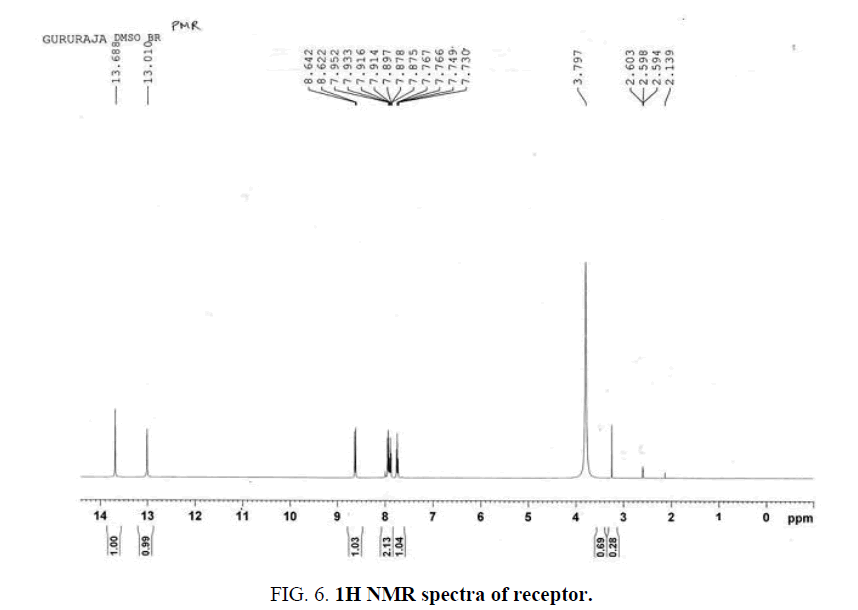
Figure 6: 1H NMR spectra of receptor.
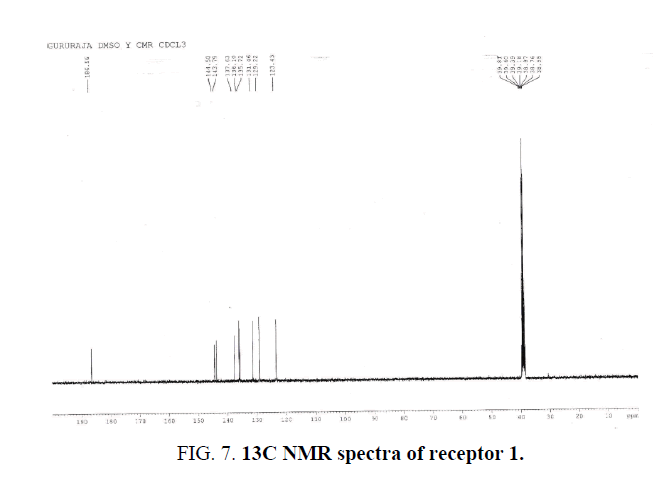
Figure 7: 13C NMR spectra of receptor 1.
Light yellow solid; Yield 76%; mp 172°C (decomposes); FTIR (KBr): cm-1 3261 (s, b), 3045 (w, b), 1724 (s), 1593 (m), 1469 (m), 1408 (m), 1366 (m), 1234 (m), 1085 (m), 962 (m), 881 (m), 719 (w), 690 (w); 1H NMR (400 MHz, DMSO-d6) δ 7.74 (1H, t, J=7.6 Hz), 7.85 (1H, t, J=7.6 Hz), 7.92 (1H, d, J=7.6 Hz), 8.63 (1H, d, J=7.6 Hz), 13.0 (1H, s), 13.7 (1H, s). 13C NMR (75 MHz, DMSO d6) δ 123.4, 129.2, 131.5, 135.7, 136.1, 137.6, 143.8, 144.50, 186.6. Anal calcd for C9H6N2O3, C 56.85, H 3.18, N 14.73; found C 57.23, H 3.56, N 15.34.
