Editorial
, Volume: 25( 3)A Systematic Review of the Impact of Pneumatic Tube System Transport on Blood Chemistry and Hematology Parameters
- *Correspondence:
- Ausmolo Angelie Jae T
Department of Medical Science, Far Eastern University, Manila, Philippines
Tel: 9060834927
E-mail: ausmoloajae@gmail.com
Received: December 28, 2022, Manuscript No. TSAC-22-84928; Editor assigned: December 30, 2022, PreQC No. TSAC-22-84928 (PQ); Reviewed: January 13, 2023, QC No. TSAC-22-84928; Revised: February 28, 2023, Manuscript No. TSAC-22-84928 (R); Published: March 10, 2023, DOI: 10.37532/0974-7419.2023.23(1).234
Citation: Jae AAT, Felice PJD, Dominique AMH, et al. A Systematic Review of the Impact of Pneumatic Tube System Transport on Blood Chemistry and Hematology Parameters. Anal Chem Ind J. 2023;23(1):234.
Abstract
Introduction: Today, many hospitals use a Pneumatic Tube System (PTS) to quickly convey specimens to the lab. This study examined the clinical and practical impacts of PTS in delivering specimens. Blood chemistry and hematologic parameters were compared between PTS and manual transfer to determine whether there was a significant increase or decrease in its values. The study evaluated the following blood chemistry parameters such as LDH, K, AST, ALP, ALT and total bilirubin and hematologic parameters such as red cell indices (MCV, MHC, and MCHC), RBC count, hemoglobin and hematocrit, hemolysis rate and platelet count. The data are gathered to evaluate if PTS provides the same specimen integrity as the manual technique. In this project, the researchers will conduct a systematic review utilizing the PRISMA 2020 reporting checklist.
Methodology: Studies obtained from online databases were utilized. A set of criteria for selecting prospective studies were established. The studies should be published from 2017 to October 2022, must assess the effects of PTS by comparing them to manually transported samples and must be fully accessible. Subgroup analysis and cumulative analysis were conducted to establish transparency of the results.
Discussion: A total of 18 studies were evaluated. Two of these studies deal with blood chemistry, five with hematology and eleven with factors related to both blood chemistry and hematology. Among all the parameters evaluated, only LDH, potassium and hemolysis index showed clinical significance between the samples transported via PTS and manually delivered. The other chemistry parameters were also increased but were not clinically significant. Whereas, hematologic parameters have only limited studies available that can show that it is of clinical significance. However, most included studies utilized a small sample size and limited population, restricting further analysis of the obtained results. Additionally, some were hindered due to insufficient data and information. Moreover, the technical aspects that can cause the results to differ are not provided because the PTS utilized to modify acceleration and deceleration, which are not sufficiently described. Some research claimed that their study was restricted to PTS effects alone.
Keywords
Pneumatic Tube System (PTS); Hematologic; Deceleration; Subgroup analysis; Cumulative analysis
Introduction
The laboratory testing is divided into pre analytical, analytical or testing and post-analytical or reporting phases. The pre analytical phase consists of the test request, collection, handling, transportation and storage of the specimen, thus considering it the most vulnerable to committing errors. Today, there are growing numbers of hospitals that utilize a Pneumatic Tube System (PTS) for transporting specimens to the laboratory as an alternative method of rapidly delivering specimens within the hospital. Kapoula, et al. states that PTS aids in decreasing the turnaround time, increasing the speed of tasks and increasing the efficiency among laboratory staff. Despite that, the use of PTS as specimen transport remains controversial as it may cause alterations in the parameters of routine blood specimens, which in turn could affect the reliability of results. The study conducted by Herman, et al. shows a falsely increased LDH after going through PTS transport. Thus, this study evaluated the clinical and practical effects of PTS in delivering specimens, wherein the results can serve as a basis for reviewing PTS before utilizing one.
Studying the effect of PTS on blood specimens will aid in determining whether PTS can serve as an alternative transport for manual couriers by identifying the parameters most affected by PTS. At the time of this research, studies were performed that determined the effects of PTS on samples transported by it compared to manual transport. Results show increased or decreased parameters in blood chemistry and hematology. However, conflicting outcomes did not result in any firm conclusion. Therefore, this study aimed to answer the query by gathering previous blood chemistry and hematology studies and deriving a conclusion as to whether the PTS is efficient enough to provide the same specimen integrity as the manual method. Based on the studies gathered, this study also showed the standard and most parameters affected by PTS in blood chemistry and hematology.
A systematic review using the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2020 reporting checklist was conducted in this study, in which the researchers obtained various literatures from different databases and followed the PRISMA guidelines. The gathered literature was subjected to eligibility criteria to answer whether blood chemistry and hematological parameters are affected when transported to the PTS.
Objectives
This systematic review aims to: Determine the possible effects of PTS compared to those specimens manually delivered to the clinical laboratory on the following blood chemistry parameters:
• Lactate Dehydrogenase (LDH)
• Potassium (K)
• Aspartate aminotransferase (AST)
• Alkaline Phosphatase (ALP)
• Alanine Transaminase (ALT)
• Total bilirubin; and
Determine the possible effects of PTS compared to those specimens manually delivered to the clinical laboratory on the following hematologic parameters:
• Red cell index.
• Mean Corpuscular Volume (MCV).
• Mean Corpuscular Hemoglobin (MCH).
• Mean Corpuscular Hemoglobin Concentration (MCHC)
• Red Blood Cell (RBC) count.
• Hemoglobin and hematocrit.
• Hemolysis rate.
• Platelet count.
Literature Review
Pneumatic tube system
The Pneumatic Tube System (PTS) is a piece of laboratory equipment in which specimen containers are pushed forward through a complex network of tubes by compressed air or by a vacuum. It can be used by hospitals to speed up the process of collecting and processing samples. According to Ding, changes may occur in the blood chemistry parameters, especially the hemolysis index, due to the variation in speed and length of the PTS. Additionally, Poletaev indicated that proper handling and transportation should be done with specimens. Nevertheless, the rate of change in the velocity of the PTS can affect the specimen integrity.
Blood chemistry
In the study of Cakirca, et al. the medical staff manually transferred each pair of 148 samples. The other 148 samples were transported with PTS, of which 113 samples were transported without PTS sponge rubber inserts (PTSws). No significant changes were observed in hemolysis rate and biochemical analyte concentrations between blood samples delivered manually and using PTS. In terms of Hemolysis Rate, potassium, and LDH levels, it was revealed that the samples conveyed using PTS without sponge-rubber inserts differed significantly from those transferred manually and with PTS. As a result, sponge-rubber inserts are required in PTS to prevent blood samples from becoming hemolyzed.
