Research
, Volume: 19( 2) DOI: 10.37532/0974-7451.2023.19(2).264Removal of Chromium (VI) from Printing Waste using Activated Natural Zeolite
- *Correspondence:
- Adi Ilcham
Department of Chemistry,
Bandung Institute of Technology,
Kota Bandung,
Indonesia;
E-mail: adi_ilcham@upnyk.ac.id
Received: January 15, 2020, Manuscript No. tses-23-6412; Editor Assigned: January 21, 2020, Pre-QC No. tses-23-6412 (PQ); Reviewed: February 04, 2020, QC No. tses-23-6412 (Q); Revised: June 30, 2023, Manuscript No. tses-23-6412 (R); Published: July 28, 2023. DOI: 10.37532/0974-7451.2023.19(2).264
Citation: Ilcham A. Removal of Chromium (VI) from Printing Waste using Activated Natural Zeolite. Environ Sci Ind J. 2023;19(2):264.
Abstract
Increasing industrial activity is producing increasing amounts of waste. Waste from the printing industry can harm the environment as it contains chromium, a toxic metal pollutant. There are two oxidative forms of chromium in the aquatic environment: Cr (VI), which is highly toxic and Cr (III), which an essential element for humans and animals. This study investigates the removal of Cr (VI) from liquid waste using activated natural zeolites as an adsorbent. The effects of activator concentration, contact time and stirring speed on Cr (VI) removal were studied. We used an artificial liquid waste made from a mixture of K2Cr2O7, water and used newspapers. The artificial liquid waste was placed in beakers with zeolite adsorbent and stirred at various speeds for various lengths of time. Then, the Cr (VI) content was analyzed using a UV-Vis spectrophotometer. The optimal conditions were determined to comprise natural zeolite crushed to 40 mesh size and activated by 0.75 N NaOH and stirring for 60 min at 120 rpm. This procedure removed 87.60% of the Cr (VI) from the liquid waste.
Keywords
Waste; Natural zeolite; Activation; Time contacting; Stirring
Introduction
Although increasing industrialization brings prosperity, it can also cause environmental problems such as air, water and soil pollution. For example, the liquid waste produced by the printing industry contains environmentally hazardous materials such as Cobalt (Co), Manganese (Mn), Lead (Pb), Zinc (Zn) and Chromium (Cr). Chromium is a dangerous metallic pollutant that is carcinogenic, mutagenic and highly toxic. The metal has two oxidative forms, namely, Cr (III) and Cr (VI). In the form of Cr (VI) (or chromium-6), the metal is very toxic, irritating and corrosive and can cause inflammation of the skin membrane. Besides, the presence of Cr (VI) in the human body can cause damage to the nasal bones and lungs and cause cancer. In contrast, Cr (III) is non-toxic and is an essential element for humans and animals.
Reducing the Cr (VI) content in waste can be achieved by several methods, such as ion exchange, precipitation, neutralization, biosorption and adsorption. The adsorption method is efficient, simple, relatively cheap and effective at low concentrations. Natural zeolite can be used as an adsorbent for removing metal pollutants from waste. Since its structure is hollow, zeolite can be used as a molecular filter. In addition, it can be used for ion exchange and as a catalyst. Normally, the active surfaces of natural zeolite are not very large. To improve zeolite’s adsorption capacity, it requires surface activation, which can be done in two ways: Physical and chemical activation [1]. Physical activation is carried out through heating so that the water trapped in zeolite pores is removed. Using the method, the surface area of natural zeolite can be increased. However, the heating of zeolite must be controlled, as overheating will damage its surface.
Chemical activation is intended to modify pore surfaces and remove impurities. Activation of zeolites can be achieved using NaOH at concentrations of 0.1 N-11 N. Zeolite impurities such as SiO2 cannot be dissolved in water but can be removed by reaction with NaOH solution. This study aims to study the removal of Cr (VI) from printing industry waste using activated natural zeolites. The influences of activator concentration, zeolite-waste contact time and stirring speed are considered. Natural zeolite from Ende-Flores, Indonesia, was characterised and activated with acid solution in order to absorb Cr (VI) from aqueous solution. This zeolite is naturally occurring and contains 75% quartz and 50% mordenite. While kinetic adsorption modelling worked well with the pseudo-second-order model, isothermal adsorption followed the Langmuir model, with the maximum adsorption (Qmax) of NZ and ANZ being 0.189 mg/g and 1.040 mg/g, respectively.
