Original Article
, Volume: 12( 1)Hydrogen Production from Non-Conventional Biomass Pyrolysis
- *Correspondence:
- Juarez A ,Instituto de Química Aplicada, Universidad del Papaloapan, Campus Tuxtepec, Circuito Central # 200, Col. Parque Industrial, C.P. 68301, Tuxtepec, Oax, México Tel: 01 (287) 8759240 Ext. 220; E-mail: eajuarez@unpa.edu.mx
Received: February 16, 2017 Accepted: March 22, 2017 Published: March 30, 2017
Citation: Navarro-Mtz AK, Urzua-Valenzuela M, Morelos-Pedro MA, et al.. Hydrogen Production from Non-Conventional Biomass Pyrolysis. Inorg Chem Ind J. 2017;12(1):107.
Abstract
Biomass is a potential energy resource that can be converted to hydrogen and is available from a wide range of sources. In this study, non-conventional biomass sources: soybean (Glycine max.), cassava (Manihot esculenta) and banana rachis (Musa paradisiaca L.) had been tested for hydrogen production from biomass pyrolysis. The influence of biomass microstructure and reducing sugar content on the hydrogen production was studied through a high-energy ball-milling pre-process of the soybean source. The results indicate that the maximum production of hydrogen was obtained at 275°C independently of the biomass source. The biomass microstructure and reducing sugar content does not seem to have an effect on hydrogen production from biomass pyrolysis. Soybean thermal stability is increased during the ball-milling process. High-energy ball-milling can be a good, easy and low cost method to increase biomass reducing sugar content.
Keywords
Hydrogen production; Biomass pyrolysis; High-energy ball milling; Reducing sugar C
Introduction
The world´s energy need is certain to increase with growth in population. Primary energy sources take many forms, including fossil fuels (petroleum, coil, natural gas), nuclear energy or renewable sources (wind, solar, hydropower, geothermal, hydrogen, etc). From those, it is known that fossil fuels sources are being consumed at a higher rate than they are being discovered [1]. Also, the continued use of fossil fuels is threatened by increasing concentrations of carbon dioxide (CO2) in the atmosphere, air pollution and concerns over global warming [2]. Alternative renewable fuels are at present not competitive with fossil fuels in terms of cost and production capacity. Finding viable alternative sources of clean energy to satisfy the demand is one of the biggest challenges. Hydrogen production is one of the most promising alternative energy technologies because it is a clean, renewable and environmentally friendly fuel [3]. It is not primary energy existing freely in nature. Hydrogen is a secondary form of energy that has to be manufactured like electricity; therefore, it is an energy carrier [4]. Hydrogen gas is being explored for use in combustion engines and fuel cell electric vehicles due to hydrogen has a high energy yield of 122 kJ/g, which is 2.75 times greater than hydrocarbon fuels [5]. Hydrogen can be used as a fuel directly in an internal combustion engine not much different from the engines used with gasoline [6].
Well-known methods for hydrogen production are water electrolysis, hydrocarbon steam reforming or auto-thermal processes. However, biomass is a potential energy resource. Depending on the pathways used to generate energy from biomass (e.g. physical, thermal, chemical, and biological conversion), biomass could be converted to heat, electricity, solid fuels (coal), liquid fuels (bio-oil, methanol and ethanol) and gas fuel (hydrogen) [7]. Biomass is a renewable material containing appreciable quantities of hydrogen, oxygen and carbon. It is available from a wide range of sources such as animal wastes, municipal solid wastes, crop residues, short rotation woody crops, agricultural wastes, sawdust, aquatic plants, waste paper, corn, and many more. For hydrogen productions, the current biomass technologies include: pyrolysis, gasification, combustion and reforming (the most studied worldwide) [8]. From those, the biomass pyrolysis is the simplest and cheapest method for energy conversion. The pyrolysis is the thermal decomposition of biomass under anaerobic conditions. Pyrolysis is also a previous step to other thermo conversion processes, such as combustion and gasification.