Additionally, Kapoula measured the biochemical parameters through a meta-analysis, where potassium, LDH and AST showed statistically significant higher values when transported using PTS than hand courier methods. Furthermore, the increase in LDH levels cannot be only accounted to the acceleration of the sample when it is transported through PTS but due to the sudden deceleration that occurs during the process, also referred to as shock force. It was mentioned that PTS with high speed and long distances, particularly PTS that ranges from 200 meters-400 meters, may lead to higher LDH. Therefore, it was suggested that hospitals should validate their PTS systems. Similarly, Ding, et al. have found insignificant changes in hemolysis, potassium and ALT levels in blood samples transported manually compared to those transported via PTS. However, the level of AST varies depending on the distance. There will be no significant difference between the two modes of transportation if the distance is <250 meters. Nonetheless, it will elevate the AST level for blood samples transported via PTS for longer distances. They also observed an elevated amount of LDH in blood samples transported using PTS than in manual transportation (95% CI; 0.06-0.34). They have identified that the elevated level was associated with a speed of over six m/s and a long transportation distance (>250 m) in both protected and unprotected blood samples. This alteration in the level of LDH shows mild hemolysis in the transported blood samples since percent hemolysis of red blood cells can cause an 18% increase in the activity of LDH. All things considered, it was concluded that there is a relationship between mild hemolysis in the transported blood samples and the PTS. Supplementarily, Mullins and Bruns discovered that the samples showed evidence of hemolysis upon inspection when subjected to PTS. In analyzing the hemolysis index, LDH and potassium, it was observed that LDH (p-value=<0.01) and potassium (p-value=<0.02) have increased results and are considered statistically significant. However, only minimal differences can be observed between the hemolysis indices of the two transport methods compared to manually transported specimens.
Furthermore, a study by Lee, et al. showed that the levels of chemical parameters such as ALT, ALP, potassium and total bilirubin showed no statistical differences for samples delivered through PTS compared to the hand delivered specimens. However, parameters such as LDH and AST (p-value=0.000) concluded that statistically significant differences were observed in samples transferred via PTS compared to hand delivered samples when tested for these. Moreover, Petit, et al. studied the preanalytic influence of PTS in biochemistry analytes potassium, LDH and AST. The samples are from 14 individuals. They stated that two PTS delivered samples for LDH level yielded results beyond the recommended guidelines one with a higher value than the manually transported samples, while the other had a lower value. However, potassium and AST both fall between the acceptability thresholds. On the other hand, Herman, et al. determined that LDH in plasma was falsely elevated after undergoing PTS due to mechanical agitation from transport, which caused a rise in plasma LDH concentrations. Furthermore, Pupek, et al. mentioned that the modern PTS is suitable for transporting samples for routine chemistry and hematological laboratory testing. The PTS affected ALP, bilirubin and Potassium were statistically significant. Nevertheless, the hematologic and chemistry parameters evaluated showed clinically significant differences between PTS and manual courier delivery.
Contrastingly, the prospective analysis of Gils, et al. showed no changes potassium and LDH between PTS and manual transport. However, in their retrospective study, they found substantial variations in the levels of potassium and LDH both before and after PTS installation. The projected differences were not, however, clinically significant. Furthermore, the researchers found no indication of PTS induced hemolysis and clinically significant alterations indicating an acceleration dependent influence on the quality of blood samples.
Additionally, Kumari, et al. concluded that samples delivered through PTS are reliable when subjected to several corrective measures to eliminate pre-analytical errors. The researchers collected 100 blood samples from healthy individuals; in which one of the samples will be transported using PTS, whereas the other will be transported manually. Accordingly, the samples underwent two analysis phases before and after applying corrective actions. In phase one, the levels of chemical parameters such as ALT and total bilirubin showed no statistically significant difference between the two transportation modalities. However, the samples were characterized by hemolysis with increased levels of LDH, Potassium and AST compared with the manually transported samples (p-values=0.001, 0.000, 0.025, 0.047, respectively). Thus, indicating a statistically significant difference between the two transportation methods. Contrastingly, no statistically significant differences were denoted between the paired samples in phase two after implementing the corrective measures.
Furthermore, Nybo, et al. evaluated a total of 39 studies. Only 12 studies focused on inpatients, primarily intensive care unit patients. Regarding clinical chemistry parameters, one of their studies mentioned that the potassium, LDH and AST transported via PTS were increased. According to one of the studies analyzed by the researchers, the degree of hemolysis is 532 fold greater, and there are changes in potassium, LDH and ALT. Similarly, one of their studies revealed a significant change in LDH, although potassium showed a minor upward trend. On the other hand, another study revealed that hematology samples did not differ. However, LDH varied considerably across all wards. Another study also concluded that sample processing had small but substantial effects on LDH and potassium results. In addition, a study revealed that at high speeds of PTS transport, all three indices of hemolysis, potassium and LDH are elevated. Lastly, one study showed an increased LDH, although the changes would not affect the clinical interpretation.
While Farnsworth, et al. aimed to validate PTS by assessing the effects of its forces, such as the number and magnitude of acceleration, on plasma LDH, hemolysis index and potassium levels; the researchers concluded that there was a significant variation in the magnitude of acceleration within a single PTS route on a day to day basis. Moreover, a linear relationship was evident between a false increase of LDH levels by ≥ 20% and acceleration in two of the PTS routes. Moreover, it was noted that blood samples transported by PTS increased hemolysis index by 85.7% and potassium levels as well.
Lastly, Garcia, et al. evaluated the use of PTS transportation on blood components, namely, Packed Red Blood Cells (PRBCs), Thawed Fresh Plasma (TFP), Cryoprecipitate (CR) and Platelet Concentrate (PC) and compared it to courier transportation. A total of 23 PRBCs, 50 units of TFP, 30 units of CR and 10 units of PC were assessed. Hemolysis parameters such as potassium, AST and LDH revealed no significant difference between pre and post-transport values in both transport routes for PRBCs. However, the potassium levels exhibited a more significant difference in the values before and after transportation via PTS, although this difference was not statistically significant. In addition, no disparities were observed in the laboratory parameters of blood components between the two transportation systems, as these were characterized to maintain stable temperatures throughout the process. It was also verified that the transport time via PTS was shorter than that of the courier.
Hematology
For routine laboratory parameters, Kapoula, et al. analyzed the measurements of hematologic parameters in relation to the impact of using PTS as a medium in transporting specimens by reviewing prospective studies. Of the 24 eligible studies, 14 discussed the hematologic parameters. From that, the research findings of the meta-analysis state that there is no statistically significant difference between PTS samples and manually transported samples with regard to hematologic parameters such as Complete Blood Count (CBC), specifically RBC count, hemoglobin, hematocrit, MCV, MCH, MCHC and platelet count. The analysis of the studies indicates that there are differences between PTS transport and handheld transportation. In contrast, the speed and distance of the PTS may contribute to the changes in the laboratory parameters. Factors such as rapid acceleration or sudden deceleration could lead to hemolysis, while many switches and bends of the PTS may cause mechanical trauma leading to cell damage.
Similarly, the study of Subbarayan, et al. utilized 75 randomly selected pairs of samples collected from inpatients, outpatient and ICU patients through the venipuncture technique. PTS with a special foam tube was utilized to transport each pair of samples while the remaining samples were manually transported to the laboratory. The samples were subjected to CBC, which was compared and ascertained that RBC, hemoglobin and MCH have no significant difference except that MCV has decreased significantly in PTS. In contrast, there was a significant increase in MCHC and platelet count. Nonetheless, the researchers concluded that the impact of PTS on the said hematologic parameters is clinically insignificant.