The ANZ was also helpful for the adsorption of Cr (VI). Because of their high toxicity and propensity to accumulate through the food chain, toxic heavy metals like Cr, Ni, Cd, Pb, Hg, Zn, Co and Cu from industrial effluents pose a serious environmental risk when released into natural water bodies.One of the most infamous heavy metals is chromium, which is released by a variety of industries, including those that deal with leather and tanning, manufacturing, catalysts and pigments, fungicides, glass, photography, ceramics, crafts and electroplating [2]. In its compounds, chromium forms the oxidation states +2, +3 and +6. While +1, +4 and +5 oxidation states are uncommon, the +3 and +6 oxidation states are the most frequently seen in chromium compounds.
Hexavalent chromium, sometimes known as Cr (VI), is the most well-known kind of hazardous chromium. The IARC has categorised metallic chromium and chromium (III) in group 3 (not classifiable as to their carcinogenicity to humans) and chromium (VI) in group 1 (carcinogenic to humans) [3]. Therefore, before wastewater is discharged into the aquatic system, the removal of chromium (VI) from it is of utmost importance. In order to remove Cr (VI) from water and wastewater, conventional treatment technologies have been developed. These include reduction followed by chemical precipitation, ion exchange, membrane separation, electrocoagulation, nanoparticles, dialysis/electrodialysis and adsorption/filtration [4]. Efficiency and efficacy of these systems are frequently constrained by capital and operating expenses, particularly when significant volumes of effluents are involved.
Materials and Methods
Standard curve
The chromium content in the waste was observed using standard curves constructed as follows. Firstly, a mother liquor was made by dissolving an amount of known K2Cr2O7 (Merck) in aquadest [5]. The solution then was diluted and tannin was added as a stabilizer until a solution pH of 10.5 was obtained. The solution was shaken to homogenize it and then its absorbance was measured.
Activation of natural zeolites
Natural zeolite chunks were obtained from Klaten and then crushed and sieved at a size of 40 mesh. Then zeolite was chemically activated by soaking for 24 h in sodium hydroxide solution at various concentrations. Next, the soaked zeolite was filtered using filter paper, then rinsed until the zeolite reached a neutral pH. Finally, the zeolite was dried at 105°C in an oven [6].
Main experiment
In this study, metal pollutant removal was studied using an artificial form of waste. The artificial waste was obtained by mixing K2Cr2O7 in water and used newspaper at a ratio of 0.5 g K2Cr2O7 to 5 L of distilled water and 100 used newspaper. Before Cr (VI) was removed from the waste, the content of chromium in the solution was observed according to its absorbance in a UV-Vis spectrophotometer at a wavelength of 486 nm [7]. To deplete Cr (VI), the waste and activated zeolite (adsorbent) were placed in a beaker and stirred. The chromium content in the processed waste was then observed using the UV-Vis spectrophotometer.
Results and Discussion
standard curve was plotted by mapping the relationship between the known chromium concentrations in the solution and their absorbance values. The standard curve was used to observe the absorbance of the solution and determine its chromium content. The initial concentration of Cr (VI) in the artificial waste was 0.9360 mg/L. The natural activated zeolite had a surface area of 27.5 m2/g [8].
Effect of activator concentration
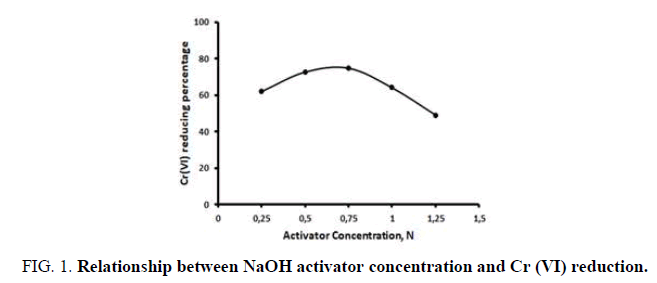
Natural zeolites have many impurities that cover the surfaces of their pores. Therefore, the pores must be modified to increase the adsorption capacity. To activate natural zeolite, activators must be applied, as reported by. In this study, to observe the influence of activator concentration on chromium reduction, we used 8 g natural zeolite of 40 mesh size and 80 ml sodium hydroxide as an activator [9]. The solution was made in various concentrations. The activated natural zeolite was then applied to the artificial waste and stirred for 30 min at 120 rpm. The results are shown in Figure 1.
