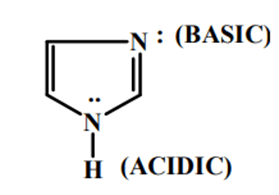Research
, Volume: 19( 1)Imidazole: Chemistry, Synthesis, Properties, Industrial Applications and Biological and Medicinal Applications.
- *Corresponding Author:
- Sai P Katke,
Department of Chemistry,
University of Mumbai,
Mumbai,
India,
E-mail: sai.katke99@gmail.com
Received: 24 November, 2022; Manuscript No. TSES-23-85413; Editor assigned: 29 November, 2022; Pre QC No. TSES-23-85413; Reviewed: 13 December, 2022; QC No. TSES-23-85413; Revised: 03 January, 2023; Manuscript No: TSES-23-85413 (R); Published: 31 January, 2023; DOI: 10.37532/environmental-science.2023.19.1.257
Citation: Katke SP. Imidazole: Chemistry, Synthesis, Properties, Industrial Applications and Biological and Medicinal Applications. Environ Sci Ind J. 19(1): 257
Abstract
Imidazole is a five-membered planar ring that is solvable in water and other polar diluents. In the arena of five membered heterocyclic structures, the imidazole nucleus exhibits a range of properties. Favoured structures have been identified and nitrogen-including heterocyclic structures have been connected to a wide range of biological activities. Imidazole is an entity that has been synthesised in many of its derivative forms over the last few years; the entity has piqued the curiosity of numerous medicinal chemists who want to investigate its many pharmacological potentials. The chemistry of imidazole and its synthetic derivatives is reviewed in this article. A review of the literature on several research papers and other publications that give thorough information on the chemistry of imidazole. As a result, the goal of this article is to examine published research on the introduction, chemistry, synthesis, properties, industrial, biological and medicinal applications with efficacy of imidazole derivatives in order to better understand and explore their various properties and pharmacological potentials.
Keywords
Synthesis; Industrial; Medicinal; Biological; Imidazole; Heterocyclic compounds
Introduction
Heterocycles are one of the most complicated and fascinating disciplines of chemistry, with a wide range of structural variety and economic use as medicinal agents. For more than a century, heterocycles have provided significant benefits to medication discovery from a biologic and manufacturing standpoint, as good as to the knowledge of life procedures and attempts to improve the quality of life. Among the many heterocycles, nitrogen-comprising heterocyclic molecules have piqued the curiosity of scholars during periods of organic synthesis advancement. The imidazole ring is a five-membered ring structure with 3C and 2N atoms, with N present in the first and third positions and containing a hydrogen attachment sphere and an electron contributor nitrogen system [1].
Imidazole-containing medicines have a greater reach in clinical medicine for treating a variety of conditions. Heinrich Debus synthesised imidazole for the first time in 1858, but several imidazole products had been found as young as the 1840’s. To create imidazole, he employed glyoxal and formaldehyde in ammonia. Imidazole possesses anticancer, b-lactamase inhibitors, carboxypeptidase inhibitors, hem oxygenase inhibitors, antiaging agents, decoagulants, antibacterial, fungicidal, antiviral, antitubercular, antidiabetic, along with antimalarial activities, among others.
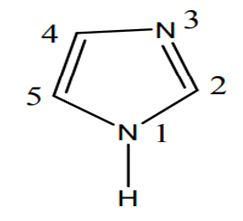
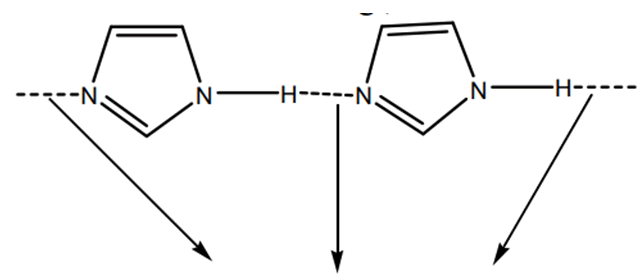
Imidazole is an extremely polar fragment with an estimated dipole of 3.61D that is entirely solvable in water [2]. The existence of a sextet of electrons, involving of two electrons from the protonated nitrogen atom and one from each of the outstanding four molecules in the ring, organises the chemical as aromatic. Imidazole is amphiprotic, which means it may behave as an acid as well as a base. The primary location is N-3 (FIG. 1).
