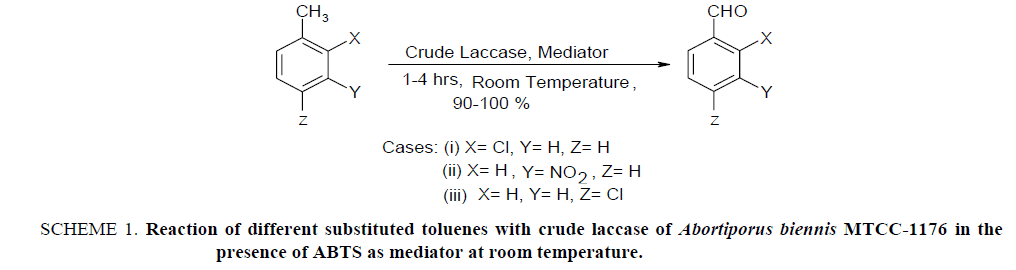Original Article
, Volume: 13( 4)Synthetic Applications of Crude Laccase from Abortiporus biennis MTCC-1176
Manisha Sharma, Pankaj Kumar Chaurasia and Sudha Yadava
Department of Chemistry, D.D.U. Gorakhpur University, Gorakhpur, Uttar Pradesh, India
- *Corresponding Author:
- Pankaj Kumar Chaurasia
Department of Chemistry, D.D.U. Gorakhpur University, Gorakhpur 273009, Uttar Pradesh, India
Tel: 0551 234 0363; E-mail: pankaj.chaurasia1987@rediffmail.com
Received Date: June 24, 2017 Accepted Date: July 24, 2017 Published Date: July 27, 2017
Citation: Manisha S, Pankaj Kumar C, Sudha Y. Synthetic Applications of Crude Laccase from Abortiporus biennis MTCC-1176. Biotechnol Ind J. 2017;13(4):143
Abstract
This communication reports a new laccase as an effective biocatalyst, obtained from the liquid culture growth medium of the indigenous fungal strain Abortiporus biennis MTCC-1176 in the selective synthesis of substituted benzaldehydes from respective substituted toluenes. Selective bioconversions of 3-nitrotoluene to 3-nitrobenzaldehyde, 2-chlorotoluene to 2-chlorobenzaldehyde and 4-chlorotoluene to 4-chlorobenzaldehyde have been performed in the presence of ABTS as mediator molecule within 1 h to 4 h at room temperature.
Keywords
Laccase; Abortiporus biennis; Mediator; Toluene; Benzaldehyde; ABTS
Introduction
Laccase [E. C. 1.10.3.2] belongs to a group of multicopper containing oxidases [1,2] and catalyzes [3-5] the four-electron reduction of molecular oxygen to water. Laccase was first reported in Japanese lacquer tree Rhus vernicifera [6]. Little is known about higher plant laccases, probably due to their presence in cell walls. Laccases are the lignolytic enzymes and abundantly occur in the fungal systems [7]. Laccase is also reported in bacteria Azospirrullum lipoferum [8] which was the first laccase producing bacteria. They are also found in Streptomyces spec. [9,10] and Anabaena azollae [11]. In addition to fungi, plants and bacteria, the presence of laccases has also been reported in wasp venom [12] as well as in insects [13].
To perform their catalytic functions, laccases depend on Cu atoms that are distributed at the three-different copper centres viz. type-1 or blue copper centre, type-2 or normal copper centre and type-3 or coupled binuclear copper centres, differing in their characteristics electronic paramagnetic resonance (EPR) signals [14,15]. The organic substrate is oxidized by one electron at the active site of the laccase generating a reaction radical which further reacts non-enzymatically. The electron is received at type 1 Cu and is shuttled to the trinuclear cluster where oxygen is reduced to water.
Fungal laccases act as potential synthetic biocatalysts. They are stable and have high catalytic efficiency. After the development of redox mediators [16,17] such as ABTS (2, 2’ [Azino-bis-(3-ethylbonzthiazoline-6-sulphonic acid) diammonium salt]), HOBT (1-hydroxybenzotriazole) etc., which have tendency to enhance the catalytic substrate range of laccases, synthetic and industrial applications of laccases have become, surprisingly, enhanced. The ability of laccases to catalyze the oxidation of various phenolic as well as non-phenolic compounds, coupled to the reduction of molecular oxygen to water makes it valuable from the point of view of their commercial applications [4,18-20]. During the last two decades, laccases have turned out to be the most promising enzymes for industrial uses [19,20] having applications in food, pulps, paper, textile, and cosmetics industries and in synthetic organic chemistry such as biotransformation’s, coupling reactions, drug syntheses etc. [21-42].
