Research
, Volume: 16( 1) DOI: 10.37532/0974-7451.2020.16(1).105Integrated Approach to Remove Iron, Arsenic, and Manganese from Water Using Manganese Greensand and Other Adsorbent
- *Correspondence:
- Ananta Saikia
Defence Research Laboratory
Tezpur, Assam, India
Tel: 9435485784
E-Mail: saikiaananta@yahoo.com
Received: February 09, 2020; Accepted:February 13, 2020; Published: February 28, 2020
Citation: Saikia A, Agnihotri G, Raul KP, et al. Integrated Approach to REMOVE IRON, ARSENIC and Manganese from Water using Manganese Greensand and Other Adsorbent. Indian J Environ Sci. 2020;16(1):105.
Abstract
An efficient system has been designed and developed to study the removal of some heavy metals like Fe(II), As(III) and Mn(II) from water considering effect of competing ions. The integrated approach to remove contaminants from water successfully proved at filtration rate 300 Lhr-1. Sand was coated with ferrous sulphate and used as principal adsorbent. The batch adsorber was designed with different compartment containing limestone, iron fillings, coated sand, manganese greensand and granular activated charcoal as filtration media and removal efficiency of iron, arsenic and manganese was monitored. The system showed the removal of Fe(II), As(III) and Mn(II) up to 0.3 mgL-1, 10 μgL-1 and 0.03 mgL-1, respectively from initial spiked water having 30 mgL-1(iron), 1400 μgL-1(arsenic), and 5 mgL-1 (manganese), with pH range of 6.8 to 8.2. It was found that removal of heavy metals from water is not pronouncedly effected in the presence of competing ions.
Keywords
Water; Health; Removal; Resources; Environmental
Introduction
Water is a vital component not only in sustaining life but also for the economic growth of any nation. India is one of the countries having high potential and rich water resources. Population, explosion, economic progress, poor management and contamination of water sources are the main reasons for the scarcity of assured quality of water. The supply of safe water in adequate quantities to all communities is an immediate need for human society. A major threat to human health in India is the poor drinking water quality. Iron, manganese, and arsenic are at the top of the list of emerging threats to the quality of water for domestic consumption.
In India, North Eastern states along with Jharkhand, Bihar, West Bengal, Madhya Pradesh, Uttar Pradesh, etc. are badly affected by groundwater contamination by arsenic and iron [1].
Arsenic is ubiquitous in the earth’s crust and is highest in marine shale materials, magmatic sulfides, and iron ores where arsenic occurs as arsenopyrite (FeAsS), realgar (AsS), and orpiment (As2S3) [2,3]. Throughout the world, arsenic is creating potentially serious environmental problems for humans and other living organisms. Arsenic related problems are found in ground water supply systems and are caused by natural processes such as mineral weathering and dissolution [4]. Human activities such as mining wastes, petroleum refining, sewage sludge, agricultural chemicals, ceramic manufacturing industries, and coal fly ash are also responsible for arsenic contamination [5-8]. Millions of people in West Bengal, Bangladesh and the North-Eastern region of India are affected by drinking water withdrawn from wells that contain 100 μgL-1-500 μgL-1 arsenic contaminated ground water [9]. Owing to epidemiological evidence linking arsenic and cancer, the safe limit of arsenic in drinking water was reduced from 50 μgL-1 to 10 μgL-1 in 1993 by WHO [10].
Arsenic has a wide range of oxidation states of arsenic -3,0,+3 and +5 of which +3 and +5 are very common. It can readily change its valency and chemical form in the environment [11]. At present many approaches such as adsorption, ion-exchange, reverse osmosis, Nanofiltration, coagulation (co-precipitation), membrane distillation, biological methods, and photocatalytic oxidation are increasingly being used for the removal of arsenic from water body [12-14]. Adsorption on the iron oxide coated sand method is one of the emerging technologies for arsenic removal [15,16]. While As(V) is more stable in oxidizing conditions, but As(III) is stable in reductive conditions [17]. The literature says that As(III) is best removed at pH 7.5 [18].