Additionally, Lee, et al. was able to compare the laboratory results of the PTS method in hand delivered groups with the blood samples collected from 56 randomly selected patients. The laboratory results showed statistically insignificant differences between PTS and hand delivered transport procedures for RBC, hemoglobin, MCV, MCH, MCHC and Platelet count. The researchers emphasized how essential it is for each laboratory to verify the consistency of the laboratory results acquired from samples by the modes of transportation.
Petit, et al. also determined the preanalytic effects of PTS on hematologic parameters. The parameters showed no difference outside the normal range. In addition, the researchers performed smears for both transportations and observed the hemolysis index and morphology of RBCs in blood between PTS and manually. The researchers indicated that no differences are in the 14 samples analyzed.
Furthermore, Pupek, et al. stated in their study that the hemoglobin of blood samples transported via PTS reached a statistically significant difference (p-value=0.012) compared to manually transported samples. Despite the results, the researchers noted that PTS provides a reliable sample delivery for routine hematologic laboratory tests.
In another study conducted by Wang, et al. the findings regarding the hemolysis rate showed one hemolyzed sample from a 500 m PTS group and a 40% hemolysis on samples from the 1000 m PTS group. Supplementarily, Nybo indicated an increase in the hemolysis index for most types of PTS stated in the study. Despite that, the findings are seldom related to any clinical significance. In addition, Garcia, et al. analyzed hemoglobin and hemolysis index. It demonstrates that both transport routes exhibited no significant difference between pre and post-transport values for hemoglobin and hemolysis index.
In the experimental study of Enko, et al. the samples were drawn from 49 ICU patients. Three of the six specimens drawn from each patient were transported by PTS, while the other three were carried by hand. The study showed that there are statistically insignificant changes between the platelet counts of samples transported using PTS and hand carried methods.
Contrastingly, Poletaev, et al. studied the effect of PTS on platelet count under Light Transmission Aggregometry (LTA), wherein the study revealed no significant difference in platelet count. Similarly, a study conducted by Slavik, et al. also analyzed the effect of PTS by measuring the LTA parameters. The platelet count ranged from 221 to 282 × 109/L or approximately 250 × 109/L. Despite the increase, the researchers concluded that it is safe to transport platelets at a controlled acceleration from 3 m/s to 6 m/s in the PTS.
Methodology
The study investigated the possible effects of the Pneumatic Tube System (PTS) as a form of laboratory specimen transportation, specifically for blood samples, in laboratory measurements. The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2020 were utilized as this is the foundation and guide for the composition of this study. Although, certain adjustments may happen to make it more suitable. The literature found was subjected to specific criteria to fit the scope of the study. Primarily, the chosen literature should be full text articles. An essential criterion for the journals included must compare the results of specimens transported via PTS with those transported manually. In addition to this, the tests analyzed should include the hematologic and blood chemistry parameters included in this study. Moreover, the specific criteria for selecting relevant literature were indicated under the eligibility criteria section of the research.
Numerous related literatures are required for this study, which would be acquired from several databases to widen the number of articles obtained. Databases were utilized to search for keywords. The Mendeley reference manager was utilized to manage the references used more quickly and effectively. The researchers also manually assessed the reference list of the included journals to determine whether there are other studies deemed to be eligible for the systematic review.
Studies relevant to the investigation were taken from PubMed, Science Direct, DeGruyter, Taylor and Francis online, Oxford Academic and Thieme. The Google Scholar advanced search was also utilized to obtain more substantial evidence to support the research question and objectives. The following search strategies were used for the chosen databases and Google Scholar: (“pneumatic tube system” or “pneumatic tube transportation” or “transportation of red cell units”) and (“routine hematology parameters” or “blood chemistry parameters” or hemolysis or hemolysis rate or “red cell indices” or “MCV” or “MCH” or “MCHC” or “red blood cell count” or “hemoglobin” or “hematocrit” or “platelet count”) and (“Lactate Dehydrogenase” or LDH or “Potassium” or K or “Aspartate aminotransferase” or “AST” or “Alkaline Phosphatase” or ALP or “total bilirubin” or “Alanine Transaminase” or ALT) (Figure 1).
Research design
This study utilized a secondary research design, precisely a systematic review approach. It gathered publications that satisfied the inclusion criteria set and were found to be relevant in addressing the objectives of this study. Using the PRISMA framework, the researchers synthesized and evaluated the data gathered from several studies.
Eligibility criteria
Inclusion criteria: The PRISMA 2020 guidelines, which are exclusively for systematic review, are the main basis of this paper. International studies published from January 2017 to October 2022 were used. This paper involves studies that only assess the effects of PTS in blood chemistry and hematology tests by comparing them to manually transported samples, in which LDH, Potassium, AST, ALP, ALT and total bilirubin are the parameters included in blood chemistry; while red cell index (MCV, MCH, MCHC), RBC count, Hgb, Hct, hemolysis rate and platelet count are the parameters considered in hematology. The articles must be fully accessible and gathered from chosen credible sources such as Google Scholar, PubMed, Science Direct, deGruyter, Taylor and Francis online, Oxford Academic and Thieme.
Exclusion criteria: The excluded articles are studies that determine the transmission of pathogens in PTS and incomplete access to the full text of the article where the abstract is only available; those studies that exceeded five years of publication were rejected. In addition, items related to meta-analysis studies in PRISMA 2020 guidelines were not included (Figure 2).
Variable description and operational definition
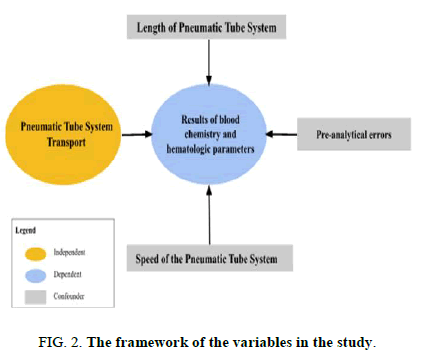
Variable description:
- • Independent variable: Pneumatic tube system transport.
- • Pre-analytical errors.
• Speed of the pneumatic tube system.
• Length of the pneumatic tube system.
• Dependent variable: Results of blood chemistry and hematologic parameters.
• Confounders:
Operational definition
• Pneumatic tube system: Laboratory equipment used to transport blood specimens from one place, usually a bloodcollection site, to the clinical laboratory for blood testing.
• Manual transport: Method of delivering blood specimens to the laboratory using a hand delivered system.
• Blood tests: Qualitative and quantitative analysis of the blood sample to measure different analytes such as blood cellsand chemicals.
• Blood chemistry parameters: Chemical substances in blood samples that are measured using blood chemistry tests,such as electrolytes, enzymes, glucose, fats and proteins.
• Hematological parameters: Blood components, including white blood cells, red blood cells, hemoglobin, hematocritand hematological indices.
• Hemolysis: Destruction of red blood cells that can be due to a patient’s condition, sample collection errors or conditionduring the transportation of the blood sample.
• Contaminants: Any unwanted physical, chemical, biological or radiological substances that could interfere with pre-analytical blood tests.
• Pre-analytical errors: Errors that occur before the analytical portion of the testing, which could alter the results.Examples include incorrect sampling time, under filled or overfilled tubes, patient identification errors, incorrecthandling of specimens, sample collection and patient preparation errors.
• Speed of the pneumatic tube system: Rate, in meters/seconds, at which the pneumatic tube system can operate.