Results and Discussion
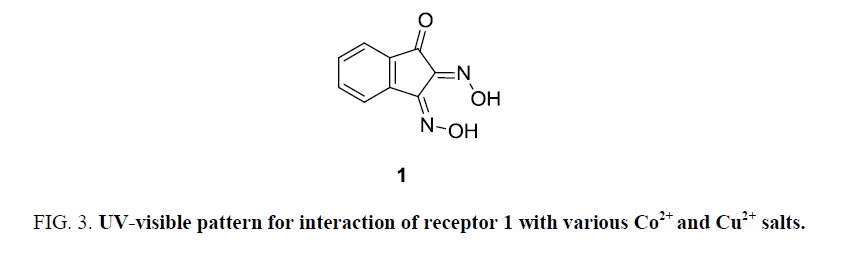
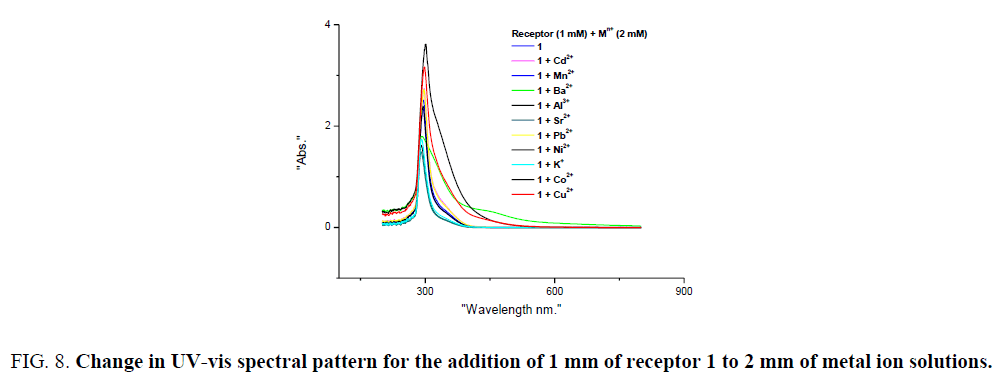
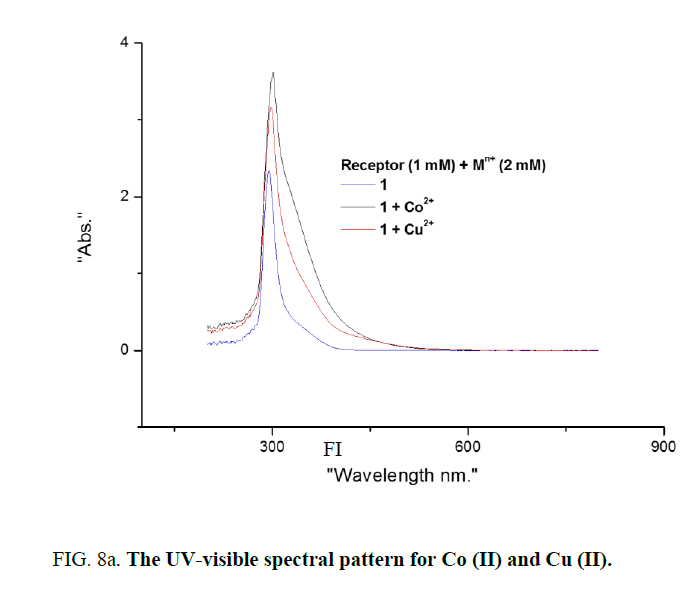
Binding properties of receptor 1 with various metal ions initially investigated with UV-visible absorption studies. Spectral properties of receptor 1 were analyzed with various solvents like ethanol, ethanol/water, methanol, methanol/water, acetonitrile, acetonitrile, acetone and as solubility is more ethanol was chosen as a solvent of choice for further screening of receptor 1. One mm solution of receptor 1 in ethanol with 2 equivalents of various above mentioned metal ions were mixed in proper proportion. Absorption pattern obtained is distinct for Co (II) and Cu (II) compared with the solution of other ions (Figure 8). Greater intensity of colour change is observed for Co (II) and Cu (II) is also reflected in UV-visible absorption studies (Figure 8a).
Figure 8: Change in UV-vis spectral pattern for the addition of 1 mm of receptor 1 to 2 mm of metal ion solutions.