The objective of this communication was to obtain crude laccase from the liquid culture growth medium containing natural lignin substrate bagasse/wheat straw of Abortiporus biennis MTCC-1176 and to demonstrate the selective bioconversions of 3-nitrotoluene to 3-nitrobenzaldehyde, 2-chlorotoluene to 2-chlorobenzaldehyde and 4-chlorotoluene to 4-chlorobenzaldehyde in the presence of ABTS as mediator molecule. Though, this type of work has been done previously, however, during study authors have found that laccase obtained from different sources have different ability to oxidize different types of toluenes. Laccases obtained from some sources have very less efficiency to oxidize these compounds due to which they cannot be properly used for such type of synthetic reactions. In this order, authors have found another effective fungal source which secrete laccase and this laccase have been effectively utilized for such type of synthetic reactions in the presence of mediators like previously reported laccases.
Experimental
Materials
4-Chlorotoluene was from Sigma Chemical Company, St. Louis (USA). 2-Fluorotoluene, 2-chlorotoluene, 2, 2’-Azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) diammonium salt (ABTS) and 2, 6-dimethoxy phenol (DMP) were from Fluka, Chemi new Ulm (Switzerland).
Preparation of crude laccase
The fungal strain was procured from the Microbial Type Culture Collection Center and Gene Bank, Institute of Microbial Technology, Chandigarh (India) and was maintained on agar slant as reported in MTCC catalogue of strains-2000 [43-46].
In order to find the crude laccase, A. biennis MTCC-1176 was grown in five 100 mL culture flasks each containing 25 mL sterilized growth medium reported by Coll et al. [47] (medium consisted of glucose 10.0 g, asparagine 1.0 g, yeast extract 0.5 g, MgSO4.7H2O and FeSO4.7H2O, 0.01 g in 1.0 L of Milli-Q water) with 500 mg of the good inducer bagasse particles/wheat straw particles, under stationary culture condition in a BOD incubator at 30°C. Since, the maximum activity of the laccase is generally found between 6th-10th days [27,39-42] of the inoculation of the fungal mycelia, all the cultures in the five flasks were pooled; mycelia were removed by filtration through four layers of cheese cloth on the 7th day. The culture filtrate was centrifuged using refrigerated centrifuged Sigma (Germany) model 3K-30 at 12500 rpm for 20 min at 4°C in order to remove another particle types impurities present in the culture liquid. This culture filtrate has been used as a crude laccase for synthetic applications.
Enzyme assay
The assay solution 1.0 mL for DMP as the substrate [40] contained 1.0 mM DMP in 50 mM sodium malonate buffer pH 5.0 at 37°C. The reaction was monitored by measuring the absorbance change at ?=468 nm and using the molar extinction coefficient [26] value of 49.6 mM-1 cm-1.
Syntheses in the presence of ABTS
The bioconversion of 2-chlorotoluene to 2-chlorobenzaldehyde [25-27] was done in 100 mM sodium acetate buffer pH 4.5 containing 20 mM toluene in 20 mL of dioxane, 0.1 mM ABTS and 1000 μL of crude laccase kept in a 100 mL conical flask which was stirred vigorously for 240 min (completion of the reaction is confirmed by the UV/Vis spectrophotometer Hitachi (Japan) model U-2900). The reaction solution was extracted thrice with ethyl acetate. 20 μL of the ethyl acetate extract was injected in Waters HPLC Model 600E using spherisorb C18 5 UV, 4.5 × 250 mM column. The eluent phase was methanol at the flow rate of 0.5 mL/min. The detection was made using Waters UV detector model 2487 at λ=254 nm.
The biotransformations of 3-nitrotoluene to 3-nitrobenzaldehyde and 4-chlorotoluene to 4-chlorobenzaldehyde were also studied using the same method described above except time of stirring the reaction solutions were 120 min and 200 min, respectively.
Isolation: Since only small amounts of chemical auxiliaries are applied which remain in the aqueous phase after extraction of the aldehydes with an organic solvent (ethyl acetate), very pure compounds are obtained requiring no further purification. During these oxidations, no side reactions occur because of the high specificity of laccase as biocatalyst. Thus, authors have used ethyl acetate as organic solvent for the extraction of products and found the almost pure substituted benzaldehydes in high yields (average yield was >90%).