• Length of the pneumatic tube system: Measurement of the distance between one end of the pneumatic tube systemand the other in meters.
Data management and analysis
A data management plan describes how researchers were able to manage data during and after the research process. Moreover, a good data management plan is fundamental for high quality research. This paper is not a traditional narrative literature review because this was conducted using the PRISMA 2020 reporting checklist. The data went through inclusion and exclusion processes and underwent revision and input in Microsoft Excel. After organizing and scanning the data, all variables related to the objectives were evaluated.
Eight reviewers work independently to look for the literature, analyze full articles of eligible studies, collect data from various electronic databases and assess the study’s risk of bias. The data comprises the title of the study with the reference link, publication year, author/s of the study, parameters discussed in the study, PTS characteristics, including the speed and length of PTS, and the study results and findings (Tables 1 and 2). Risk of Bias in Systematic reviews (ROBIS) was used to assess the study risk of bias, ROBIS is a new tool for assessing the risk of bias in systematic reviews; it is composed of 3 phases: (1) assess the relevance, (2) identify concerns with the review process and (3) judge the risk of bias. Any disagreements were addressed through discussion with the additional four reviewers. The results of the search and selection are described using the PRISMA flow diagram. Finally, the findings of the study are presented in a descriptive or narrative format.
| Study | PTS speed (m/s) and length (m) | Sample size | Parameters discussed |
|---|---|---|---|
| Cakirca | Length: 170 m Speed: 5 m/s |
148 blood samples |
|
|
|||
|
|||
|
|||
|
|||
| Ding | Length: 250 m Speed: 6 m/s |
3,121 blood samples |
|
|
|||
|
|||
|
|||
| Farnsworth | Length: Not reported Speed: 6.096 m/s |
|
|
|
|||
| Garcia | Length: Approximately 463 m Speed: 3 m/s-6 m/s |
|
|
|
|
||
|
|
||
|
|||
| Gils | Length: 73 m-297 m Speed: 7.4 m/s-10.9 m/s |
|
|
|
|
||
| Herman | Not reported |
|
|
|
|||
| Kapoula | Length: 42.6 m-1,423 m Speed: 2.0 m/s-13.7 m/s |
|
|
|
|
||
|
|
||
| Kumari | Length: 300 m Speed: 3-6 m/s |
200 paired samples |
|
|
|||
|
|||
|
|||
|
|||
|
|||
| Lee | Length: 106 m Speed: 7 m/s-10 m/s |
56 paired blood samples |
|
|
|||
|
|||
|
|||
|
|||
|
|||
| Mullins | Not reported |
|
|
|
|
||
|
|||
| Nybo | Not reported |
|
|
|
|
||
|
|
||
|
|
||
|
|||
| Petit | Length: 685 m Speed: 5 m/s |
14 paired blood samples |
|
|
|||
|
|||
| Pupek | Length: Not reported Speed: 6.1 m/s |
41 paired blood samples from healthy hospital staff volunteers |
|
|
|||
|
TABLE 1. Characteristics of the thirteen studies included in blood chemistry.
| Study | PTS speed (m/s) and length (m) | Sample Size | Parameters discussed |
|---|---|---|---|
| Enko | Length: Not reported Speed: 4 m/s |
|
|
| Farnsworth | Length: Not reported Speed: 6.096 m/s |
|
|
|
|||
| Garcia | Length: Approx. 463 m Speed: 3 m/s-6 m/s |
|
|
|
|
||
|
|
||
|
|
||
|
|||
| Gils | Length: 73 m-297 m Speed: 7.4 m/s-10.9 m/s |
|
|
|
|||
| Herman | Length: Not reported Speed: Not reported |
|
|
|
|||
|
|||
| Kapoula | Length: 42.6-1 m, 423 m Speed: 2.0 m/s-13.7 m/s |
|
|
|
|
||
|
|
||
|
|
||
| Kumari | Length: 300 m Speed: 3 m/s-6 m/s |
|
|
| Lee | Length: 106 m Speed: 7 m/s-10 m/s |
|
|
| Mullins | Length: Not reported Speed: Not reported |
|
|
|
|||
|
|||
| Nybo | Length: Not reported Speed: Not reported |
|
|
|
|||
|
|||
|
|||
|
|||
| Petit | Length: 685 meters Speed: 5 m/s |
|
|
|
|||
|
|||
| Poletaev | Length: 125 m Speed: 7 m/s |
|
|
| Pupek | Length: Not reported Speed: 6.1 m/s |
|
|
|
|||
|
|||
|
|||
| Slavik | Length: 13, 425 m Speed: 1st mode: 6 m/s 2nd mode: 3 m/s to 6 m/s |
|
|
| Subbarayan | Length: Not reported Speed: 5 m/s |
|
|
|
|||
|
|||
|
|||
|
|||
|
|||
| Wang | Length: |
|
|
| Short distance PTS: 500 m | |||
| Long distance PTS: 1000 m | |||
| Speed: 7 m/s |
TABLE 2. Characteristics of the sixteen studies included in hematology.
Discussion
Study selection and excluded studies
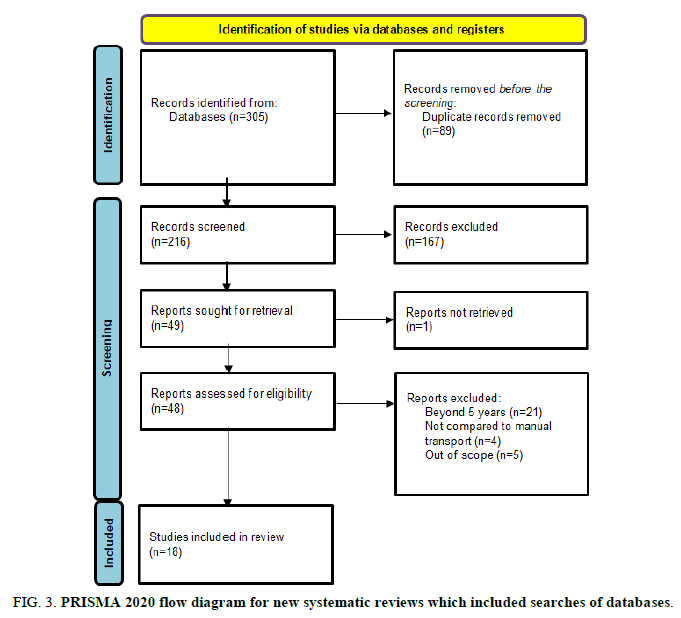
A total of 305 papers were collected through database searching. Following a screening procedure, 89 duplicate records were eliminated, leaving a total of 216 papers for the records. 49 papers are sought for retrieval after 167 entries are rejected following the filtering of article titles and abstracts. One paper could not be retrieved because the entire text could not be located. In total, 48 studies are assessed for eligibility criteria; however, despite discussing potential impacts of specimens transferred via PTS, 30 papers were eliminated following screening. These were disregarded, nevertheless, as 21 papers had publication dates that were more than five years old. In the nine articles, the information needed for the investigation was not sufficiently provided (e.g., there was no comparison of specimens submitted utilizing PTS with those manually transported and not specific to those parameters focused on the study). Therefore, 18 papers were included utilizing the PRISMA 2020 guidelines and the inclusion criteria set, of which two studies are related to blood chemistry, five studies are related to hematology and 11 studies are related to both blood chemistry and hematology, resulting in a total of 13 studies included in blood chemistry parameters and 16 studies included in hematologic parameters (Figure 3 and Table 3).