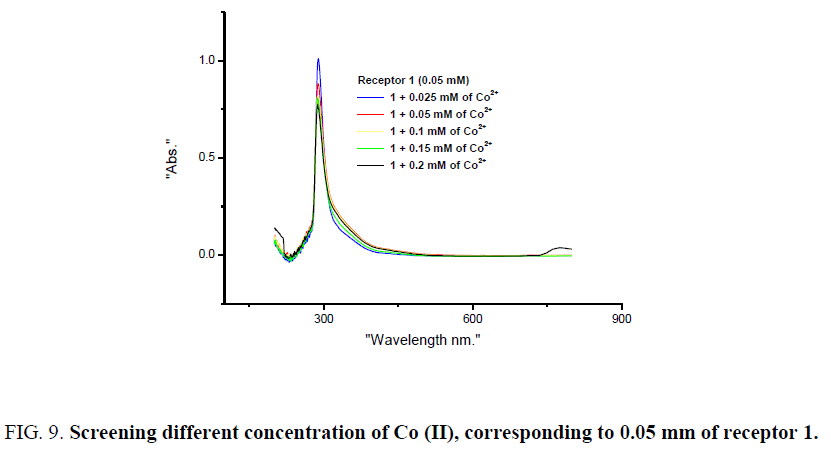
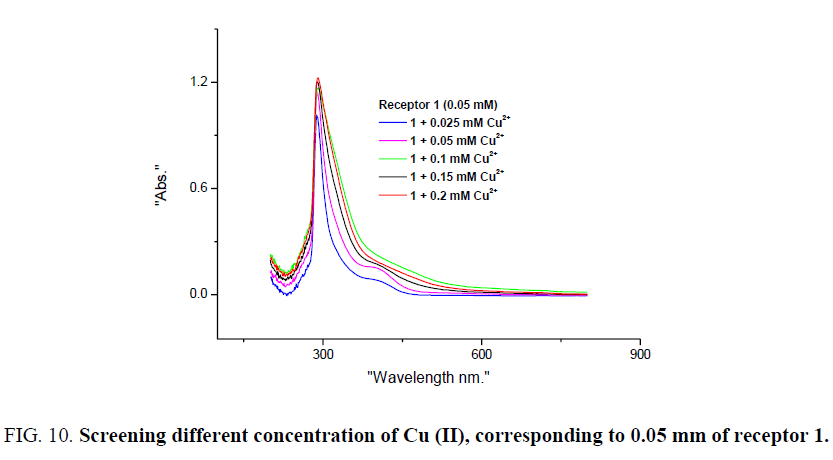
The scope of visual detection of these metal ions with receptor 1 was further screened for different concentration of metal ions. Corresponding to 0.05 mm of receptor 1, metal ion concentration such as 0.025, 0.05, 0.1, 0.15, 0.2 mm were considered. When the CoCl2 solution was mixed in proper portion to receptor 1, detectable colour change observed corresponding to 0.15 mm of the CoCl2 solution. UV-visible spectral studies reveal an interesting trend, upon addition of 0.025 to 0.2 mm solution of CoCl2 to receptor 1, the absorbance peak at 288 nm gradually decreased and colour change from colourless to coloured was clearly observed corresponding to 0.15 mm of CoCl2 with receptor 1. The decrease in absorbance and colour change corresponding to 288 nm may be attributed to the effect of ligand-based transition [20] on complex formation (Figure 9). However when the concentration of the CuCl2 solution was varied with receptor 1, detectable colour change observed with 0.05 mm of the CuCl2 solution. Spectral studies reveal notable trend for Cu (II), upon addition of 0.025 to 0.2 mm solution of CuCl2 to receptor 1 absorbance peak at 288 nm gradually increased and detectable colour change was observed with 0.05 mm of CuCl2 and receptor 1. Increase in absorbance corresponding 289 may be attributed to π–π* transition (Figure 10). Intermolecular charge transfer (ICT) band at 530-550 nm are generally observed for Co (II) and Cu (II), however, sensitivity of the instrument used was inadequate to record such weak transition for these dilute solutions.
Further optical studies were performed by varying concentration of receptor 1. 0.10 mm of CoCl2 and CuCl2 were considered with 0.025 to 0.20 mm of receptor 1. When Different concentration of receptor 1 were added to 0.10 mm of Co (II), detectable colour change is observed corresponding to 0.025 mm of receptor 1, signifying the lower detection limit of receptor 1 for visual detection of Co (II) and on similar experiments for Cu (II) lower detection limit was found to be 0.10 mm of receptor 1. UV-visible studies reveal notable results as varying the concentration of receptor 1, the intensity of absorbance corresponding 289 due to π–π* transition increases in both the cases (Figure 11 and Figure 12). Spectral studies on the screening of different concentration of receptor 1, Co (II) and Cu (II) it may be proposed that structure of charge transfer complex formed from these two metal ions bears different interactions with ligand and structure. Experimental methods to find stoichiometric ratios and study of type interaction and complex formation between receptor 1, CoCl2, and CuCl2 is in progress.