Imidazole is observed in a variety of key genetic compounds. The most prevalent amino acid is "histidine," which has an imidazole on the side chain [3]. Histidine is found in many proteins and enzymes and is required for the form and binding capabilities of haemoglobin. Histidine may be decarboxylated to become histamine, another ubiquitous biological molecule. Imidazole is used in the decontamination of His-tagged proteins using restrained Metal Attraction Chromatography (IMAC).
Materials and Methods
Imidazole is a component of theophylline, which is present in tea leaves and coffee beans and activates the central nervous system. Apart from its usage in pharmaceuticals, imidazole has a variety of industrial uses [4]. It has been significantly utilised as a rust inhibitor on certain change metallic element, such as copper. Copper corrosion prevention is critical, especially in aquatic methods where copper conduction declines owing to corrosion. Many commercial and technologically important chemicals include imidazole products. The thermostable Polybenzimidazole (PBI) is a fire retardant that comprises imidazole fused to a benzene ring and connected to benzene. Imidazole can also be obtained in a type of substances used in photography and electronics [5].
Chemistry of imidazole
Imidazole is a chemical with the method C3N2H4. It is a white or colourless substance that evaporates in water to form a moderately basic mixture. It is an aromatic heterocycle categorised as a di-azole in chemistry, with non-adjacent nitrogen molecules in meta-substitution. The imidazole ring is found in many organic compounds, particularly alkaloids. This imidazole has the same 1,3-C3N2 ring but different substituents. This ring structure is found in several critical biological developing components, including histidine and the associated hormone histamine. Various medications, including antifungals, antibiotics in the nitroimidazole group, and the sedative midazolam, consist of an imidazole ring [6].
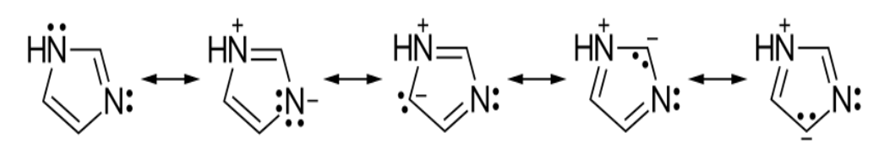
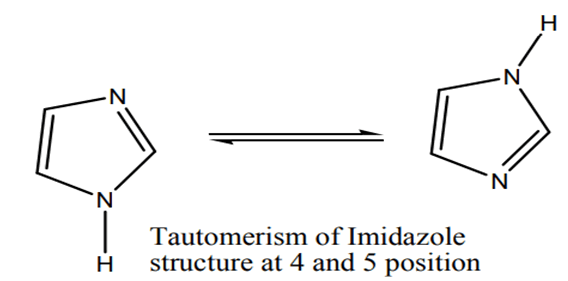
Imidazole is a planar 5-membered ring with two parallel tautomeric types due to hydrogen binding to one or both nitrogen atoms. Imidazole is a highly polar chemical with a dipole moment of 3.67 D and is very solvable in water. The existence of a planar ring containing 6 -π electrons classifies the chemical as aromatic (FIG. 2). The following are some imidazole resonance structures:
Imidazole is amphiprotic, which means it can act as together an acid and a base. Imidazole has a pKa of 14.5, making it somewhat a lesser amount of acidic than carboxylic acids, phenols, as well as imides but marginally more acidic than alcohols. The proton that is linked to nitrogen is the acidic proton. Deprotonation produces the symmetrical imidazolide anion [7]. The conjugate acid's pKa as a base (referred to as pKBH+ to avoid misunderstanding) is around 7, this makes imidazole roughly sixty times more basic than pyridine. The nitrogen with the lone pair is the fundamental site. Protonation produces the symmetrical imidazolium cation [8].