Groundwater may contain ferrous iron at concentrations up to several milligrams per liter without discoloration or turbidity in the water. High iron content in drinking water is a major problem in most parts of the North-Eastern region of India [19,20]. Once brought to the surface, with time the iron will come out of solution giving the water an undesirable reddish-brown color. Furthermore, the presence of iron gives water a taste described as metallic, astringent or medicinal. Iron also causes other aesthetic problems such as laundry, walls and plumbing fixtures at levels above 0.1 mgL-1. Treatment requirements for the removal of dissolved iron from water are well understood.
Very often the presence of manganese in water is ignored as its characters are much in similarity with iron. Manganese also has dark brownish-black appearances and health hazards too. Manganese is commonly found in two forms e.g. Mn2+ which is soluble in water (colorless water) and Mn4+ which is not soluble in water (MnO2) (cause turbidity in water). Manganese is generally found in groundwater as Mn2+ while in the surface it becomes MnO2. Chronic exposure to excessive manganese levels can lead to a variety of psychiatric and motor disturbances and even influence copper and iron metabolism in the body [21,22]. Various techniques have been adopted for the removal of manganese as well as iron from contaminated water [23-26].
In view of the above, it is very essential to ensure the removal of Fe, As and Mn from the water before it is being supplied for human consumption as well as for domestic uses. In the present scenario various systems are available for the removal of individual ions from water, but the requirement of integrated removal system for ions like Fe, As and Mn has been arising at higher filtration rate.
In the present study, individual as well as integrated removal of Fe, As and Mn from the contaminated water have been carried out along with the study of effects of competing ions using various filtering media at filtration rate 300 Lhr-1
Experimental
Materials and methods
A stock solution of 1000 μgmL-1 arsenic was prepared from As2O3 using double distilled water. A stock solution of 1000 μgmL-1 iron was prepared from FeSO4•7H2O using double distilled water. The stock solution of 1000 μgmL-1 manganese was prepared from MnSO4•H2O using double distilled water. All other reagents were of analytical grade (Merck, India) and used without further purification. Limestone was obtained from the Mawlong mining site, Meghalaya, India. Limestone showed the presence of CaCO3 and traces of silica. The weight percentages of the elements are shown in TABLE 1.
| Element | Weight% | Atomic% |
|---|---|---|
| C | 6.1 | 10.24 |
| O | 55.91 | 70.25 |
| Si | 0.32 | 0.23 |
| Ca | 37.67 | 18.98 |
Table 1: Weight percent of elements present in limestone.
Iron and manganese were estimated by flame AAS (LabIndia AA 7000) according to standard methods [27]. Arsenic was measured with the help of a hydride generation system on the same instrument.
Preparation of iron oxide coated sand: Iron oxide coated sand was prepared using a procedure similar to the described elsewhere [15,16]. Washed and dried river sand of geometric mean size 0.8 mm was mixed with 10% of ferrous sulphate solution of pH 10-11. The mixture was then dried in an oven at 110°C for 14 hours. The coated sand was washed with distilled water until the runoff was clear. Then the mixture was dried at 105°C and stored in capped bottles.
Manganese greensand: Manganese greensand is a zeolite mineral called glauconite, processed with manganese sulphide or manganese sulphate, and potassium permanganate in alternating steps to produce a black precipitate of manganese dioxide on the granules. It is used as a filter media, operated the same as a rapid sand filter except that it can be also regenerated after its capacity decreases. Manganese greensand is a unique medium used in conjunction with a filtration system to oxidize, precipitate and remove Iron and manganese.
Manganese greensand offers advantages over other iron and manganese removal media: (a) it has an optimum grain size and shape to retain oxidation precipitation products of iron and manganese, (b) all grains have the same finite homogeneous coating, which is firmly attached, (c) it has unequaled oxidation-reduction buffer capacity, and can tolerate a slight over or underfeed of continuously fed oxidants, (d) manganese oxide coating is not removed during backwashing or during the water-saving, but more physically demanding, air/water washing and (e) manganese greensand is not a proprietary medium of any equipment manufacturer.