Based on visual detection and spectral screening studies detection limit is found to be 1.5 × 10-4 M Co (II) and 5 × 10-6 M for Cu (II) in an aqueous system. Further stability of the coloured solution formed due to receptor 1, CoCl2 and CuCl2 were analysed at different pH. Solutions ranging from pH 2 to 10 were analysed and colour remained stable. Receptor 1 was also considered with different anions for detection of anions in an aqueous medium, however, no noticeable colour change is observed.
To assess the potential of receptor 1 as a colourimetric sensor, we investigated the interaction of receptor 1 with various Co (II) and Cu (II) salts. 0.1 mm of CoSO4, Co (NO3)2, Co (CH3COO)2 were separately added to 0.05 mm of receptor 1. Similarly, 0.1 mm of CuSO4, Cu (NO3)2, Cu2 (CH3COO)4 were added to 0.05 mm of receptor 1. The remarkable colour change was observed in all the cases and spectral studies also support the optical properties of the receptor 1 with various Co (II) and Cu (II) salts. Absorbance pattern was a similar and small increase in intensity for CH3COO- salt of the metals is attributed to the presence of CH3COO- ion (See ESI –S1).
Further, as a part of the application, visual recognition using organic receptor could be exploited for reliable and selective detection of Co (II) and Cu (II) in aqueous solution. Development of filter paper-based “dip-stick” appears to be an attractive technique that does not need any equipment [30]. Accordingly, filter paper-based strips similar to that of pH paper were developed. In the presence of other cations and anions, the receptor was found to be selective towards Cu (II) and Co (II) hence it can be exploited for the real-time detection of these cations. Dipsticks of this receptor were prepared by dissolving 0.1 g of the receptor 1 in 30 mL of ethanol. The above mixture is then heated to get a concentrated solution of the receptor. Whatmann filter paper of desired shape and size were dipped into this solution and air dried for several days. These dried dipsticks were ready for the visual detection of Co (II) and Cu (II).
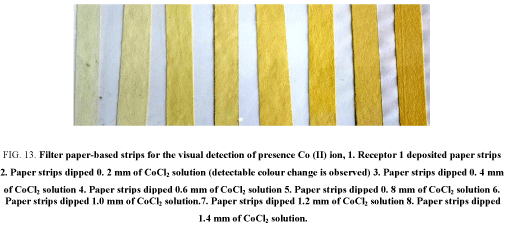
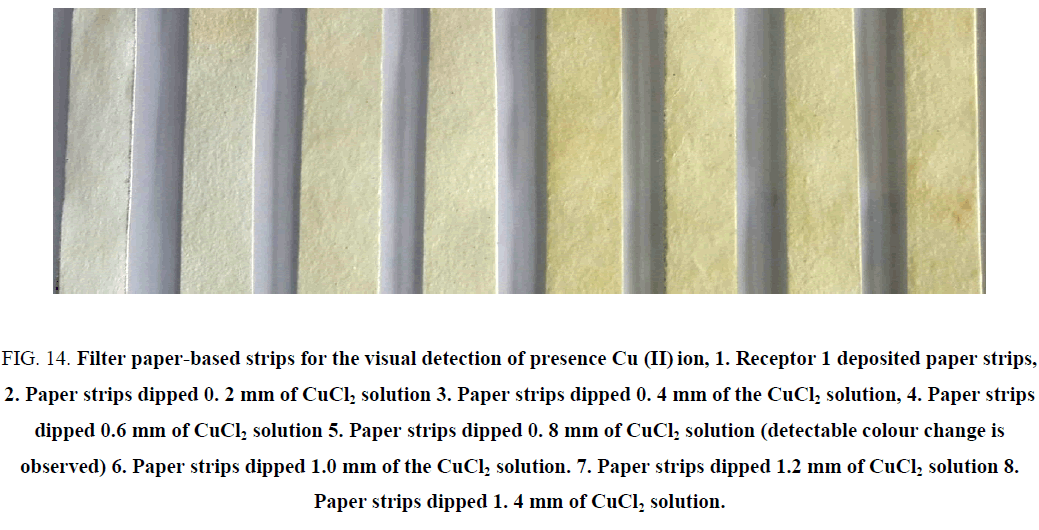
Solutions of Co (II) and Cu (II) (as their chlorides) of varying concentrations such as 0.2, 0.4, 0.6, 0.8, 1.0, 1.2 and 1.4 mm were prepared. When these dipsticks were tested for the presence of Co (II) and Cu (II), a colour change from colourless to yellow colour indicated the presence of these cations. However, for the same concentration of metal ions, a distinct colour change was observed in case of Co (II) corresponding to 0.2 mm concentration as compared to 0.6 mm Cu (II) concentration. Hence this difference in intensity of colour could be exploited to distinguish between Co (II) and Cu (II) (Figure 13 and Figure 14).