Besides impurities, the pores inside natural zeolites are covered by metal oxides, which can be improved by activation. Activation of the adsorbent can improve the adsorption of metals or carbon. Activation of the adsorbent’s surface increases its surface texture allowing larger pore surfaces to attract more adsorbate.
As shown in Figure 1, the greatest decrease in Cr (VI) was obtained using natural zeolite that was activated using a 0.75 N NaOH solution. At higher concentrations there is less reduction in Cr (VI). This is likely due to the condition of the zeolite [10]. When the zeolite is more alkaline, the Cr (VI) will be released more easily.
Effect of stirring speed
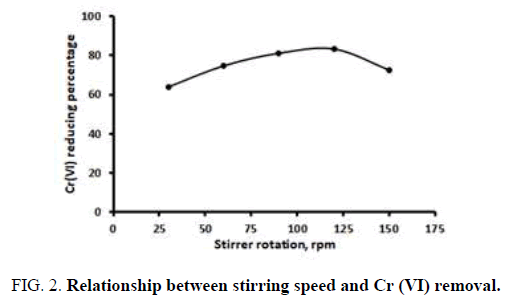
We next investigated the effect of stirring speed on the removal of Cr (VI) from liquid waste. For this, we used 8 g natural zeolite of 40 mesh size that was activated using 0.75 N NaOH. The adsorption process was carried out for 30 min at various stirring speeds. The results are shown in Figure 2.
Figure 2 shows how stirring speed affects Cr (VI) adsorption. Starting at 30 rpm, chromium adsorption increased with stirring speed. Adsorption consists of both physical and chemical adsorption but, in this case, it is almost all physical adsorption. When the liquid is stirred, turbulence is formed in the solution, which increases contact between the adsorbate and adsorbent. The ability of zeolite to adsorb Cr (VI) reaches a maximum at 120 rpm, at which point 83.31% is adsorbed. At greater speeds, the adsorption capacity decreases and adsorbate that has been absorbed is released again.
Effect of contact time
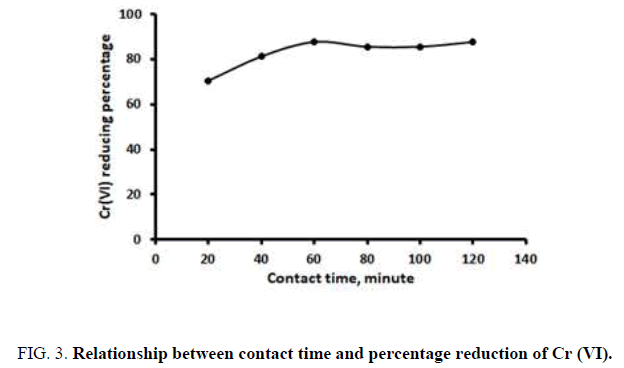
Contact time plays a role in the adsorption of metal pollutants from waste. To study the effect of contact time, we used 8 g activated zeolite of 40 mesh size, an activator concentration of 0.75 N NaOH and stirring speed of 120 rpm [11]. The results are shown in Figure 3.
Figure 3 shows that the highest percentage of Cr (VI) reduction was 87.60%, which occurred at 60 min contact time. The Cr (VI) content in liquid waste tended to be constant at contact times of 80, 100 and 120 min. From this result, it is assumed that the adsorption of Cr (VI) in artificial waste will remain constant after 60 min. After 60 min, further adsorption is difficult due to the saturated condition of the zeolites [12]. In the saturated state, the zeolite pores are filled with adsorbate so no further adsorption can occur. This result is similar to that reported in, where zeolite adsorption reaches saturation at 60 min.
Isotherm study
To understand the pattern of Cr (VI) adsorption in the adsorbents, two adsorption isotherm models were studied: The Langmuir isotherm and Freundlich isotherm models. The Langmuir isotherm model assumes that adsorption takes place through a single layer, while in the Freundlich model it occurs through many layers [13]. Therefore, the experimental data were plotted in accordance with the two isotherm models: Equation (1) for the Langmuir model and equation for the Freundlich model.