Experiments
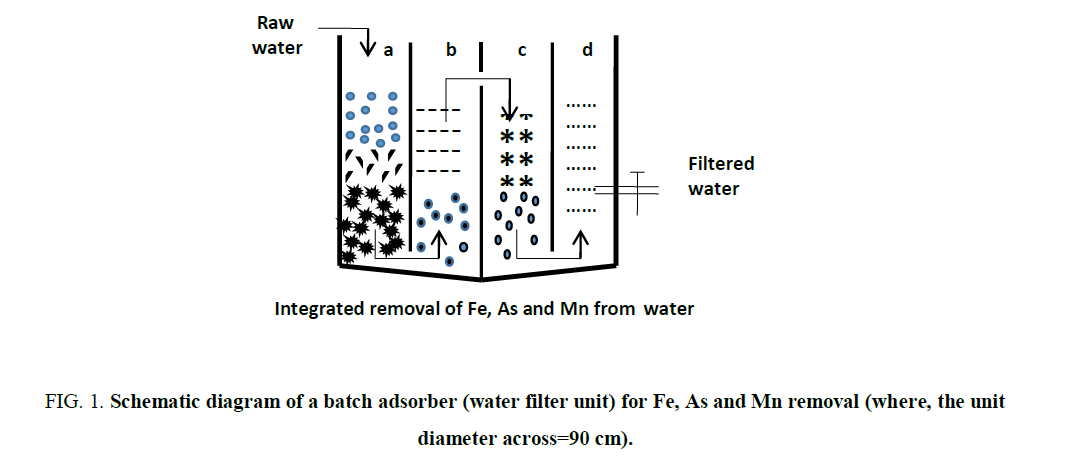
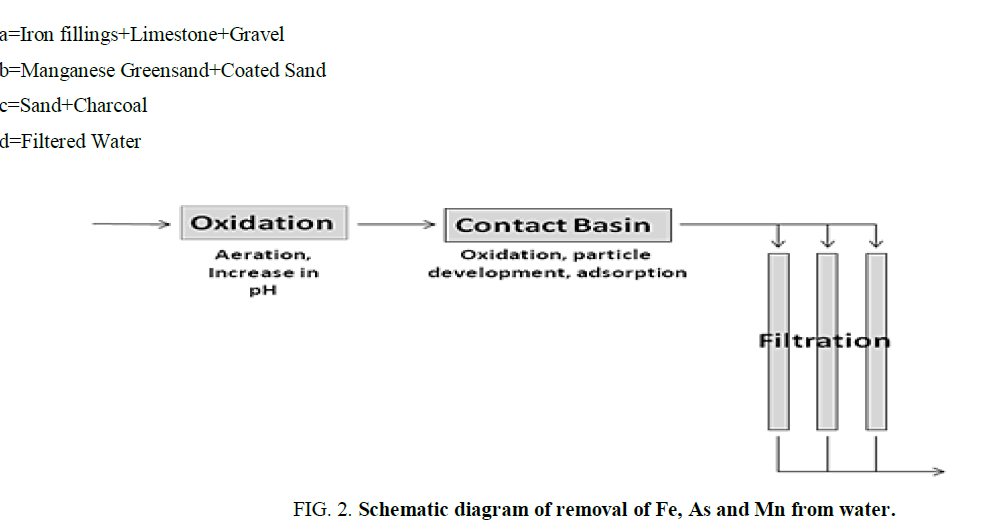
The raw water was spiked with varying concentrations of iron, arsenic, and manganese. This water has been passed through the three chambers containing different filtering media as shown in FIG. 1. The simple representation of the process is shown in the schematic diagram FIG. 2.
Figure 1. Schematic diagram of a batch adsorber (water filter unit) for Fe, As and Mn removal (where, the unit diameter across=90 cm).
The filtered water gets collected in the last chamber and then analyzed for the presence of Fe, As and Mn. Particle size and bed volume of the filter medium used in the chambers are shown in TABLE 2. The experiments have been carried out using various spiked water containing individual as well as a mixture of Fe, As and Mn ions.
| Filter medium used | Particle size (mm) |
Bed volume (m3) |
|---|---|---|
| Coated gravel | 40-60 | 0.020 |
| Limestone | 15-20 | 0.020 |
| Iron coated sand | 0.8-1.2 | 0.068 |
| Manganese green sand | 0.5-1.0 | 0.007 |
| Granular charcoal | 4.0-6.0 | 0.008 |
| Sand | 0.8-1.2 | 0.077 |
Table 2: Particle size (20 kilograms of Iron filling) and bed volume of the filter medium used in the column.