Synthesis of imidazole
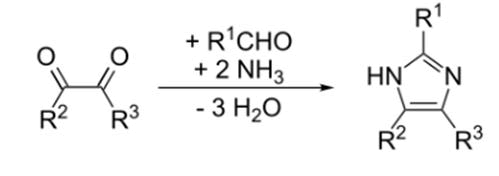
Imidazole was initially published in 1858 by the scientist Heinrich Debus, while several imidazole products had been identified as early as the 1840’s [9]. It was discovered that glyoxal, formaldehyde, and ammonia concentrate to generate imidazole (glyoxaline, as it was formerly named). Despite its poor yields, this procedure is still utilised to generate C-substituted imidazole (FIG. 3).
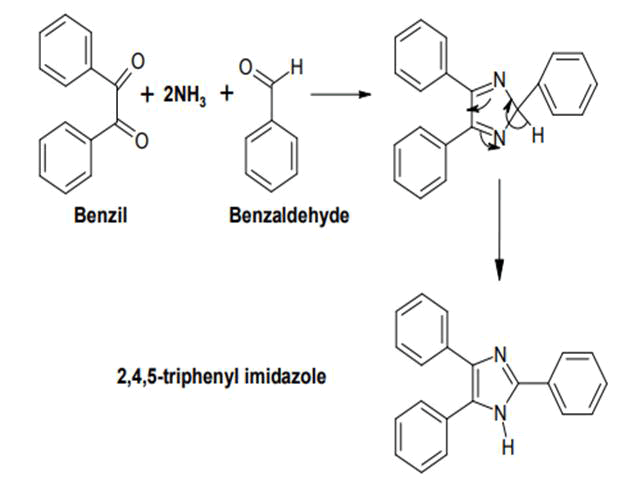
In one microwave alteration, the reactants are benzil, benzaldehyde and ammonia in glacial acetic acid, yielding 2,4,5-triphenylimidazole ("lophine"). Aside from the Debus technique, there are several more ways to manufacture imidazole. By altering the functional groups on the reactants, many of these amalgamations may also be utilized to other substituted imidazole and imidazole derivatives. These approaches are generally classified based on which and how many bonds are formed to generate the imidazole rings [10].
Results and Discussion
Numerous methodologies are available for synthesis of imidazole as, radiszewski production, dehydrogenation of imidazolines, after alpha halo ketones, wallach production, etc.
Radiszewski synthesis
It is produced by compressing a dicarbonyl chemical such as glyoxal, a-keto aldehyde, or a- diketones in conjunction with an aldehyde in the existence of ammonia, for example, benzyl with benzaldehyde and two molecules of ammonia. Formamide is frequently used as a replacement for ammonia (FIG. 4).
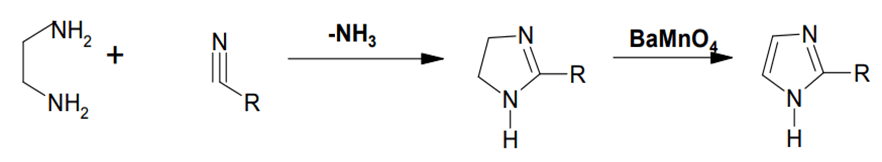
Imidazoline dehydrogenation
Knapp and colleagues described the use of a gentler component, barium manganate, in the transition of imidazolines to imidazole in the presence of sulphur. On reaction with BaMnO4, imidazolines derived from alkyl nitriles and 1,2 ethane diamines give 2-substituted imidazole (FIG. 5).