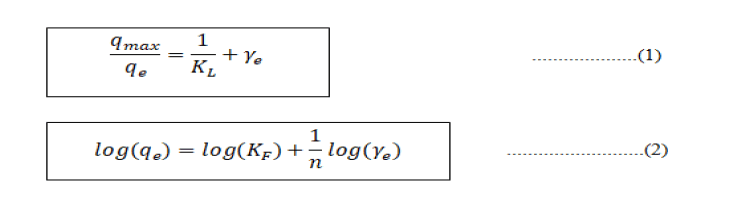
Mapping of the experimental results and isotherm equations are represented in Figure 3. These graphs show that the data tend to follow the Langmuir isotherm [14]. The results explain that the adsorption of Cr (VI) takes place through a single-layer mechanism, as reported by other researchers. The experimental results also show that the maximum adsorption power is 0.07152 mg of Cr (VI) per gram zeolite [15].
Conclusion
This study investigated the removal of Cr (VI) from artificial printing waste. The results show that zeolite of 40 mesh size can remove 87.60% of the heavy metal Cr (VI) content from liquid waste. This result can be achieved using natural zeolite activated by NaOH at a concentration of 0.75 N. Maximum removal of Cr (VI) from liquid waste occurred at a stirring speed of 120 rpm and contact time of 60 min.
References
- Liu Y, Deng L, Chen Y, et al. Simultaneous photocatalytic reduction of Cr (VI) and oxidation of bisphenol A induced by Fe (III)-OH complexes in water. J Hazard Mater. 2007;139(2):399-402.
[Crossref] [Google Scholar] [PubMed]
- Jorfi S, Ahmadi MJ, Pourfadakari S, et al. Adsorption of Cr (VI) by natural clinoptilolite zeolite from aqueous solutions: Isotherms and kinetics. Pol J Chem Technol. 2017;19(3):106-114.
- Majlesi Nasr M, Rahmani K, Rahmani H, et al. Removal of chromium (VI) by modified zeolite with nanoscale Zero-Va lent Iron (nZVI) from water. J Health. 2017;8(3):280-288.
- Wirawan SK, Sudibyo H, Setiaji MF, et al. Development of natural zeolites adsorbent: Chemical analysis and preliminary TPD adsorption study. J Eng Sci Technol. 2015;10(Spec. issue 4):87-95.
- Ademiluyi FT, David-West EO. Effect of chemical activation on the adsorption of heavy metals using activated carbons from waste materials. Int Sch Res Not. 2012.
- Djaeni M, Kurniasari L, Purbasari A, et al. Activation of natural zeolite as water adsorbent for mixed-adsorption drying. Int Conf Mater Eng Reg Confer Mater (RCM) 2010;25(3):24-26.
- Ruthven DM. Principles of adsorption and adsorption processes. John Wiley and Sons; 1984.
- Pang M, Kano N, Imaizumi H. Adsorption of chromium (VI) from aqueous solution using zeolite/chitosan hybrid composite. J Chem Eng. 2015;9(7):433-441.
- Kuic D, Simonic M, Furac L. Batch adsorption of Cr (VI) ions on zeolite and agroindustrial waste. Chem Biochem Eng. 2017;31(4):497-507.
- Dehghani MH, Sanaei D, Ali I, et al. Removal of chromium (VI) from aqueous solution using treated waste newspaper as a low-cost adsorbent: Kinetic modeling and isotherm studies. J Mol Liq. 2016;215(4):671-679.
- Neolaka YA, Kalla EB, Supriyanto G, et al. Adsorption of hexavalent chromium from aqueous solutions using acid activated of natural zeolite collected from Ende-Flores, Indonesia. Ras J Chem. 2017;10(2):606-612.
- Namasivayam C, Sureshkumar MV. Removal of chromium (VI) from water and wastewater using surfactant modified coconut coir pith as a biosorbent. Bioresour Technol. 2008;99(7):2218-2225.
- Wang S, Peng Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem Eng J. 2010;156(1):11-24.
- Ma H, Yang J, Gao X, et al. Removal of chromium (VI) from water by porous carbon derived from corn straw: Influencing factors, regeneration and mechanism. J Hazard Mater. 2019;369:550-560.
[Crossref] [Google Scholar] [PubMed]
- Othmani A, Magdouli S, Kumar PS, et al. Agricultural waste materials for adsorptive removal of phenols, chromium (VI) and cadmium (II) from wastewater: A review. Environ Res. 2022;204:111916.
[Crossref] [Google Scholar] [PubMed]