Aeration is one of the main step involved in the experimental process, which is used (a) primarily to provide oxygen from the atmosphere for the oxidation of Fe and Mn and to liberate H2S and CO2 from water, (b) to release taste and odour producing substances e.g., H2S or some of the volatile substances liberated by algae growths or incidental to the decomposition of organic matter, (c) to enhance the taste of water. Water devoid of dissolved air/oxygen has a flat taste which is replaced by a fresh taste on aeration of water.
Various types of aerators are in use viz. spray aerators, waterfall aerators, cascade aerators, diffused air aerators, etc. The filtration rate of 300 Lhr-1 has been found to be optimum for the removal of the selected metal ions. Filtration rate less than 300 Lhr-1 does not affect significantly the removal capacity, whereas an increase up to 350 Lhr-1 had also not much effect on removal efficiency, but flow rate more than 400 Lhr-1 largely affected the removal depending upon the concentration of the contaminants. This may be due to less contact time of the contaminants and adsorbents during the process.
Results
The integrated approach was applied in the batch adsorber system keeping in view the simultaneous steps involved in the chemistry of iron, arsenic, and manganese.
Chemistry of iron removal
• The oxidation of iron ions raises the pH of the water
• Ferrous iron (Fe2+) is oxidized to ferric iron (Fe3+)
4Fe2+ (aq)+O2 (g)+4H+ (aq) → 4Fe3+ (aq)+2H2O (l)
• If pH is greater than ~3.6, ferric iron will hydrolize to form ferric hydroxide
Fe3+ (aq)+3H2O (l) →Fe(OH)3 (s)+3H+ (aq)
Chemistry of iron and arsenic removal
• Oxidation of Ferrous iron (Fe2+) to ferric iron (Fe3+)
• Further adsorption of As onto Fe(OH)3 precipitate
• Further reactions
α-FeOOH+H2AsO−+3H+ → FeH2AsO4+2H2O
α-FeOOH+H3AsO3+2H+ → FeH2AsO3+2H2O
• pH needs to be around 7.3
• In oxygen-rich environments where aerobic conditions persist, arsenate [As(V)] is prevalent and exists as a monovalent (H2AsO4−) or divalent (HAsO42−) anion, whereas, arsenite [As(III)] exists as an uncharged molecule (H3AsO3) and anionic (H2AsO3−) species in moderately reducing environment where anoxic conditions persist
• Similar to iron, manganese removal by the physical-chemical way can be carried out by the oxidation of Mn2+ to Mn4+, which precipitates as manganese dioxide (MnO2). The precipitates are then separated from water by filtration on green sand
• For manganese removal only, manganese dioxide (MnO2) is used as an adsorbent according to the following reaction
• Mn + MnO2 (s) → 2MnO (s)
Removal of arsenic
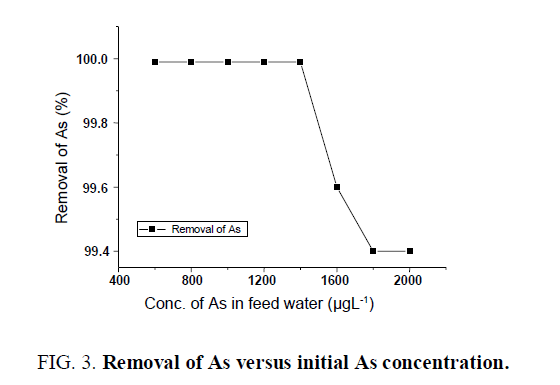
The removal of arsenic was studied using granular activated charcoal and Iron oxide-coated sand in the filtration system with a flow rate of 300 Lhr-1 FIG. 3. It was found that the percentage removal of as is achieved by up to 99.99% with feed water up to 1400 μgL-1. The experiment was conducted by using limestone in the first compartment of the filtration unit, without the addition of iron salts like FeSO4 and lime water (pH range 6.8 to 8.2). The increase in percentage removal is may be due to the use of iron oxide coated sand with which strong interaction occurs with arsenic.