Results and Discussion
Laccase have different synthetic applicability due to which they are promising enzymes in research. Selective bioconversion of aromatic methyl group to aldehyde group is an important application out of its different valuable synthetic applications. The chemical routes of these conversions are inconvenient because methyl groups are preferably converted into carboxylic acids and it is very difficult to stop the reaction at aldehyde stage. Moreover, they require drastic reaction conditions which pollute the environment. The conversion done with laccase occurs under milder conditions, yield is >93% and the process is eco-friendly. The use of crude laccases for this purpose has been studied [25,26] in the presence of mediator molecule like 2, 2’-Azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) diammonium salt (ABTS) [25]. A number of work have been done on this topic, however, the purpose of this work was to identify a new laccase from another fungal source which can perform these types of synthesis effectively. In this order, authors have find successfully that laccase secreted from Abortiporus biennis MTCC-1176 can be effectively utilized as a biocatalyst for the synthesis of aromatic aldehydes from toluenes in the presence of the mediator molecule which was tested using 2-chlorotoluene, 3-nitrotoluene and 4-chlorotoluene as substrates given in Scheme 1.
Scheme 1: Reaction of different substituted toluenes with crude laccase of Abortiporus biennis MTCC-1176 in the presence of ABTS as mediator at room temperature.
Infrared (IR) spectroscopy (KBr, ν, cm-1)
Identification and characterization of products obtained during enzymatic reaction were analyzed on the basis of IR-results given below: ~3037 cm-1, ~2846 cm-1, ~1699 cm-1, and ~1378 cm-1, expect 1511 cm-1, 1326 cm-1 band which were additional in case of 3-nitrobenzaldehyde may be due to presence of nitro group. In above IR results bands near ~2846 cm-1 were due to the aldehydic C-H stretching and near ~1699 cm-1 were due to conjugated aldehydic C=O stretching which confirm the products formation.
1H NMR (CDCl3)
For 2-chlorobenzaldehyde: δ=9.43 (s, 1H), 7.83 (db, 1H), 7.71 (db, 1H), 7.28 (t, 1H) and 6.89 (t, 1H).
For 3-nitrobenzaldehyde: δ=9.49 (s, 1H), 8.42 (s, 1H), 8.31 (db, 1H), 7.81 (t, 1H), and 8.25 (db, 1H).
For 4-chlorobenzaldehyde: δ=9.39 (s, 1H), ~7.86 (db, 2H) and ~7.47 (db, 2H).
A singlet δ=9.31-9.49
(1H) for each biotransformed product shows that all the compounds containing aromatic methyl group have been converted into their aldehyde group.
Synthesized products were given in Table 1 with the yields of products.
| Reactants | Products | Yield (%) |
|---|---|---|
| 3-Nitrotoluene | 3-Nitrobenzaldehyde | 96 |
| 2-Chlorotoluene | 2-Chlorobenzaldehyde | 92 |
| 4-Chlorotoluene | 4-Chlorobenzaldehyde | 93 |
Table 1. Enzymatically synthesized benzaldehydes from their respective toluenes in the presence of mediator with yields.
Conclusion
This communication shows the successful bioconversions of aromatic methyl group of different substituted toluenes to corresponding aldehyde group in the presence of ABTS as mediator molecule using crude laccase of A. biennis MTCC-1176. Yields were excellent and all the syntheses were done at room temperature and pressure. In this way this communication provides another fungal source which secretes effective laccase that have strong tendency to oxidize different substituted toluenes.
Acknowledgements
The authors acknowledge the financial support of DST Lucknow in the form of project CST/SERPD/D-1223 dated 13 Sept 2012. Authors are also thankful to Department of Chemistry, DDU Gorakhpur University for providing useful facility for the work.
Refrences
- Hoegger PJ, Kilaru S, Jomes TY, et al. Phylogenetic comparison and classification of laccase and related multicopper oxidase protein sequences. FEBS J. 2006;273:2308-26.
- Messerschmidt A. Multi-Copper Oxidases, World Scientific, Singapore, 1997.
- Riva S. Laccases: Blue enzymes for green chemistry. Trends in Biotechnol. 2006;24(5):219-26.
- Baldrian P. Fungal laccases – Occurrence and properties. FEMS Microbiol Rev. 2006;30:215-42.