Several non-conventional biomass sources have been suggested as promising option of biomass for its direct use as fuels and/or its refinement to chemical feedstocks, such as hydrogen, through gasification or other similar process. In this study, soybean (Glycine max.), cassava (Manihot esculenta) and banana rachis (Musa paradisiaca L.) had been tested for hydrogen production from biomass pyrolysis.
Experimental Procedure
Non-conventional biomass sources
Soybean (Glycine max): The soybean is the seed of the legume plant Glycine max. This plant is an important part of diets in many Asian countries. The composition of mature soybean is about 38% protein; 30% carbohydrate; 18% oil; and 14% moisture, ash, and hull. The commercial soybean is just the dried seeds. There are several methods to release amino acids and reducing sugars from soybean; the most used method is the acid hydrolysis [9,10]. Soybean subjected to enzymatic or acid hydrolysis has been used as a bacteriological nutrient media [11]. The hydrolysis process generates nutrients that support the growth of microorganisms. Therefore, in this study is tested soybean for hydrogen production from biomass pyrolysis. Commercially organic soybean was purchased in a natural health food store. In the present study, three types of samples of soybean were tested: soybean, soybean meal (donated by the CINVESTAV, Bioprocess Laboratory [11] and ball-milled soybean.
Cassava (Manihot esculenta): Cassava (Manihot esculenta) is a perennial woody shrub in the Euphorbiaceae (spurge family) originated in western and southern Mexico but now grown in tropical and sub-tropical areas worldwide for the edible starchy roots (tubers). Cassava is also known as yuca, manioc and tapioca. The use of native cassava is limited; nevertheless, its functionality is favored as modified starch, thus increasing its industrial applications. The cassava (Manihot esculenta) used in this study was purchased to a local farmer in Tuxtepec, Oaxaca, Mexico.
Banana rachis (Musa paradisiaca L.): One of the main economic activities of Southern Mexico is the production and commercialization of banana (Musa paradisiaca L.). However, once the fruit is harvested the banana rachis is disposed without any further use; as many other agricultural wastes. Banana rachis (Musa paradisiaca L.) used in this study was provided by a farmer from the community of Santa María Jacatepec, Oaxaca, Mexico [12].
Sample preparation
High-energy ball-milling: The production of soybean meal requires a process of drying, bleaching, fat extraction and grinding while the soybean only requires the drying process, reducing considerably the cost and energy used in the whole process. However, it is expected than the hydrogen production using soybean meal will be greater than the soybean. Therefore, in this study a high-energy ball-milling pre-process was applied to the soybean. High-energy ball-milling generates physical and chemical transformations of substances induced by mechanical energy [13].
Soybeans were used as starting material. The high-energy ball-milling treatment was carried out in air (in hermetically sealed vials) in a planetary ball mill (Retsch PM400/2). Vials (250 ml) and balls (9 of 2 cm) of stainless steel grinding materials were used. A 400 rpm rotation rate and a weight ratio of 1:50 sample: milling balls (5.5 g: 250 g) were used. The grinding times tested were 2 min and 20 min. The temperature during milling was not measured, but the vials could be touched directly after milling.
Drying process: The cassava and the banana rachis have a high content of humidity; therefore, a drying pre-process was necessary. The raw material was washed, cut into slices and placed on stainless steel trays. The drying process was carried out in an oven at 65°C until a constant weight was achieved; raw material completely dehydrated. The drying process lasts 24 hours. After drying, the raw material was stored in hermetically sealed bags to avoid moisture absorption.
Pyrolysis and hydrogen quantification
The biomass pyrolysis was carried out in an electric furnace (Lindberg-Blue TF55035C). A porcelain sample holder was used to place 1.0 g of biomass. The sample holder was introduced into the quartz tube (1.25 inches of internal diameter) of the electric tubular furnace in a horizontal position. A stream of helium gas was fed through the quartz tube at 60 mL/min. The system temperature was maintained at 250°C, 275°C and 300°C for 30 min in each experiment. A scheme of the system used for the pyrolysis and hydrogen quantification is shown in Figure. 1.