Removal of manganese
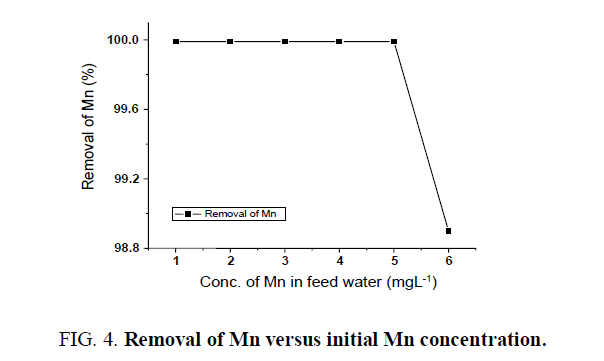
The study for the removal of Mn was carried out using greensand at 300 Lhr-1 filtration rate. The initial conc. of manganese was varied from 1 to 6 mgL-1 and it was observed that the batch adsorber system is efficient to reduce up to 99.99% leaving a trace amount of manganese in filtered water (FIG. 4). The higher percentage removal of Mn was achieved due to the application of greensand as a medium.
Removal of iron
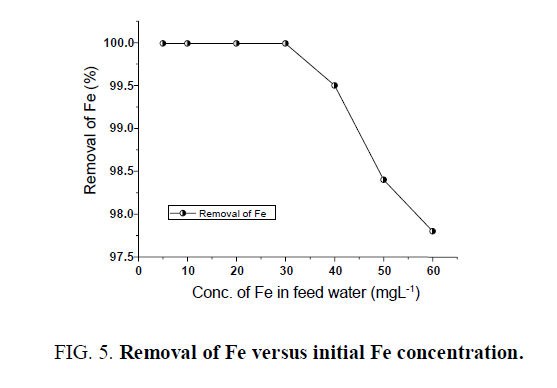
The removal of iron was studied at a filtration rate of 300 Lhr-1. From the experimental study, it was observed that the removal of iron can be achieved up to 98.0% from initial conc. of 50 mgL-1 to less than 1 mgL-1 ( FIG. 5). Also, it is showing removal up to 0.3 ppm with initial conc. of 30 mgL-1. The water filtered through the filtration system can be used for drinking purposes as iron is within the desirable limit (WHO/USEPA).
Integrated removal of iron, arsenic, and manganese
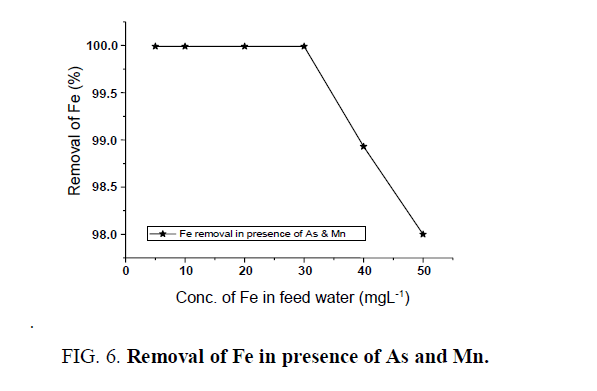
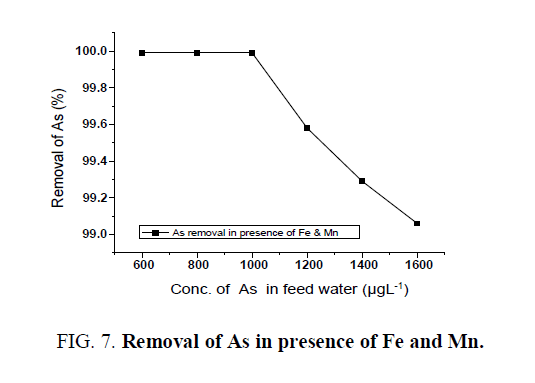
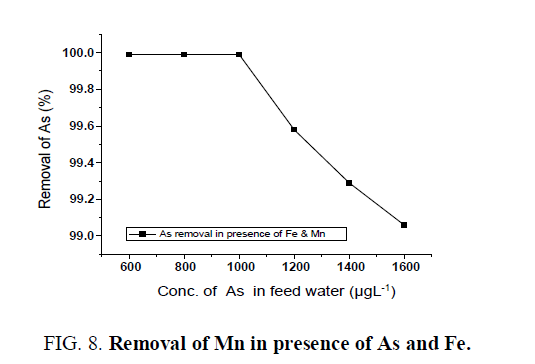
Different concentrations of Fe, As and Mn were taken in feed water and passed through the batch adsorber to see the removal efficiency of the unit at 300 Lhr-1 filtration rate. Feedwater containing a mixture of Fe, As and Mn ions in various concentrations were tested for removal efficiency and it has been observed that all contaminants in the filtered water are within desirable limit (WHO/USEPA). Iron was removed from 50 mgL-1to less than 1 mgL-1 in the presence of manganese and arsenic ( FIG. 6). Maximum 99% arsenic removal was observed from the initial arsenic concentration of 1600 μgL-1 in the presence of other ions like Mn and Fe ( FIG. 7). The trend was also followed in case of manganese removal along with iron and arsenic ( FIG. 8)
Effect of competing ions
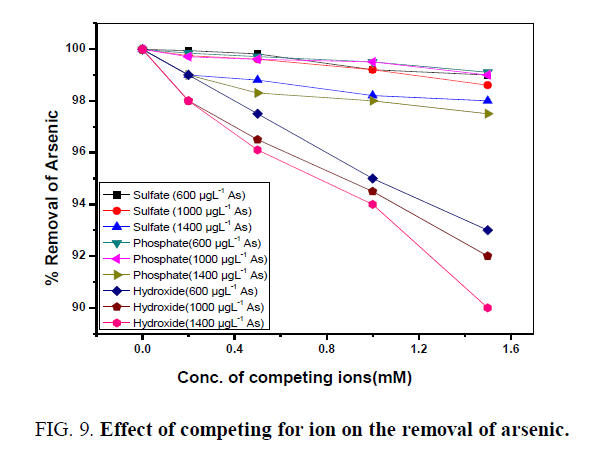
The contaminated drinking water may contain several common other anions, viz., OH-, SO42- and PO43- which can compete with the arsenic during sorption process and hence the adsorption was studied in the presence of competing anions with varying initial concentrations of these ions viz., 0.2, 0.5, 1.0 and 1.5 mM keeping the initial arsenic concentration of 600 μgL-1, 1000 μgL-1and 