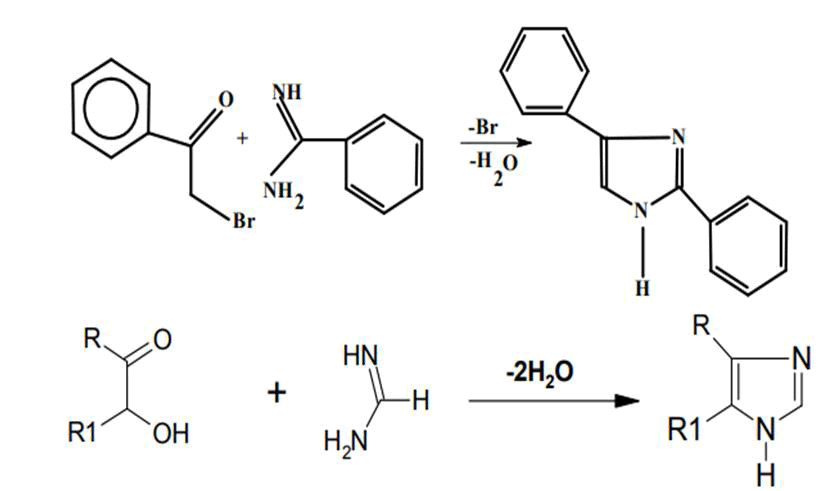
By alpha-halo ketone
This effect includes an imidine interacting with alpha-halo ketones. This approach has remained used effectively to synthesise 2,4- or 2,5-biphenyl imidazole, phenacyl bromide, as well as benzamidine. It also yields 2,4-diphenyl imidazole. Similarly, imidazole is formed when amidine interacts with acyloin or alpha halo ketones (FIG. 6).
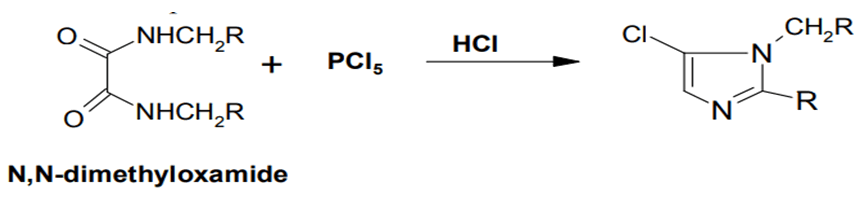
Synthesis of wallach
While N, N′ -dimethyl oxamide is considered with phosphorus pentachloride, a chlorine-including molecule is formed, which may then be reduced with hydroiodic acid to get N-methyl imidazole. Under the similar conditions, N, N′-diethyl oxamide is transformed to a chlorine molecule, which after reduced yields 1-ethyl-2-methyl imidazole. The chlorine chemical has have being identified as 5- chloral imidazole (FIG. 7).
Properties of imidazole
Reactivity: Imidazole has qualities that are comparable to both pyrrole and pyridine. Because it is part of the aromatic sextet, the electrophilic component would strike the unshared electron pair on N-3 but not that on the 'pyrrole' nitrogen. While the imidazole ring is vulnerable to electrophilic attack on an annulated carbon, it is much less likely to be involved in a nucleophilic substitution process unless the ring contains other strongly electron revoking substituents. In the deficiency of such triggering, C-2 is the site most vulnerable to nucleophilic assault. In benzimidazoles, the fused benzene ring offers enough electron with drawl to accept a variation of nucleophilic substitution consequences at C-2 (FIG. 8).
The total reactivity of imidazole and benzimidazole is mentioned to by sets of resonance form in which the dipolar donors have a limited role. These anticipate electrophilic assault in imidazole at N-3 or any ring carbon molecule, nucleophilic attack at C-2 or C-1, as well as the molecule's amphoteric character. The nucleophilic attack at C-2 is expected in benzimidazole. When compared to the neutral molecule, the sense of benzimidazole ion at the C-2 site with nucleophiles is developed.