Hydrogen quantification was performed using a mixture of sodium acetate (CH3COONa) and acetonitrile (CH3CN) as a dissolvent at a rate of 2/100 wt/v using 5 steps reactors connected in series to capture the biomass pyrolysis gas, Figure. 1. The pyrolysis study was done at three temperatures 250°C, 275°C and 350°C. At the end of each reaction time, the reaction medium of each step reactor was titrated with 0.1 M NaOH solution and phenolphthalein as indicator to quantify the hydrogen production.
The production of hydrogen was evaluated by titration of sodium acetate (CH3COONa) with sodium hydroxide (NaOH). The acid is generated by the reaction between the molecular hydrogen (H2) generated during the pyrolysis process and the sodium acetate, according to Eq.(1):
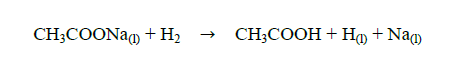 (1)
(1)
The titration was carried out with 0.1M NaOH solution. The moles of atomic hydrogen produced were determined with Eq. (2):
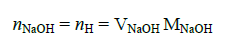 (2)
(2)
where nNaOH and nH are the moles of sodium hydroxide and hydrogen, while VNaOH and MNaOH are the volume and concentration. of sodium hydroxide used in the titration. From those moles and using the molecular weight of hydrogen the mass was estimated.
Characterization techniques
The morphology and characteristics of the surface of the samples were observed by Scanning Electron Microscopy using a Jeol JSM-6390 LV and in a FEI-Helios Nanolab 600. The samples were placed on a 30 nm carbon electrically conductive tape or covered with a gold layer. X-ray powder diffraction (XRPD) patterns were collected in air and at ambient temperature in a Bruker D-8 Advance diffractometer with the Bragg-Brentano θ-θ geometry and Cu Kα radiation. The 2θ-range explored was 10–80° with 0.05° step size, 10s counting time, in a continuous mode and spinning of 15 rpm.
Thermogravimetric (TGA) and differential thermal analysis (DTA) were carried out using a Perkin Elmer STA 6000 simultaneous thermal analyzer, with N2 atmosphere and a temperature range between 30°C and 700°C at 10º/min.
Soybean ball-milled samples were quantified the reducing sugar. Reducing sugar quantification was done with the DNS (ácido dinitrosalisílico) technique [14]. The color intensities were measured at 540 nm using glucose as a standard. The acid hydrolysis was done in soybean to compare the reducing sugar concentration obtained by ball-milling. The acid hydrolysis was done at 120°C and 1.3 Kg/cm2 during 20 min, however with the heating and cooling time the process takes more than 1 h.
Results and Discussion
As mentioned above, the production of soybean meal requires complex processes that increase the cost and energy demand. However, this process is fundamental if the soybean is to be used as a culture media in order to release amino acids and reducing sugars that support the growth of microorganisms. However, nothings is known about the production of hydrogen from soybean pyrolysis or the effect of particle size and reducing sugar concentration on the process. Therefore, in this study the production of hydrogen from pyrolysis of soybean with different modification is studied: soybean (dried seeds), soybean meal (previously characterized [11]) and soybean processed in a high-energy ball-milling.
Scanning Electron Microscopy images (SEM) of soybean, soybean meal and soybean ball milled are shown in Figure. 2. The soybean granules are much bigger than the soybean meal. It can be seen that the acid hydrolysis treatment done during the soybean meal process decrees the ductility in the material. Flat surfaces are associated with fragile fractures. On the other hand, ball milled soybean show different microstructure depending of the milling time, Figure. 2 C-D. High-energy ball-milling generates physical and chemical transformations of substances induced by mechanical energy; in simple systems the effect is very well documented [15,16], however, in complex organic matrices that is not the case. One of the early uses of ball-milling was reduce the size particle and it is what happen with the soybean in this study. In Figure. 2 C-D a clearly soybean particle size reduction is observed as a function of milling time. After 2 min of milling the average soybean particle size is bigger than 5 microns, while at 20 min it decrease below the 5 microns.