- Dwivedi UN, Singh P, Pandey VP, et al. Structure-function relationship among bacterial, fungal and plant laccases. J Mol Cat B: Enzymatic. 2011; 68(2):117-128.
- Yoshida H. LXIII. Chemistry of lacquer (Urushi). Part I. Communication from the Chemical Society of Tokio Chem Soc. 1883;43:472-86.
- Messerschmidt A, Huber R. The blue oxidases, ascorbate oxidase, laccase and ceruloplasmin. Modelling and structural relationships. Eur J Biochem. 1990;187:341-52.
- Givaudon A, Effose A, Faure D, et al. Polyphenol oxidase in Azospirillum lipoferum isolated from rice rhizosphere: Evidence for laccase activity in non-motile strains of Azospirillum lipoferum. FEMS Microbiol Lett. 1993;108:205-10.
- Arias ME, Arenas M, Rodroguez J, et al. Kraft pulp biobleaching and mediated oxidation of a nonphenolic substrate by laccase from streptomyces cyaneus CECT 3335. Appl Envioron Microbiol. 2003;69:1953-8.
- Suzuki T, Endo K, Ito M, et al. A thermostable laccase from Streptomyces lavendulae REN-7: Purification, characterization, nucleotide sequence, and expression. Biosci Biotechnol Biochem. 2003;67(10):2167-75.
- Malliga P, Uma L, Subramanian G. Lignolytic activity of the cyanobacterium Anabaena azollae ML2 and the value of coir waste as a carrier for BGA biofertilizer Microbios. 1996;86:175-83.
- Parkinson N, Smith I, Weaver R, et al. A new form of arthropod phenoloxidase is abundant in venom of the parasitoid wasp Pimpla hypochondriaca. Insect Biochem Mol Biol. 2001;31:57-63.
- Thomas BR, Yonekura M, Morgan TD, et al. A trypsin-solubilized laccase from pharate pupal integument of the tobacco hornworm, Manduca sexta. Insect Biochem. 1989;19:611-22.
- Solomon EI, Baldwin MJ, Lowery MD. Electronic structures of active sites in copper proteins: Contributions to reactivity. Chem Rev. 1992;92:521-42.
- Bento I, Carrondo MA, Lindley PF. Reduction of dioxygen by enzymes containing copper. J Biol Inorg Chem. 2006;11:539-47.
- d’Acunzo F, Galli C. First evidence of catalytic mediation by phenolic compounds in the laccase-induced oxidation of lignin models. Eur J Biochem. 2003;270:3634-40.
- Morozova OV, Shumakovich GP, Shleev SV, et al. Laccase-mediator systems and their applications: A review. Appl Biochem Microbiol. 2003;43:523-35.
- Wandrey C, Liese A, Kihumbu D. Industrial biocatalysis: Past, present, and future. Org Proc Res Develop. 2000;4(4):286-90.
- Couto SR, Herrera JLT. Industrial and biotechnological applications of laccases: A review. Biotechnol Advan. 2006;24:500-13.
- Xu F. Applications of oxidoreductases: Recent progress. Ind Biotechnol. 2005;1:38-50.
- Coniglio A, Galli C, Gentili P, et al. Oxidation of amides by laccase-generated aminoxyl radicals. J Mol Cat B: Enzymatic. 2008;50(1):40-9.
- Mikolasch A, Niedermeyer TH, Lalk M, et al. Novel penicillins synthesized by biotransformation using laccase from Trametes spec. Chem Pharm Bull. 2006;54(5):632-8.
- Mikolasch A, Niedermeyer THJ, Lalk M, et al. Novel cephalosporins synthesized by amination of 2,5-dihydroxybenzoic acid derivatives using fungal laccases. Chem Pharm Bull. 2007;55(3):412-6.
- Mikolasch A, Hammer E, Jonas U, et al. Synthesis of 3-(3,4-dihydroxyphenyl)-propionic acid derivatives by N-coupling of amines using laccase. Tetrahedron. 2002;58:7589-93.
- Potthast A, Rosenau T, Chen C-L, et al. Selective enzymic oxidation of aromatic methyl groups to aldehydes. J Org Chem. 1995;60(14):4320-1.
- Fritz-Langhals E, Kunath B. Synthesis of aromatic aldehydes by laccase-mediator assisted oxidation. Tetrahedron Lett. 1998;39(33):5955-6.