Figure 13: Filter paper-based strips for the vi sual detection of presence Co (II) ion, 1. Receptor 1 deposited paper strips 2. Paper strips dipped 0. 2 mm of CoCl2 solution (detectable colour change is observed) 3. Paper strips dipped 0. 4 mm of CoCl2 solution 4. Paper strips dipped 0.6 mm of CoCl2 solution 5. Paper strips dipped 0. 8 mm of CoCl2 solution 6. Paper strips dipped 1.0 mm of CoCl2 solution.7. Paper strips dipped 1.2 mm of CoCl2 solution 8. Paper strips dipped 1.4 mm of CoCl2 solution.
Figure 14: Filter paper-based strips for the visual detection of presence Cu (II) ion, 1. Receptor 1 deposited paper strips, 2. Paper strips dipped 0. 2 mm of CuCl2 solution 3. Paper strips dipped 0. 4 mm of the CuCl2 solution, 4. Paper strips dipped 0.6 mm of CuCl2 solution 5. Paper strips dipped 0. 8 mm of CuCl2 solution (detectable colour change is observed) 6. Paper strips dipped 1.0 mm of the CuCl2 solution. 7. Paper strips dipped 1.2 mm of CuCl2 solution 8. Paper strips dipped 1. 4 mm of CuCl2 solution.
The model waste solution was prepared to contain different cations such as Cu (II), Co (II), Pb (II), Ni (II), and Fe (II) employed for the visual detection of the presence of Co (II) and Cu (II). It can be seen that these dipsticks show a detectable colour change for Co (II) rather than Cu (II) [31]. Although the change in colour is same for Co (II) and Cu (II) change in case of Co (II) seems to be more intense and distinct than Cu (II) [31].
Conclusion
We have presented the synthesis and application of simple ninhydrin derivative ‘indantrione-1, 2-dioxime’ for selective visual detection of Co (II) and Cu (II). This receptor can also be applied in solvents like methanol, methanol/water, ethanol, ethanol/water, acetonitrile and acetone. Detection of heavy metal Co (II) and Cu (II) in the aqueous medium is significant as presences of these above critical level pose significant environmental and occupational hazards. Receptor interaction and structure of complex is appealing and binding of Co (II) and Cu (II) with receptor could be basis for difference in intensity of colour developed by dipsticks for Co (II) and Cu (II) and these solid optical sensors can exploited for detection of these heavy metal ions in industrial waste water.
References
- Abdullah MA, Prasad AD. Biosorption of nickel (II) from aqueous solutions and electroplating wastewater using tamarind (Tamarindus indica L.) bark. Australian Journal of Basic and Applied Sciences. 2010;4(8):3591-601.
- Mudgal V, Madaan N, Mudgal A, et al. Effect of toxic metals on human health. The Open Nutraceuticals Journal. 2010;3(1):94-9.
- Schenck JF, Zimmerman EA. High-field magnetic resonance imaging of brain iron: Birth of a biomarker? Nmr Biomed. 2004;17(7):433-45.
- Zilm ME, Chen L, Sharma V, et al. Hydroxyapatite substituted by transition metals: Experiment and theory. Phys Chem Chem Phys. 2016;18(24):16457-65.
- Maiga A, Dlallo D, Bye R, et al. Determination of some toxic and essential metal ions in medicinal and edible plants from Mali. J Agric Food Chem. 2005;53:2316-21.
- Yeung HYA, New EJ, Chang CJ. A selective reaction-based fluorescent probe for detecting cobalt in living cells. Chem Commun. 2012;48:5268-70.
- Selden A. Norberg C, Karlson SC, et al. Cobal release from glazed earthenware: observation in case of lead poisoning. Environ Toxicol Pharmacol. 2007;23:129-31.
- Abebe FA, Tribal CS, Ramakrishna G, et al. ‘Turn-on’ fluorescent sensor for the selective detection of cobalt and nickel ions in aqueous medium. Tetrahedron Lett. 2011;52:5554-58.
- Stern BR. Essentiality and toxicity in copper health risk assessment: Overview, update and regulatory considerations. Toxicol Environ Health A. 2010;73:114-27.