Figure 2: Scanning electron micrographs of (A) soybean, (B) soybean meal, (C) soybean ball-milled 2 min, (D) soybean ball-milled 2 min.
Powder diffraction patterns of soybean meal, soybean and soybean ball-milled (2 and 20 min) are shown in Figure. 3 . A typical diffraction pattern of an amorphous material is observed on these samples. This behavior was expected since the soybean composition is mainly protein, carbohydrate, oil and moisture. However, after 20 min of milling the reflection around 20° in 2-theta looks like it is sharpening. This may represent a local ordering. Further milling time is required to corroborate it.
Figure 3: X-ray diffraction patterns of soybean meal, soybean and soybean ball-milled (2 and 20 min).
Due that the pyrolysis is the thermal decomposition of biomass, study the thermal behavior of the starting material is very important. Thermogravimetric (TGA) and differential thermal analysis (DTA) curves of soybean meal, soybean and soybean ball-milled (2 min and 20 min) are shown in Figure. 4. The TGA curves, Figure. 4A , show a similar behavior. Two weight loss regions can be observed. The first one, around 100°C, is associated to water loss, around 8% weight loss. The endothermic peak observed at the same temperature in the DTA curves, Figure. 4C, confirms this.
Figure 4: (A) Thermogravimetric curves (TGA), (B) first derivative of the TGA curves and (C) differential thermal analysis (DTA) curves of soybean meal, soybean and soybean ball-milled (2 min and 20 min) are shown.
The second region is between 220°C to 400°C where the weight loss gets up to 60%, this region is associated with the degradation of the soybean [17]. The DTA curves show an increasing endothermic heat flow that is consisting with the energy needed to degrade the soybean. After the soybean degradation process, just inert carbonaceous residues remains which need higher energy to keep degrading. The effect of the ball milling seems to be the increment of soybean thermal stability. No crystallization or phase transition processes were observed.
The first derivative of the TGA curve allows viewing the start and the end of each event of mass loss, indicating the temperature range where the decomposition reaction occurs. In the first derivative of the TGA curves, Figure. 4C, can be seen clearer the two regions observed in TGA curves. Especially in the second region it can be seen that the mayor weight loss is associated with two different events, one at around 250°C and the other above 300°C. As mentioned above, the soybean composition is mainly protein, carbohydrate, oil and moisture. The moisture is lost around 100°C while the oil around 360°C. [17]; thus it could be that the event at 250°C is associated with the degradation of protein while the event at 300°C is associated with the degradation of carbohydrate. Here is evident that the effect of the ball milling on soybean is to increase the thermal stability.
Once that the thermal behavior of soybean was studied, the other important point to consider is the reducing sugar concentration. The concentration of reducing sugar from soybean (S), soybean meal (SM), acid hydrolysis soybean (SH), acid hydrolysis soybean meal (SMH), soybean ball milled (S2' and S20'), as well as cassava (C) are shown in Figure. 5 . As expected, the reducing sugar concentration in soybean meal (SM) is higher than in soybean (S) as a consequence of the bleaching, fat extraction and grinding process involved. Additional acid hydrolysis breaks further the soybean increasing further the reducing sugar, almost double in both cases. However, with just 2 min of high-energy ball-milling the concentration of reducing sugar in the sample (S2') is similar than the concentration in the acid hydrolysis soybean (SH) sample. This means that after 2 min of milling, using balls of 20 mm of iron, it is released almost the same amount of reducing sugar obtained with 1 h of acid hydrolysis. After 20 min of milling (S20') the concentration increases more than four times comparing with soybean and it is even higher than cassava concentration (C). Thus high-energy ball-milling can be a good, easy and low cost alternative method to generate reducing sugar.
Figure 5: Reducing sugar concentration from soybean (S); soybean meal (SM); acid hydrolysis soybean (S(H)); acid hydrolysis soybean meal (SM(H)); soybean ball-milled for 2 (S2') and 20 (S20') min); and assava (C).