1400 μgL-1 at 25°C.FIG. 9 shows the efficiency of the removal capacity of arsenic by the water purification system in the presence of other competing anions. With the increase in the concentration of these anions, the removal of arsenic from the water was decreased. It may be due to competition among them for the sites on the sorbent surfaces, which in turn is decided by the concentration, charge, and size of the anions. The presence of competing anions like phosphate ion has a significant effect on arsenic adsorption by coated sand followed by hydroxide and sulphate ion respectively [28]. The percentage of arsenic removal decreased sharply after 0.5 mM concentration of competing ions and the trend was followed in all cases. The concentrations of competing anions in this study were far higher than those likely to be encountered in groundwater. Thus, coated sand was able to remove arsenic from water over a broad range of pH in the presence of all interfering ions except the high concentration of phosphate ion.
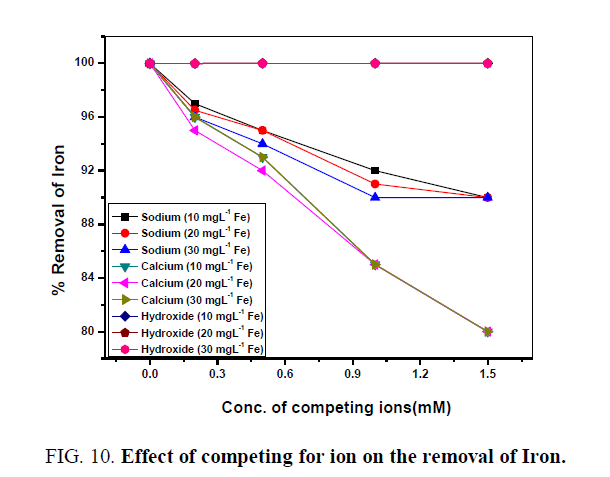
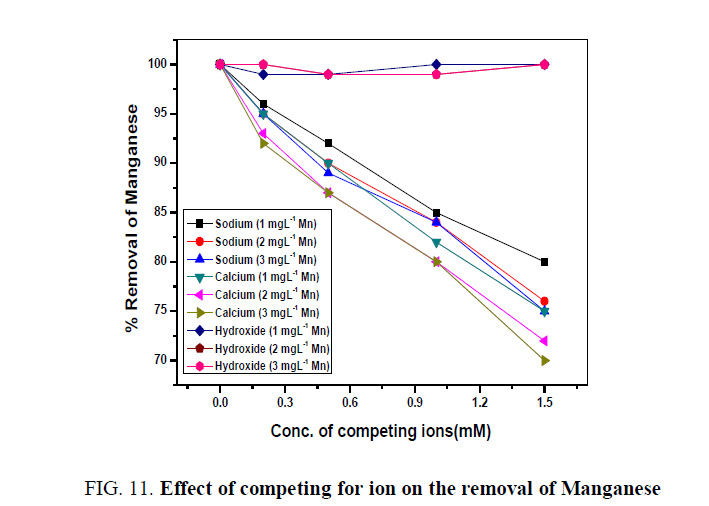
Foreign ions like Ca2+, Na+, and OH- ions were used as competing cations to study the sorption of iron and manganeseseparately by the coated sand because they are found in the ground water by a high concentration [29,30]. The obtained data were represented in FIG. 10 and FIG. 11 for Fe3+ and Mn2+ respectively. Generally, it is observed that the presence of Ca2+ and Na+ cations as competing cations decrease the adsorption of Fe3+ and Mn2+ by the coated sand. But in the presence of hydroxide ion, adsorption of Fe3+ and Mn2+ were increased. This is probably due to the formation of ferric hydroxide and manganese hydroxide respectively. The adsorption of Fe3+ ion is decreased by increasing the concentration of both competing ions, this indicates that Ca2+ and Na+ cations have a competing effect on the sorption of Fe3+ by the experimental material [31]. FIG. 11 illustrates that the percent uptake of Mn2+ ion decreases sharply with increasing the concentrations of Ca2+ and Na+ cation as a competing ion. It could be attributed to the fact that the two cations are more active than the Iron and Manganese ion, probable they have nearly similar ionic radii (Ca2+=0.990A, Mn2+=0.80A, Fe3+=0.660A, Na+=0.90A) [32].