- Tchounwou P, Newsome C, Williams J, et al. Copper-induced cytotoxicity and transcriptional activation of stress genes in human liver carcinoma (Hep G (2)) cells. Biol Med. 2008;10:285-90.
- Jarup L. Hazards of heavy metal contamination. British Medical Bulletin. 2003;68(1):167-82.
- Sud D, Mahajan G, Kaur MP. Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions: A review. Bioresource Technol. 2008;99(14):6017-27.
- Kato T, Nakamura S, Morita M. Determination of nickel, copper, zinc, silver, cadmium and lead in seawater by isotope dilution inductively coupled plasma mass spectrometry. Analytical Sciences. 1990;6(4):623-6.
- Yin BC. Zuo PH, Zhong H, et al. DNAzyme self-assembled gold nanoparticles for determination of metal ions using fluorescence anisotropy assay. Anal Biochem. 2010; 401:47-52.
- Hong S, Kang T, Moon J, et al. Surface plasmon resonance analysis of aqueous copper ions with amino-terminated self-assembled monolayers. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2007;292(2-3):264-70.
- Gattas-Asfura KM, Leblanc RM. Peptide-coated CdS quantum dots for the optical detection of copper (II) and silver (I). Chem Commun. 2003:21;2684-85.
- Zhang YJ, He XP, Hu M, et al. Highly optically selective and electrochemically active chemosensor for copper (II) based on triazole-linked glucosyl anthraquinone. Dyes and Pigments. 2011;88(3):391-5.
- Hu BS, Lu QP, Wang Y. BODIPY modified 9-cycloheptatrienylidene fluorine derivatives: fluorescent’ turn on’ for detecting Cu (II) acidity independence. Sens Actuators B. 2012. 168:310-17.
- Xu Z, Yoon J, Spring DR. A Selective and ratiometric Cu2+ fluorescent probe based on naphthalimide excimer. Chem. Commun. 2010:46; 2563-65.
- Choi NYJ, You YW, GR, et al. Novel selective colourimetric chemosensor for cobalt ions in a near perfect aqueous solution. Sensors and Actuators B. 2016;223:234-40.
- JinPark GL, You J, Nguyen G, et al. Dual chemosensor for Zn (II) and Co (II) in aqueous media and living cells: Experimental and theoretical studies. Sensors and Actuators B. 2016;223:509-19.
- Jung SH, Park MH, Lee HJ, et al. Selective removal and quantification of Cu (II) using fluorescent iminocoumarin functionalised magnetic nanosilica. Chem Commun. 2012; 48:5082-84.
- Gunnlaugsson TL, PJ, Murray SN. Highly selective colorimetric naked eye Cu (II) detection using an azobenzene chemosensor. Org Lett. 2004;6:1557-60.
- Guo QZ, Chen QW, Duan MX. Highly selective visual detection of Cu (II) utilizing intramolecular hydrogen bond-stabilised merocyanine in aqueous buffer solution. Org Lett. 2010;12:2202-05.
- Wu SP, Du KJ, Sung YM. Colorimetric sensing of Cu (II): Cu (II) induced deprotonation of an amide responsible for color changes. Dalton Transactions. 2010;39(18):4363-8.
- Kumar AK, Diwan V, Upadhyay KK. Highly sensitive and selective naked eye detection of Cu2+ in aqueous medium by a ninhydrin -quinoxaline derivative. Sen Actuators B. 2013;176:420-27.
- Kumar AK, Upadhyay KK. Ninhydrin based colorimetric molecular switch for Hg2+ and CH3COO-/F-. Tetrahedron Lett. 2011;52:6809-13.
- Suzuki M, Kobayashi K. Cryst. Polymorphism in solvate crystals of indantriione 1, 2-dioxime. Growth Des. 2011;11:1814-20.
- Zhong BL, Chen G. Triethylammonium (indane-1, 2, 3-trione 1, 2-dioximato- 2N1, O2) (indane-1, 2, 3-trione 2-oximato 1-oxime- 2N1, O2) nickel (II). Acta Cryst. 2012;68:421.
- Xiong JJH, Zhang PC, CY, et al. Colorimetric detection of Cu (II) in aqueous solution and on the test kit by 4-aminoantipyrine derivative. Sensors and Actuators B. 2016;226:30-36.
- Suzuki M, Kobayashi K. Crystal Polymorphism in solvate crystals of indantriione 1, 2-dioxime. Growth Des. 2011;11:1814-20.