| Study | Parameters | p-value | Effect (Significant/Not significant) |
|---|---|---|---|
| Cakirca | LDH | 0.001 | Significant |
| Potassium | 0.001 | Significant | |
| AST | 0.019 | Significant | |
| ALT | 0.448 | Not significant | |
| Total bilirubin | 0.405 | Not significant | |
| Ding | LDH | Not reported | Significant (≥ 6 m/s speed and ≥ 250 m length) Not significant ( <6 m/s and <250 m length) |
| Potassium | 0.24 | Not significant | |
| AST | 0.02 | Significant | |
| ALT | 0.96 | Not significant | |
| Farnsworth | LDH | Not reported | Significant |
| Potassium | 0.0 | Significant | |
| Garcia | LDH | 0.666 | Not significant |
| Potassium | 0.345 | Not significant | |
| AST | 0.796 | Not significant | |
| Gils | LDH | 0.4819 | Not significant |
| Potassium | 0.3856 | Not significant | |
| Herman | LDH | 0.6 | Not significant |
| Kapoula | LDH | <0.0001 | Significant |
| Potassium | 0.002 | Significant | |
| AST | 0.003 | Significant | |
| Kumari | LDH | 0.218 | Not significant |
| Potassium | 0.042 | Significant | |
| AST | 0.042 | Significant | |
| ALP | 0.667 | Not significant | |
| ALT | 0.983 | Not significant | |
| Total bilirubin | 0.06 | Not significant | |
| Lee | LDH | 0.000 | Significant |
| Potassium | 0.366 | Not significant | |
| AST | 0.000 | Significant | |
| ALP | 0.415 | Not significant | |
| ALT | 0.303 | Not significant | |
| Total bilirubin | 0.180 | Not significant | |
| Mullins | LDH | <0.01 | Significant |
| Potassium | <0.02 | Significant | |
| Petit | LDH | Not reported | Significant |
| Potassium | Not reported | Not significant | |
| AST | Not reported | Not significant | |
| Pupek | LDH | 0.648 | Not significant |
| Potassium | 0.610 | Not significant | |
| AST | 0.882 | Not significant | |
| ALP | 0.009 | Significant | |
| ALT | 0.778 | Not significant | |
| Total bilirubin | 0.027 | Significant |
TABLE 3. Summary of parameters and p-value of studies for blood chemistry parameters.
Blood chemistry parameters
Lactate Dehydrogenase (LDH): From the 12 evaluated studies that deal with clinical chemistry parameters, four studies suggest that there is a clinically significant difference between the levels of lactate dehydrogenase in blood samples transported through PTS and manual transport, with a p-value of 0.001, <0.0001, 0.000 and <0.01 respectively [1-3]. On the other hand, five studies stated that there is no clinically significant difference between samples transported with the use of PTS and manual transport, with a p-value of 0.666, 0.4819, 0.6, 0.218 and 0.648 consecutively. The PTS used has a varying length from 42.6 m to 1,423 m with a speed ranging from 2 m/s to 10.9 m/s, whereas one study did not report the length nor the speed of the PTS utilized.
Potassium (K): Eleven research studies investigated the impact of PTS on potassium levels. Statistically significant differences were found in five studies with p-values of 0.001, 0.0, 0.002, 0.042 and <0.02, respectively [4,5]. Whereas five studies found no statistically significant differences with p values of 0.24, 0.345, 0.3856, 0.366 and 0.610, respectively. However, one study has not reported the p-value but indicated that potassium levels had no significant impact on PTS transport. On the other hand, nine studies have speed ranges from 3 m/s to 13 m/s and two studies have not reported such characteristics. In addition, each study used a different length of PTS, nine studies reported the following length of the PTS: 170 m, 250 m, 6.096 m, 463 m, 73 m-297 m, 42.6 m-1 m, 423 m, 106 m, 106 m and 685 m, respectively. However, the length of the PTS was not reported in two studies [6-8].
Aspartate Aminotransferase (AST): Eight of the 12 studies on blood chemistry examined the potential impact of PTS on aspartate Aminotransferase (AST). Two of these studies showed no statistically significant differences between the PTS and manual transportation groups, with a p-value of 0.796 and 0.882. One study with no reported p-value noted that PTS had no significant effect on this parameter. Whereas, five studies have observed a statistically significant effect with a p-value of 0.019, 0.02, 0.003, 0.042 and 0.000 respectively [9]. The speed set to operate the PTS ranges from 3 m/s-10 m/s, while one study did not include the speed of PTS. Six studies utilized a PTS with a length varying from 106 m-685 m, while two studies did not report the length of the PTS.
Alkaline Phosphatase (ALP): There are three papers that studied the effects of PTS on the level of ALP. The p-value for this parameter from the three studies was 0.667, 0.0415 and 0.009, respectively. All of these show statistically insignificant differences (p>0.05) between PTS and manual transportation. The speed of the PTS used ranges from 3 m/s-10 m/s, while the lengths were 106 m and 300 m. However, one study did not report the length of PTS [10].
Alanine Transaminase (ALT): Five out of twelve blood chemistry studies reviewed the parameter ALT. The p-values of these are as follows: 0.448, 0.96, 0.983, 0.303 and 0.778. Based on these values, it shows that there is no statistically significant change in the discussed parameters via PTS compared to manual transportation. The length of the PTS utilized in the studies has an average of 219 m, wherein one study did not report such system characteristics. On the other hand, the speed of the equipment has an average of 6.02 m/s, and all studies reported such characteristics.
Total bilirubin: Out of 12 studies related to blood chemistry, four studies about the possible effect of PTS analyzed total bilirubin. Three studies showed no statistically significant difference between PTS and manual transportation, with p-values of: 0.405, 0.06 and 0.180, respectively, while one study shows statistical significance with a p-value of 0.027. The speed of the PTS used was reported and ranged from 3 m/s to 10 m/s and the length of the PTS was also reported by three studies, 220 m, 300 m, and 106 m consecutively. However, one study did not report the length of the PTS used (Table 4) [11].