Discussion and Conclusion
The removal of As along with Fe and Mn can be achieved even without the addition of ferric salts and limewater. The integrated removal approach can be achieved up to desirable limits by involving simultaneous steps keeping in view the reciprocal chemistry of the metals removed. Apart from just experiments, a practical device can be operated using simple material to get portable water. Competing ion like phosphate has a significant effect on arsenic removal followed by hydroxide and sulphate ion respectively. The removal of iron decreased sharply in the presence of Na+ and Ca2+ ion. A trend was highly followed by Mn2+ also. But in the presence of hydroxide ion removal of both Fe2+ and Mn2+ have been increased pronouncedly, because of the formation of the corresponding hydroxide which in turn gets deposited over adsorbent. The batch absorber system is thus efficient to remove As, Mn and Fe separately as well as altogether in the presence of competing ions.
Acknowledgement
The authors are thankful to DRDO HQs, New Delhi for providing necessary financial support to carry out the research work.
References
- Srikanth R. Challenges of sustainable water quality management in rural India Current Science. 2010;97(3):317-25.
- Tanaka T. Distribution of arsenic in the natural environment with emphasis on rocks and soils. Appl Organometall. Chem. 1988;2(4):283-95.
- Huang SY, Chang CS, Tang JL, et al. Acute and chronic arsenic poisoning associated with the treatment of acute promyelocytic leukaemia. Brit J Haematology. 1998;103(4):1092-95.
- Igor RA, Luiz ED, Jaime WV, et al. Annual Report, Institute of Science and Technology for Mineral Resource, Water and Biodiversity, 2010.
- Webb SM, Gaillard JO, Ma LQ, et al. XAS Speciation of Arsenic in a hyper-accumulating fern. Env Sci and Tech. 2003;37(4):754-60.
- Manning BA, Goldberg S. Adsorption and stability of Arsenic(III) at the clay mineral-water interface. Env Sci and Tech. 1997;31(7):2005-11.
- Grossl PR, Eick M, Sparks DL, et al. Arsenate and chromate retention mechanisms on Goethite 1 surface structure. Env Sci and Tech. 1997;31(2):315-20.
- Thirunavukkarasu OS, Viraraghavan T, Subramanian KS. Removal of arsenic in drinking water by iron oxide-coated sand and ferrihydrite. Water Quality Res J Can. 200;36(1):55-70.