Base on the thermal behavior of soybean, three temperatures were chosen to study the hydrogen production from biomass pyrolysis: 250°C, 275°C and 300°C. The selection was done considering the main degradation region ( Figure. 4A); specifically at temperatures between the two events observed in Figure. 4C. The hydrogen production from pyrolysis are shown in Table 1 (250°C), Table 2 (275°C), Table 3 (300°C) and Figure. 6. From the tables it can be seen that 5 steps reactors connected in series to capture the biomass pyrolysis gas is not enough ( Figure. 1). Which means that more hydrogen was produced and it was not properly quantified. Further steps reactors are needed to exhaust the hydrogen produced.
Figure 6: Hydrogen production from pyrolysis at different temperatures of soybean (S); soybean meal (SM); soybean ball-milled for 2 (S2') and 20 (S20') min); cassava (C); and banana rachis (BR).
| T1= 250°C | VStep VNaOH/mL |
Vtotal VNaOH/mL |
WtH2/mg | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Soybean meal | 8.4 | 4.1 | 3.5 | 2.8 | 3.1 | 21.9 | 1.10 |
| Soybean | 7.5 | 4.2 | 3.5 | 2.4 | 2.4 | 20.0 | 1.01 |
| Soybean 2 min milling | 6.9 | 3.7 | 2.9 | 2.1 | 2 | 17.6 | 0.89 |
| Soybean 2 min milling | 8.5 | 4.4 | 3.9 | 2.7 | 2.3 | 21.8 | 1.10 |
| Cassava | 10.6 | 4.2 | 2.5 | 2.2 | 2.3 | 21.8 | 1.10 |
| Banana Rachis | 12.8 | 3 | 2.8 | 2.9 | 2.9 | 24.4 | 1.23 |
Table 1. Hydrogen production from non-conventional biomass pyrolysis at 250°C.
| T2= 275°C | VStep VNaOH/mL |
Vtotal VNaOH/mL |
WtH2/mg | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Soybean meal | 11.2 | 5.8 | 6.2 | 6 | 6.2 | 35.4 | 1.78 |
| Soybean | 12 | 7.4 | 7 | 6.2 | 6.2 | 38.8 | 1.96 |
| Soybean 2 min milling | 12 | 6.4 | 5.8 | 4.4 | 4.6 | 33.2 | 1.67 |
| Soybean 20 min milling | 10.8 | 5.4 | 5.4 | 4.4 | 5 | 31 | 1.56 |
| Cassava | 19.4 | 7.8 | 6.6 | 6.6 | 5.3 | 45.7 | 2.30 |
| Banana Rachis | 15.7 | 4.5 | 4.9 | 3.7 | 4.1 | 32.9 | 1.66 |
Table 2. Hydrogen production from non-conventional biomass pyrolysis at 275°C.
| T3= 300°C | VStep VNaOH/mL |
Vtotal VNaOH/mL |
WtH2/mg | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Soybean meal | 10.4 | 1.8 | 3.8 | 1 | 0.5 | 17.5 | 0.88 |
| Soybean | 8.3 | 3.3 | 2.1 | 1 | 0.7 | 15.4 | 0.78 |
| Soybean 2 min milling | 9.5 | 6.1 | 17.8 | 3.2 | 1.8 | 38.4 | 1.94 |
| Soybean 20 min milling | 7.4 | 16.6 | 1.6 | 0.9 | 1.3 | 27.8 | 1.40 |
| Cassava | 12.8 | 3.1 | 2.5 | 2.1 | 1.9 | 22.4 | 1.13 |
| Banana Rachis | 12.8 | 3.1 | 2.5 | 2.1 | 1.9 | 22.4 | 1.13 |
Table 3. Hydrogen production from non-conventional biomass pyrolysis at 300°C.