| Study | Parameters | p-value | Effect (Significant/Not significant) |
|---|---|---|---|
| Enko | Platelet count | 0.418 (whole blood) 0.0176 (platelet rich plasma) |
Not significant (whole blood) Significant (platelet rich plasma) |
| Farnsworth | Hemolysis index | Not reported | Significant |
| Garcia | Hemoglobin | 0.369 | Not significant |
| Hematocrit | 0.336 | Not significant | |
| Hemolysis index | 0.554 | Not significant | |
| Platelet count | 0.841 | Not significant | |
| Gils | Hemolysis index | Not reported | Not significant |
| Herman | RBC count | 0.08 | Not significant |
| Platelet count | 0.004 | Significant | |
| Kapoula | MCV | 0.186 | Not significant |
| MCH | 0.13 | Not significant | |
| MCHC | 0.58 | Not significant | |
| RBC count | 0.656 | Not significant | |
| Hemoglobin | 0.866 | Not significant | |
| Hematocrit | 0.866 | Not significant | |
| Platelet count | 0.51 | Not significant | |
| Kumari | Hemolysis index | Not reported | Significant |
| Lee | MCV | 0.207 | Not significant |
| MCH | 0.481 | Not significant | |
| MCHC | 0.215 | Not significant | |
| RBC count | 0.705 | Not significant | |
| Hemoglobin | 0.782 | Not significant | |
| Hematocrit | 0.298 | Not significant | |
| Platelet count | 0.598 | Not significant | |
| Mullins | Hemolysis index | < 0.01 | Significant |
| Nybo | Hemolysis index | Not reported | Significant |
| Petit | RBC count | Not reported | Not significant |
| Poletaev | Platelet count | 2.94 | Not significant |
| Pupek | MCV | 0.165 | Not significant |
| RBC count | 0.806 | Not significant | |
| Platelet count | 0.851 | Not significant | |
| Hemoglobin | 0.012 | Significant | |
| Slavik | Platelet count | 0.7 | Not significant |
| Subbarayan | MCV | 0.001 | Significant |
| MCH | 0.5 | Not significant | |
| MCHC | <0.001 | Significant | |
| RBC count | 0.11 | Not significant | |
| Hemoglobin | 0.84 | Not significant | |
| Platelet count | <0.001 | Significant | |
| Wang | Hemolysis rate | Not reported | Not significant (500 m-PTS) Significant (1000 m-PTS) |
TABLE 4. Summary of parameters and p-value of studies for hematologic parameters.
Hematology
Red cell index: The effect of PTS on MCV was investigated in four studies. Out of these, one study showed a statistically significant difference with a p-value of 0.186, while the other three studies showed no clinical significance with p-values of 0.207, 0.165 and 0.001. On the other hand, a total of three studies investigated the effect of PTS on MCH and MCHC. The results for MCH presented p-values 0.130, 0.481 and 0.500, respectively, which indicate that there are no significant differences between the samples transported through PTS and manual transportation. The p-values of MCHC in these studies were 0.580, 0.215 and <0.001, respectively. On this basis, two studies concluded that there are no significant differences between samples transported through PTS when compared to manual transport, while one study reported an increase in the levels of MCHC transported through PTS when compared to manual transport [12-14]. Only one study indicated the length of the PTS used, which is 106 m. Lastly, the speed for the two studies ranged from 5 m/s-10 m/s, while one study did not indicate the length and speed of the PTS applied.
RBC count: Six out of 18 studies analyzed the effects of PTS on RBC count. Of which, none showed a statistically significant difference in comparison to samples delivered manually with a p-value range of 0.08 to 0.806. In the included studies, samples were transported at a speed of 5 m/s to 10 m/s, with most of the lengths unreported [15].
Hemoglobin and hematocrit: A total of 5 out of 18 studies analyzed the effect of PTS on hemoglobin and hematocrit. In the two studies that discussed hematocrit, a p-value of 0.336 and 0.866 was obtained. With that, no significant differences were found between pre and post-transportation for both PTS and manual transportation. On the other hand, four of the five studies for hemoglobin had p values of 0.369, 0.866, 0.782 and 0.84, respectively. It was concluded that the results were statistically insignificant and comparable between the transport methods. Additionally, one of the five studies for hemoglobin, with a p-value of 0.012, showed statistically significant results between PTS and manual transportation. The speed of the PTS for four of the five studies had a range of 3 m/s to 10 m/s, while one study had varying speeds of the PTS. Lastly, the length of the PTS ranges from 106 m to 463 m, which was only, indicated in two studies, while the rest did not report the length of the PTS [16].
Hemolysis index: Out of seven studies only one study found no evidence of PTS induced hemolysis. A study by Farnsworth, et al. showed the highest hemolysis index in samples transported by PTS with an 85.7% increase. Of note, a study conducted by Nybo, et al. did not report any p-values; however, the study showed an increase in hemolysis index and was considered significant. The study of Wang, et al. showed the longest length of PTS was used with a measurement of 1000 m, while two studies did not report the length of PTS. The speed of PTS ranges from 3 m/s to 10.9 m/s, while three studies did not report the speed of PTS.
Platelet count: Nine out of 18 studies analyzed the effect of PTS on platelet count. Two of these studies reported a statistically significant difference with a p-value of 0.004 with a 1-13 fold increase and <0.001, with a 1.2% difference in platelet count. One study showed an increased platelet count but is considered statistically insignificant. No significant effects were concluded for the remaining studies with a p-value of 0.418 and 0.0176, 0.841, 0.510, 0.598, 0.851 and 0.700 respectively. The speed of PTS ranges from 2 m/s to 13.7 m/s, while one study did not report the speed of PTS. The length of PTS used in the study ranges from 106 m to 13 m, 425 m, while four studies did not report the length of the PTS [17].
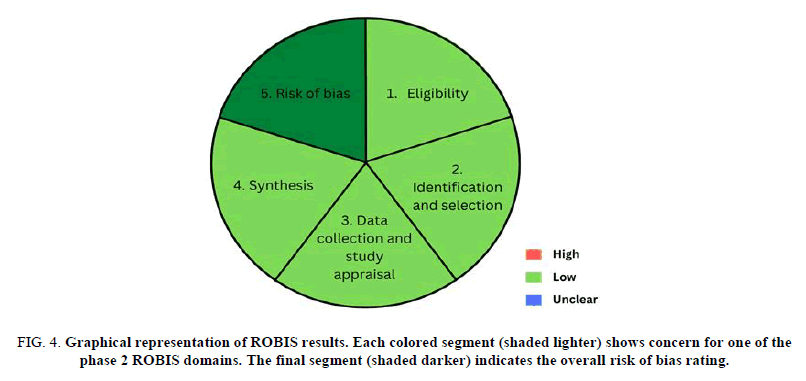
Risk of bias assessment
The assessment of the risk of bias was based on the scale recommended by the Risk of Bias Assessment tool for Systematic reviews (ROBIS). Phase 1 involved the identification of the terms in PICO criteria (Population, Intervention, Comparator and Outcomes) to determine the relevance of the review. Then, in phase 2, it was assessed that there are low concerns regarding each of the four domains. The domains include the specification of study eligibility criteria, identification and selection of studies, data collection and study appraisal and synthesis and findings. Finally, it was considered that the systematic review as a whole has a low risk of bias in phase 3 (Figure 4).
FIG 4: Graphical representation of ROBIS results. Each colored segment (shaded lighter) shows concern for one of the phase 2 ROBIS domains. The final segment (shaded darker) indicates the overall risk of bias rating.
ROBIS phase 1: Assessing relevance
PICO determines the eligibility of the study through the following criteria: “Population” was limited to blood samples of no certain individual delivered through PTS. “Intervention” has no distinguished demand, while manual transportation of specimens is included in “comparators”. Lastly, the “outcome” consists of clinical or statistically significant differences concluded from the evaluation of parameters using manual transportation and PTS [18].
ROBIS phase 2: Identifying concerns with the review process
Domain 1: Study eligibility criteria: All directional questions were answered as “yes” or “probably yes”, hence considering it with low possible concern about the study’s eligibility criteria. The authors made a profuse effort in establishing the objective of the study under chapter 1. Moreover, the study design was clearly settled in chapter 3 along with a comprehensive eligibility criterion, its inclusion and exclusion utilizing PRISMA 2020 guidelines.