Good water propllent
Imidazole have also been discovered to be effective water propellants. Imidazole, either as a set free base or as an aqueous scattering of the acid, imparts water resistance to surfaces such as timber, solid, polymers, and alloys.
Good lubricity
Imidazole is also lubricious in the formation of complexes with bentonites. Imidazole oleic acid table salts are effective lubricants. Furthermore, imidazole may be corresponding with aqueous systems. The features of film formation and corrosion inhibition lend themselves to a wide range of industrial applications. For such functions, metallic facades are treated with acid salts of imidazole. Amido-ethyl imidazole is more resistant to corrosion throughout the manufacturing process.ss
Physical characters
It is a colourless fluid with a higher boiling point of 256°C than any additional 5-membered heterocyclic mixtures due to intermolecular H-bonding and molecule linear connection (FIG. 9).
In dioxane, imidazole has a significant dipole moment of 4.8 D. Imidazole possesses amphoteric characteristics and a higher pKa than pyrazole and pyridine. Imidazole are aromatic compounds with a reverberation value of 14.2 KCal/mol, which is about half that of pyrazole. In imidazole, electrophilic substitution is common, and nucleophilic substitution occurs when an electron vacating group is present in the nucleus. Imidazole have M. pt. 90°C, are a weak base, and are tautomeric, as positions 4 and 5 are equivalent (FIG. 10).
Its spectroscopic properties are 207 nm max, I.R.=1550, 1492, 1451(cm-1)=2.30, 2.86, mass spectroscopy is examined in depth for heterocyclic complexes having one heteroatom, but not for those containing two or more heteroatoms.
Applications of industrial purpose
Imidazole has long been used as a corrosion inhibitor on changeover metallic element such as copper. Copper corrosion prevention is critical, especially in aquatic systems where copper conductivity declines owing to corrosion.
Many important industrial and technical chemicals include imidazole. The thermostable polybenzimidazole PBI is a fire retardant that comprises imidazole melded to a benzene ring and bonded to a benzene. Imidazole can also be obtained in a variety of chemicals used in photography and electronics.
Imidazole the situation has few explicit functions. It is instead a pioneer to a kind of agrichemicals, including enilconazole, climbazole, clotrimazole, prochloraz, as well as bifonazole.
Bleach activator
Gosselink Eeugene P, et al. investigated compounds containing one quaternary ammonium group, such as 1,4-[R(CH2)5N+ Me2CH2]2C6H4 2Cl, [R-p-C6H4 CH2 N+Me2(CH2)3]82Cl- or [R-p-C6H4 CH2 N+Me2(CH2)3]82Cl- or [R-p- 5R'Cl- (R=2-oxo-1-azacyclohept-1-yl carbonyl) is employed as an H2O2 source in bleaching formulations, laundry shampoos, automated dish water detergents, and other applications. The activators provide favourable rates of per hydrolysis and diacyl peroxide production.
Mildness to eyes and skin
Pomares et al. created and tested imidazoline-based surfactants on the eyes combined with skin. Surfactant compositions containing imidazolinium compounds were shown to be non-irritating to the eyes and skin. Kubo and colleagues created amido/amino acid surfactants for shampoos and conditioners. They employed a hydrolysed creation of 268 gm of 1-hydroxyethyl-2-undeculimidazoline for this purpose, which was shown to have high detergency with little irritability to the eyes and skin.
Thickeners for oil and grease
Because imidazole forms compounds with bentonites, it can be utilised as a thickening/gelling agent in paraffin wax and paint systems.
Application of paints
The addition of imidazole to paint is known to increase paint adherence even on damp surfaces. Water resistance has also been enhanced.
Corrosion inhibitor
Imidazole is frequently employed as a corrosion inhibitor in the oil sector, although its characteristics and behaviour in such a complex environment are not well understood from a scientific standpoint. Because of their low concentrations and operation in complex environments, the instrument by which these mixtures prevent corrosion is not completely understood, and their obviously good presentation has only recently been supported by experimental indication and investigated by molecular modelling techniques. For the same reasons, quantifying them in generated water is very challenging and has only lately been explored.