- Johnston R, Heijnen H, Wurzel P. Safe water technology for arsenic removal. Chapter 6, 2001.
- Bajpai S, Chaudhuri M. Removal of arsenic from ground water by manganese dioxide-coated sand. J Environ Eng. 1999;125(8):782-84.
- Mohan D, Pittman CU. Arsenic removal from water/wastewater using adsorbents. J Hazard Mater. 2007;142(1-2)1-53.
- Qu D, Wang J, Hou DY, et al. Experimental study of arsenic removal by direct contact membrane distillation. J Hazard Mater. 2009;163(2-3):874-79.
- Chen YN, Chai LY, Shu YD. Study of arsenic(V) adsorption on bone char from aqueous solution. J Hazard Mater. 2008;160(1):168-72.
- Chen W, Parette R, Zoau J, et al. Arsenic removal by iron-modified activated carbon. Water Res. 2007;41(9):1851-58.
- Joshi A, Chaudhuri M. Removal of Arsenic from ground water by iron oxide-coated sand. J Environ Eng. 1996;122(8):769-71.
- Gupta VK, Saini VK, Jain N. Adsorption of As(III) from aqueous solutions by iron oxide-coated sand. J Colloid and Interface Sci. 2005;288(1):55-60.
- Jeon CS, Baek K, Park JK, et al. Adsorption characteristics of As(V) on iron-coated zeolite. J Hazard Mater. 2009:163(2-3):804-08.
- Sim SJ, Kang CD, Lee J W, et al. Treatment of highly polluted groundwater by novel iron removal process. J Env Sci and Health. 2001;Part A36(1):25-38.
- Singh AK, Bhagowati S, Das TK, et al. Assessment of arsenic, fluoride, iron, nitrate and heavy metals in drinking water of northeastern India. Envis Bulletin: Himalayan Ecology. 2008;16(1):1-7.
- Raul PK, Umlong IM, Banerjee S. Removal of fluoride from water using iron oxide-hydroxide nanoparticles.
- J Nanosci and Nanotech. 2012;12(5):3922-930.
- Santamaria AB. Manganese exposure, essentiality and toxicity. Indian J Med Res. 2008;128(4):484-500.
- Schneider JS, Decamp E, Koser AJ. Effects of chronic manganese exposure on cognitive and motor functioning in non-human primates. Brain Res. 2006;1118(1):222-31.
- Danka B, Jan I, Karol M. The use of iron-based sorption materials and magnetic fields for the removal of antimony from water. Polish J of Environ Stud. 2015;24:1983-92.
- Mahmoud MA, Gawad EA, Hamoda EA, et al. Kinetics and thermodynamic of Fe(III) adsorption type onto activated carbon frombiomass: kinetics and thermodynamics studies. Indian J Environ Sci. 2015;11(4):128-36.
- Khadse GK, Patni PM, Labhasetwar PK. Removal of iron and manganese from drinking water supply. Water Res Manag. 2015;1(2):157-65.
- Pakniat M, Hashemnia S, khodaveisi J, et al. Removal of Cd2+ ions from water and wastewater by complex formation with cation2b and extraction the complex with magnetic nanoparticles. Indian J Environ Sci. 2016;12(4):166-73.
- AAPHA, AWWA and WPCF, Standard methods for the examination of water and wastewater., 6th ed, 1985.
- Guan X, Dong H, Ma J. Removal of arsenic from water: Effects of competing anions on As(III) removal in KMnO4–Fe(II) process. Wat Res. 2009;43(15):3891-899.
- Raul PK, Devi RR, Umlong IM. Iron oxide hydroxide nano flower assisted removal of arsenic from water. Materials Research Bulletin. 2014;49:360-68.
- Erdem E, Karapinar N, Donat R. The removal of heavy metal cations by natural zeolites. J Colloid Interface Sci. 2004;280(2):309-14.
- Choo KH, Han SC, Choi SJ. Use of Chelating Polymers to Enhance Manganese Removal in Ultrafiltration for Drinking Water Treatment. Ind Eng Chem. 2007;13(2):163.
- Raul PK, Senapati S, Sahoo AK, et al. CuO nanorods: a potential and efficient adsorbent in water purification. RSC Advances. 2014;4(76):40580-587.