Domain 2: Identification and selection of studies: Eight researchers carefully selected and evaluated the studies that were obtained and an additional four reviewers addressed any disagreements in acquiring the studies. Despite having an appropriate range of databases for published reports, the researchers lacked on utilizing electronic sources for unpublished reports. PRISMA 2020 guidelines, a supplementary method and checklist that discussed the inclusion criteria of the studies such as date, publication, format and language of the articles gathered for eligibility, was also used in this review. All things considered, there is a low possible concern with regard to assessing the identification and selection of the studies in view of the fact that three of the directional questions were answered by “yes” or “probably yes”.
Domain 3: Data collection and study appraisal: In the assessment of data collection and appraisal of studies, eight reviewers independently searched electronic databases for eligible studies using keywords that met the study's objectives and eligibility criteria. Furthermore, the reference lists of the included journals were manually assessed to discover if other studies were eligible to be incorporated in the review. Moreover, discrepancies were resolved through discussion with the addition of four reviewers. Therefore, the review was formally assessed in accordance with ROBIS criteria and thus we concluded that the said study is of low risk and concern regarding the methods used to collect data and appraise studies.
Domain 4: synthesis and findings: Studies included in this systematic review require only those that assess the effects of transportation via PTS regarding tests of blood chemistry and hematology sections through comparison with samples transported manually. A total of 72 papers were identified through database searching, wherein after screening and identification, only 18 papers were included utilizing the PRISMA 2020 guidelines and the inclusion criteria set. The standard must be met regardless of what kind of study is that of the paper. With this, most of the qualified journals were identified to only use a small sample size and populations, while some needed more data and information. Most importantly, not all studies utilized the same brand of PTS, and technical aspects were not thoroughly provided. In conclusion, it shows that there are low concerns regarding the synthesis and findings of this study after evaluation using the ROBIS criteria.
ROBIS phase 3: Judging the risk of bias
All relevant findings gathered in the study were taken into account and addressed the concerns identified in domains 1 to 4. Furthermore, all eligible studies identified were appraised and answered the study's research questions. The researchers also used the p values of the various parameters in each study to determine statistical significance. This means that the study’s conclusions were supported by evidence gleaned from a large body of research that can back up the research questions and findings.
Conclusion
As demonstrated in the findings and discussion, there are various results gathered from different studies which showed either clinically significant or insignificant differences between PTS and manually transported specimens. Blood chemistry parameters, such as LDH, potassium, AST, ALP, ALT and total bilirubin, increase in value when carried by PTS. However, the difference is deemed minor from a clinical standpoint. Few studies demonstrated clinically significant elevations of LDH and potassium. Meanwhile, in the discussion of hematologic parameters, studies that discussed the hemolysis index showed that there are clinically significant differences in its value. However, hematologic parameters such as MCV, MCHC, platelet count and hemoglobin only had a few supporting studies that showed that there are clinically significant differences, hence, it is unable to conclude that there are clinically significant differences in its value when the specimen is transported with PTS compared to manually transported samples. In this study, speed and distance must be taken into account as a consideration.
Most of the included studies indicated utilizing a small sample size in the analysis of the study as their limitation. A small sample size may lead to an increase in the margin of error and decrease the credibility of the study since it may be difficult to determine whether the results obtained by the researchers are factual.
On the other hand, specimens collected from a limited group of populations were the limitation of most studies. To cite, samples from mostly healthy volunteers or patients may not truly assess the effects of PTS. Contrarily, samples from terminally ill patients may give erroneous results when subjected to PTS.
Moreover, insufficient data and information hinder some of the studies. For instance, some studies used in the research conducted by Ding, et al. were excluded due to this limitation. In addition to this, the lack of a detailed description of the PTS alongside the technologies used to manipulate the acceleration and deceleration fails to provide technical factors that may contribute to the discrepancy of the results. Equally, the failure to assess the impact of centrifugation speed and its associated forces and the omission of certain PTS routes may affect the sample integrity. Similarly, Subbarayan, et al. excluded the effects of PTS at various levels of distance which could potentially have some effect on the samples as seen in other studies. Additionally, the lack of standardized qualifications for transporting different blood containers, as in the study by Petit, et al. of microtubes, may cause arbitrary results. Although, the study stated that there are no significant differences in potassium and AST this may be rooted in the absence of a systematized criterion for transporting microtubes via the PTS. Not to mention, the possibility of overlooking the significant difference between the samples transported manually and via PTS after obtaining commensurate within the normal range results of the compared samples.
Furthermore, many studies indicated a limitation in the pre-analytical and analytical phases. To cite, the use of various samples was not utilized in assessing the rate of hemolysis and the analysis of the blood samples in the study was also restricted to one laboratory unit. Moreover, the use of gel separator tubes was only for the analysis of LDH serum samples, while the use of other types of tubes was not evaluated for their effects on PTS.
Lastly, some studies stated that restricting their analysis on the effects of PTS as their limitation. For instance, analyzing only hemolysis sensitive and platelet specific tests despite the possibility that other laboratory variable may be associated with the acceleration forces of PTS in various ways and measuring only specific analytes sensitive to hemolysis.
There is a need for caution while assessing and interpreting the results of the involved parameters due to the limited number of included studies. In addition, small sample numbers in some research might invalidate the findings of the study. Most samples of the study transported by the PTS were monitored closely, which is not common in a natural laboratory setting. Some studies did not provide information on PTS transportation, including the number of turns, various routes and other factors. Additionally, several studies did not indicate the operating speed and distance of the PTS. Furthermore, many studies examined the suggested speed and length of their particular brand of PTS. With regard to these, it could explain why the particular research included in the analysis of the review differs from others. There is a possibility for the rate of change in the velocity of the PTS to affect the specimen integrity. Moreover, several studies were excluded because they lacked the necessary data required for this review, which might have led to a bias in selection. Lastly, the study did not include other pre-analytical variables in the blood samples, such as coagulation, but these still might be affected by PTS.
Limitations of the study
Several limitations must be acknowledged in the study. Insufficiency in the studies is mostly due to the fairly new automated method for specimen transportation, namely, the PTS. Thus, there are only a few studies that analyze the effect of PTS on laboratory parameters. In addition, other parameters, such as coagulation, were not included due to the inadequate number of studies with statistically significant results. Likewise, factors such as the brand of PTS and the type of tube used to collect the specimen were not considered for the evaluation of the findings.
Recommendations
The systematic review revealed that PTS must be verified and evaluated further regarding its specifications through close monitoring of its speed, length and other pre-analytical factors, which may significantly alter the credibility of the samples and the results. On this premise, reputable PTS producers are urged to guarantee the quality of their goods in order to increase the likelihood of distributing reliable PTS and producing clinically acceptable results. Additionally, it is advised that laboratories rethink their use of PTS when analyzing LDH, potassium, AST, ALP, ALT and total bilirubin in blood samples because it could produce erroneous increases or decreases in the findings. However, the results should be reevaluated by the staff when the use of PTS is inevitable. Lastly, future researchers are recommended to conduct further studies of the effect of PTS on other body fluids, as there are still limited studies available during this review.