Biological and medicinal significance and applications
Imidazole is included in a variety of key biologic molecules. The amino acid histidine, which possesses an imidazole side chain, is the best common. Histidine is uncovered in numerous proteins and enzymes, such as haemoglobin, where it is bound by metal cofactors. Imidazole-based histidine molecules are essential for intracellular buffering. Histamine can be formed by decarboxylating histidine. When histamine is released during an allergic reaction, it can induce urticaria (hives). Many medications include imidazole substitutes. Many fungicides, fungicidal, antiprotozoal and antihypertensive medicines include synthetic imidazole.
Imidazole is a component of the theophylline molecule, which is present in tea and coffee beans and activates the central nervous system. Mercaptopurine, an anticancer drug that works by interfering with DNA functions, contains it. Clotrimazole and other substituted imidazole are discriminatory inhibitors of nitric oxide synthase, making them promise therapeutic targets in inflammation, neurological disorders, and nervous system malignancies. Other organic effects of the imidazole pharmacophore include inhibition of translation initiation and downregulation of intracellular Ca2+ and K+ fluxes. Many systemic fungal diseases benefit from the use of substituted imidazole derivatives. Imidazole is an azole antifungal, which also contains ketoconazole, miconazole and clotrimazole.
Role of imidazole in diabetes mellitus
Imidazole's role in diabetic mellitus Diabetes mellitus, or diabetes, is a metabolic condition that develops when your blood glucose levels are too high, resulting in high blood sugar. It is most likely one of the earliest illnesses known to man, having been originally recorded in an Egyptian book some 3000 years ago.
Mehdi Adib developed and synthesised a series of fused carbazole-imidazole composites 6a-w and tested them for -glucosidase inhibitory activity. The inhibition experiment demonstrated that all the produced compounds were more powerful than the standard medication acarbose. Among the series, compound (IC50=74.0 0.7 M) was discovered to be the most potent inhibitor, having 10-fold more inhibitory action than the conventional medication (FIG. 11).
Anti-depressant action
Moclobemide is an antidepressant that is a discriminatory and flexible monoamine oxidase-A inhibitor. Hadizadeh created moclobemide derivatives by substituting the parachloro phenyl group of moclobemide and tested them for antidepressant effect in mice using a forced swimming test. Among the synthetic series, compounds were discovered to be more powerful than moclobemide (FIG. 12).
Anthelmintic imidazole
Imidazole was discovered to be less sensitive in intestinal parasites. Various genus of nematodes and trematodes are removed from different masses by class (2-alkyl benzimidazole) types. Compounds exhibit great action against nematodes and have been shown to kill many kinds of intestinal worms. It was also discovered to have action against both human and animal cestodiasis. Mebendazole, a benzimadiazole medication 12, is used to treat patients with T. Solium and T. Saginata at a dosage of 100 mg/kg (FIG. 13).
Antibacterial properties
N. Gupta developed and tested a variety of N-substituted imidazole pharma cores for antibacterial activity. Molecule 4 was discovered to be the most active antibacterial compound among the produced derivatives (FIG. 14).
Anti-fungal imidazole
In recent years, the hunt for novel antifungal agents has focused mostly on the imidazole and triazole chemistry. The azoles are a class of drugs that include a variety of 1-substituted imidazole. Imidazole has strong antifungal pharmacological and biological properties. Because of poor absorption and substantial first pass metabolism, lipophilic imidazole such as clotrimazole (I), econazole (II), and miconazole (III) displayed weak complete accessibility at the back of verbal administration, limiting their application to contemporary therapy of shallow fungal infection. Ketoconazole (IV), a more polar imidazole that was put into treatment in the late 1970’s, represented a breakthrough in antifungal illness medicine (FIG. 15).