- Sahay R, Yadav RSS, Yadava S, et al. A Laccase of Fomes durissimus MTCC-1173 and its role in the conversion of methylbenzene to benzaldehyde. Appl Biochem Biotechnol. 2012;166(3):563-75.
- Burton SG. Laccases and phenol oxidases in organic synthesis: A review. Curr Org Chem. 2003;7(13):1317-31.
- Osiadacz J, Al-Adhami AJH, Bajraszewska D, et al. On the use of Trametes versicolor laccase for the conversion of 4-methyl-3-hydroxyanthranilic acid to actinocin chromophore. J Biotechnol. 1999;72(1-2):141-9.
- Wang HX, Ng TB. Purification of a novel low-molecular-mass laccase with HIV-1 reverse transcriptase inhibitory activity from the mushroom Tricholoma giganteum. Biochem Biophys Res Commun. 2004;315(2):450-4.
- Duhe NV, Hendrix DL. Poison ivy relief composition. U.S. Pat. 4259318;1981.
- Bauer CG, Kuhn A, Gajovic N, et al. New enzyme sensors for morphine and codeine based on morphine dehydrogenase and laccase. Fresenius’ J Anal Chem. 1999;364(1-2):179-83.
- Orndorff SA. Enzymatic catalyzed biocide system. U.S. Pat. 4478683A;1984.
- Chaurasia PK, Bharati SL, Sarma C. Laccases in pharmaceutical chemistry: A comprehensive appraisal. Mini Rev Org Chem. 2016;13(6):430-51.
- Chaurasia PK, Bharati SL, Sharma M, et al. Fungal laccases and their biotechnological significances in the current perspective: A review. Curr Org Chem. 2015;19(19):1916-34.
- Chaurasia PK, Bharati SL, Yadava S, et al. Purification, characterization and synthetic application of a thermally stable laccase from Hexagonia tenuis MTCC-1119. Indian J Biochem Biophys. 2015;52(1):60-67.
- Chaurasia PK, Bharati SL, Singh SK, et al. Amination of p-hydroquinones by laccase of Xylaria polymorpha MTCC-1100. Russian J Gen Chem. 2015;85(3):683-5.
- Timo HJN, Michael L.Nuclear amination catalyzed by fungal laccases: Comparison of laccase catalyzed amination with known chemical routes to aminoquinones. J Mol Cat B: Enzymatic. 2007;45(3-4):113-7.
- Kurisawa M, Chung JE, Uyama H, et al. Laccase-catalyzed synthesis and antioxidant property of poly (catechin). Macromol Biosci. 2003;3(12):758-64.
- Hosny M, Rosazza JPN.Novel oxidations of (+)-catechin by Horseradish peroxidase and laccase. J Agric Food Chem. 2002;50(20):5539-45.
- Chaurasia PK, Yadav A, Yadav RSS, et al. Purification and characterization of laccase from Coriolopsis floccosa MTCC-1177 and its use in the selective oxidation of aromatic methyl group to aldehyde without mediators. J Chem Sci. 2013;125(6):1395-1403.
- Chaurasia PK, Yadav RSS, Yadava S. Purification and characterization of laccase secreted by Phellinus linteus MTCC-1175 and its role in the selective oxidation of aromatic methyl group. Appl Biochem Microbiol. 2013;49(6):592-9.
- Chaurasia PK, Yadav RSS, Yadava S. Selective biotransformation of aromatic methyl groups to aldehyde groups using crude laccase of Pleurotus ostreatus MTCC-1803. Int J Res Chem Environ. 2013;3(1):188-97.
- Chaurasia PK, Yadava S, Bharati SL, et al. Syntheses of aromatic aldehydes by laccase of Pleurotus ostreatus MTCC-1801. Synth Comm. 2014;44(17):2535-44.
- Chaurasia PK, Yadav RSS, Yadava S. Purification and characterization of yellow laccase from Trametes hirsuta MTCC-1171 and its application in synthesis of aromatic aldehydes. Proc Biochem. 2014;49(10):1647-55.
- Catalogue of strains-2000. 5th ed. Microbial Type Culture Collection and Gene Bank Institute of Microbial Technology, Chandigarh. p:60.
- Coll MP, Fernandez-Abalos JM, Villanueva JR, et al. Purification and characterization of a phenoloxidase (laccase) from the lignin-degrading basidiomycete PM1 (CECT 2971). Appl Environ Microbiol. 1993;59(8):2607-13.