Acknowledgements
We would like to extend our sincere gratitude to Dr. Roberto G. Manaois, president and CEO of Scientific Biotech Specialties, Inc. (SBSI), who served as our research consultant and provided crucial direction and counsel throughout this assessment. His genuineness, vision and drive have greatly boosted our morale. Despite his busy schedule, he did not pass up the chance to offer his assistance in furthering our research. Studying and working together with him was a great honor and privilege.
Additionally, we would like to express our gratitude to Dr. Sherwin N. Reyes and Mrs. Mary Denneth Fuentes-Reyes, MSMT, who served as our research professors and provided valuable advice for this work.
Finally, we would like to thank the Research Square for providing the manuscript’s preprint. The preprint of the file enables us to get community feedback and enhance the paper.
Author Contributions
M.Abiog: Conceptualization, investigation, resources, writing original draft. B. Alberto: Conceptualization, methodology,investigation, resources, project administration, writing-original draft, writing review and editing A. Ausmolo: Conceptualization,methodology, resources, project administration, visualization, writing original draft, writing review and editing E. Bilo: Conceptualization, investigation, resources, writing original draft. K. Dela Cerna: Conceptualization, investigation, resources, writing review and editing. C. de Leon: Conceptualization, methodology, resources, writing- review and editing J. Jugueta: Conceptualization, investigation, resources, writing review and editing. M. Mendoza: Conceptualization, methodology, resources, writing original draft. J. Pua: Conceptualization, investigation, resources, supervision, project administration, writing original draft, writing review and editing D. Sison: Conceptualization, methodology, resources, visualization. C. Untalan: Conceptualization, investigation, resources. L. Yumul: Conceptualization, investigation, resources, project administration, writing original draft, writing review and editing.
Conflict of Interest
The paper does not include any conflicts of interest. There is no group that supports the authors financially or otherwise. There are no connections or professional appearances that could influence the manuscript.
Data and Code Availability
The previously published papers and datasets that have been cited serve as the source of the information supporting this systematic review. The additional files provide the processed data.
Supplementary Information
Supplementary materials are provided in a research literature database exhibited through Google sheets. The database contains publications included in the study, articles that were screened and rejected and other PTS related articles that were excluded from the report. In addition, a quantitative summary of the results was also included.
Ethical Approval
This study followed the requirements and guidelines given by the research ethics review committee of Far Eastern university Dr. Nicanor Reyes medical foundation, provided that FEU-NRMF IERC 2022-0113 is the study identification. The study ensures that all ethical standards were met by the involvement of acknowledgment of the authors, literature and studies included in the APA 7 referencing format. Additionally, the data are free from plagiarism and are accurately extracted from the articles reviewed. Ethical approval is not necessary for acquiring references since these kinds of literature are publicly available.
References
- Cakirca G, Erdal H. The effect of pneumatic tube systems on the hemolysis of biochemistry blood samples. J Emerg Nurs. 2017;43(3):255-258.
- Ding X, Wen X, Wang L, et al. Effects of a pneumatic tube system on the hemolysis of blood samples: A PRISMA-compliant meta-analysis. Scand J Clin Lab Invest. 2021;81(5):343-352.
[Crossref] [Google Scholar] [PubMed]
- Enko D, Mangge H, Munch A, et al. Pneumatic tube system transport does not alter platelet function in optical and whole blood aggregometry, prothrombin time, activated partial thromboplastin time, platelet count and fibrinogen in patients on anti-platelet drug therapy. Biochem Med. 2017;27(1):217-224.
[Crossref] [Google Scholar] [PubMed]
- Farnsworth CW, Webber DM, Krekeler JA, et al. Parameters for validating a hospital pneumatic tube system. Clin Chem. 2019;65(5):694-702.
[Crossref] [Google Scholar] [PubMed]
- Garcia LO, Speransa DM, Rodrigues CB, et al. Validation of blood components transport through a pneumatic tube system. Hematol Transfus Cell Ther. 2022;44(4):519-525.
[Crossref] [Google Scholar] [PubMed]
- Gils C, Broell F, Vinholt PJ, et al. Use of clinical data and acceleration profiles to validate pneumatic transportation systems. Clin Chem Lab Med. 2020;58(4):560-568.
[Crossref] [Google Scholar] [PubMed]
- Herman DS, Toro E, Baraban EG, et al. Falsely increased plasma lactate dehydrogenase without hemolysis following transport through pneumatic tube system. J Appl Lab Med. 2019;4(3):433-438.
[Crossref] [Google Scholar] [PubMed]
- Kapoula GV, Kontou PI, Bagos PG. The impact of pneumatic tube system on routine laboratory parameters: A systematic review and meta-analysis. Clin Chem Lab Med. 2017;55(12):1834-1844.
[Crossref] [Google Scholar] [PubMed]
- Kumari S, Kumar S, Bharti N, et al. Impact of pneumatic transport system on Pre-analytical phase affecting clinical biochemistry results. J Lab Phys. 2022;15(1):48-55.
- Lee AJ, Suh HS, Jeon CH, et al. Effects of one directional pneumatic tube system on routine hematology and chemistry parameters; a validation study at a tertiary care hospital. Pract Lab Med. 2017;9:12-17.
[Crossref] [Google Scholar] [PubMed]
- Mullins GR, Bruns DE. Air bubbles and hemolysis of blood samples during transport by pneumatic tube systems. Clin Chim Acta. 2017;473:9-13.
[Crossref] [Google Scholar] [PubMed]
- Nybo M, Lund ME, Titlestad K, et al. Blood sample transportation by pneumatic transportation systems: A systematic literature review. Clin Chem. 2018;64(5):782-790.
[Crossref] [Google Scholar] [PubMed]
- Petit M, Mine L, Pascreau T, et al. Preanalytical influence of pneumatic tube delivery system on results of routine biochemistry and haematology analysis. Ann Biol Clin. 2017;75(6):703-712.
[Crossref] [Google Scholar] [PubMed]
- Poletaev AV, Koltsova EM, Ignatova AA, et al. Alterations in the parameters of classic, global and innovative assays of hemostasis caused by sample transportation via pneumatic tube system. Thromb Res. 2018;170:156-164.
[Crossref] [Google Scholar] [PubMed]
- Pupek A, Matthewson B, Whitman E, et al. Comparison of pneumatic tube system with manual transport for routine chemistry, hematology, coagulation and blood gas tests. Clin Chem Lab Med. 2017;55(10):1537-1544.
[Crossref] [Google Scholar] [PubMed]
- Slavik L, Ulehlova J, Bradacova P, et al. The modern pneumatic tube system transports with reduced speed does not affect special coagulation tests. J Med Syst 2020;44(9):142.
[Crossref] [Google Scholar] [PubMed]
- Subbarayan D, Choccalingam C, Lakshmi CK. et al. The effects of sample transport by pneumatic tube system on routine hematology and coagulation tests. Adv Hematol. 2018;2018:6940152.
[Crossref] [Google Scholar] [PubMed]
- Wang H, Wang L, Liang H, et al. Falsely decreased FVIII activity following pneumatic tube transport. Int J Lab Hematol. 2021;43(2):305-310.
[Crossref] [Google Scholar] [PubMed]