Conclusion
The imidazole moiety has received the greatest attention; several of its analogues are efficacious against a variety of clinical disorders, which are briefly covered in this article. Imidazole has a distinct niche in the field of therapeutic chemistry. Imidazole has a broad range of therapeutic products. Numerous techniques for synthesising imidazole have been devised and their diverse structure-activity relationships provide significant potential in the field of medicinal chemistry. As a result, the resolution of this article is to scrutinize the research that has stood published on the introduction, its chemistry, synthesis, properties, industrial, biological and medicinal applications with efficacy of imidazole derivatives to better understand and explore their various properties and pharmacological potentials.
Various compounds have been produced, and their efficacy is being tested therapeutically. A full evaluation of such substances is available, thanks to peer review and published research publications. Thus, imidazole is a component that has been used in the past to synthesise many compounds with diverse pharmacological properties and it can still be used in the future against various
pathological diseases and other applications. Imidazole was produced with high yields using an improved technique and was
discovered to have several industrial uses. Furthermore, the chemical structure of imidazole compounds lends itself to a wide range
of commercial uses.
Declarations
Statement of ethics
The author created all authentic work and presentations.
Statement of consent
I agree to have my research work published in the journal.
References
- Kerru N, Bhaskaruni SV, Gummidi L, et al. Recent advances in heterogeneous catalysts for the synthesis of imidazole derivatives. Synthetic Commun. 2019;49(19):2437-2459.
[Crossref] [Googlescholar] [Indexed]
- Daraji DG, Prajapati NP, Patel HD. Synthesis and applications of 2‐substituted imidazole and its derivatives: A review. J Heterocyclic Chem. 2019;56(9):2299-2317.
[Crossref] [Googlescholar] [Indexed]
- Henary M, Kananda C, Rotolo L, et al. Benefits and applications of microwave-assisted synthesis of nitrogen containing heterocycles in medicinal chemistry. RSC Advances. 2020;10(24):14170-14197.
[Crossref] [Googlescholar] [Indexed]
- Kumar P, Bansal V, Paul AK, et al. Biological applications of zinc imidazole framework through protein encapsulation. Appl Nanosci. 2015;7(6):951-957.
[Crossref] [Googlescholar] [Indexed]
- Nieri P, Carpi S, Fogli S, et al. Cholinesterase-like organocatalysis by imidazole and imidazole-bearing molecules. Scientific Reports. 2017;8:45760.
[Crossref] [Googlescholar] [Indexed]
- Kazemi M. Reusable nanomagnetic catalysts in synthesis of imidazole scaffolds. Synthetic Commun. 2020;50(14):2095-2113.
[Crossref] [Googlescholar] [Indexed]
- Yang PB, Davidson MG, Edler KJ, et al. Synthesis, properties and applications of bio-based cyclic aliphatic polyesters. Biomacromolecules. 2021;22(9):3649-3667.
[Crossref] [Googlescholar] [Indexed]
- Kumar G, Mogha NK, Kumar M, et al. NiO nanocomposites/rGO as a heterogeneous catalyst for imidazole scaffolds with applications in inhibiting the DNA binding activity. Dalton Transactions. 2020;49(6):1963-1974.
[Crossref] [Googlescholar] [Indexed]
- Al Kobaisi M, Bhosale SV, Latham K, et al. Functional naphthalene diimides: Synthesis, properties and applications. Chem Rev. 2016;116(19):11685-11796.
[Crossref] [Googlescholar] [Indexed]
- Arshad N, Rafiq M, Ujan R, et al. Synthesis, X-ray crystal structure elucidation and Hirshfeld surface analysis of N-((4-(1 H-benzo [d] imidazole-2-yl) phenyl) carbamothioyl) benzamide: Investigations for elastase inhibition, antioxidant and DNA binding potentials fors biological applications. RSC Advances. 2020;10(35):20837-20851.
[Crossref] [Googlescholar] [Indexed]