The hydrogen production results show that the pyrolysis process does not depend of particle size or reducing sugar content. The production at 250°C is similar in all the samples, around 1 mg of hydrogen. At 275°C, the production increase and then drop at 300°C. Thus the process depends on the temperature selection rather than microstructure. It is necessary to perform a thermal characterization of cassava and banana rachis to identify the optimal temperature of pyrolysis for those sources. Finally, this study show that hydrogen can by produced from non-processed waste biomass pyrolysis easily and at low cost.
Conclusion
It is possible to produce hydrogen from non-conventional biomass pyrolysis. Microstructure or reducing sugar content does not seem to have an effect on hydrogen production from biomass pyrolysis. Thus, hydrogen can by produced from non-processed waste biomass at low cost. Soybean thermal stability is increased during the ball-milling process. High-energy ball-milling can be a good, easy and low cost method to increase reducing sugar. This can be very useful to produce culture media.
Acknowledgment
The author wishes to thank Urzua-Valenzuela M., Morelos-Pedro M.A. and Roldan-Sabino C for the scholarship granted by CONACyT. The authors also acknowledge LINAN for all the analytical facilities and the support.
References
- Bermejo R. Handbook for a sustainable economy. 2014; ISBN: 978-94-017-8980-6.
- Balat M. Bioethanol as a vehicular fuel: A critical review. Energy Sources Part A. 2009;31:1242.
- Yamin JAA, Gupta HN, Bansal BB, et al. Effect of combustion duration on the performance and emission characteristics of a spark ignition engine using hydrogen as fuel. Int J Hydrogen Energy. 2000;25:581–89.
- Balat H, Kirtay E. Hydrogen from biomass e Present scenario and future prospects. Int J Hydrogen Energy. 2010; 35;7416.
- Kapdan IK, Kargi F. Bio-hydrogen production from waste materials. Enzym Micro Tech 2006;38;569-82.
- Balat M. Hydrogen in fueled systems and the significance of hydrogen in vehicular transportation. Energy Sources Part B. 2007;2(1):49-61.
- Chang A, Chang HF, Lin FJ, et al. Biomass gasification for hydrogen production. Int J Hydrogen Energy. 2011;36:14252-60.
- Libra JA, Ro KS, Kammann C, et al. Biofuels. 2011;2:71.
- Peres A, Lunelli BH, Fllho RM. AIDIC Conference Series. 2013;11:291.
- Albin DM, Wubben JE, Gabert VM. Effect of hydrolysis time on the determination of amino acids in samples of soybean products with ion-exchange chromatography or precolumn derivatization with phenyl isothiocyanate. J. Agric. Food Chem. 2000;48(5):1684-91.
- Farrera RR, Perez-Guevara F, De La Torre. Carbon: Nitrogen ratio interacts with initial concentration of total solids on insecticidal crystal protein and spore production in Bacillus thuringiensis HD-73. M. Appl Microbiol Biotechnol 1998;49(6):758-65.
- Paramo-Calderon DE, Carrillo-Ahumada J, Juarez-Arellano EA, et al. Effect of cross-linking on the physicochemical, functional and digestibility properties of starch from Macho (Musa paradisiaca L.) and Roatan (Musa sapientum L.) banana varieties. Starch/Stärke. 2016;68(7-8):584-92.
- Martinez-Garcia A, Navarro-Mtz AK, Aparicio-Saguilan A, et al. Determination of the mechanosynthesis conditions of the Mg ñ MgO reaction region. Inorganic Chemistry: An Indian Journal, 2015;10(2):34-40.
- Miller GL. Use of dinitrosaiicyiic acid reagent for determination of reducing sugar. Anal Chem 1959;31(3):426-8.
- Juarez-Arellano EA, Kakazey M, Vlasova M, et al. J. Alloys Compd. 2010;492(1-2):368-72.
- Kakazey M, Vlasova M, Dominguez-Patiño M, et al. Kinetics of physico-chemical processes during intensive mechanical processing of ZnO–MnO2 powder mixture. J Magn Magn Mater. 2011; 323(20):2429-35.
- Biernat K. Biofuels - Status and perspective. ISBN 978-953-51-2177-0; Krzyszt of Biernat (ed), InTech, 2015 p: 580.

