Research
, Volume: 19( 1)A Theoretical Analysis of a 1,3-Oxazole Derivative used as an Antifungal Active Agent that Varies with Ambient Temperature
- *Correspondence:
- Sai P Katke
Department of Chemistry, University of Mumbai, Mumbai, Maharashtra, India
Tel: 9420802742
E-mail: sai.katke99@gmail.com
Received: November 24, 2022, Manuscript No. TSES-22-81105; Editor assigned: November 28, 2022, PreQC No. TSES-22-81105 (PQ); Reviewed: December 12, 2022, QC No. TSES-22-81105; Revised: February 22, 2023, Manuscript No. TSES-22-81105 (R); Published: February 28, 2023, DOI: 10.37532/0974-7451.2023.19(1).264
Citation: Katke SP. A Theoretical Analysis of a 1,3 Oxazole Derivative Used As an Antifungal Active Agent That Varies With Ambient Temperature. Environ Sci Ind J. 2023;19 (1):264.
Abstract
Understanding the impacts of antifungal activities, prevention via oxazole derivatives with beneficial medical value in modern life. Oxazole is a five membered heterocyclic ring that has been studied in the synthesis of new compounds with intriguing medicinal properties. As a result, several researchers produced these compounds as target structures and tested them for biological activity. In this hypothetical research, we describe the theoretical interaction of 1, 3-oxazole as an antifungal agent at ambient temperature for survival, growth and substantial reduction estimate.
Keywords
1,3-Oxazole; Temperature; Anti-fungal; Heterocyclic compound; Treatments
Introduction
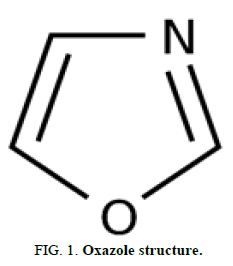
Heterocycles are a very significant type of chemicals. In fact, heterocycles account for more than half among all known chemical molecules. Many naturally occurring medicines are heterocyclic in composition. Many synthesized medications are heterocycles as well. Heterocyclic compounds are fundamental to the chemicals that enable life to exist. Heterocycles have been investigated for use in the development of pharmaceutically significant compounds. Because hundreds of azole derivatives are utilized as medications today, there has been a continuing interest in the chemistry of azoles in recent decades. Azoles are heterocyclic compounds that have a five membered ring and at least one additional noncarbon element, such as nitrogen, sulphur or oxygen. These aromatic compounds have two double bonds. These are effective and wise in the medicinal field. Oxazole is extensively used heterocyclic chemicals for the prevention of major illnesses, demonstrating antifungal biological activity in research (Figure 1).
The oxazole parental skeleton is found in the class of planar heterocycles (Figure 1).
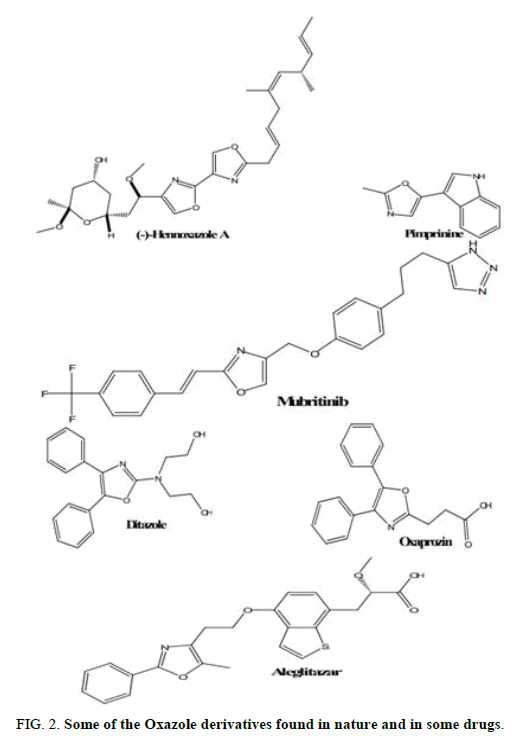
The scaffold of oxazole is unique in its structure and it is a component of various natural compounds with high biological activity, including (-)-hennoxazole a (antiviral) and pimprinine (alkaloid) [1,2]. Oxazole and its derivatives have also been included in a variety of medicinally relevant molecules, both as exploratory and advanced therapeutic candidates. Oxazole containing drugs have been used to treat diabetes II (e.g. Aleglitazar, platelet aggregation, diazole) as part of tyrosine kinase inhibitors like mubritinib and as COX-2 inhibitors like oxaprozin (Figure 2) [3-6].
This study assesses the pharmacological efficacy of oxazole derivatives as antipathogenic agents that have recently been discovered in medicinal chemistry investigations.
Antifungal: An antifungal medicine, also known as an antimitotic medication, is a fungicide or fungi static that is used to treat and prevent mycosis such as athlete's foot, ringworm, candidiasis (thrush), dangerous systemic infections such as cryptococcal meningitis and others. Only a few azole antifungals are utilized systemically in clinical trials. Ketoconazole, itraconazole, fluconazole, fosfluconazole, voriconazole, posaconazole and isavuconazole are examples [7].
Oxazole compound: Oxazole is the parent compound for a vast class of heterocyclic aromatic organic compounds. These are azoles with oxygen and nitrogen separated by one carbon. Oxazole is aromatic compounds but less so than the thiazoles. Oxazole is a weak base its conjugate acid has a pKa of 0.8 compared to 7 for imidazole.
Materials and Methods
Preparation of oxazole’s
Oxazole derivative synthesis: Oxazole’s are made up of a 5 membered doubly unsaturated ring with one oxygen atom (position 1)separated by one carbon from a nitrogen atom (position 3). The first oxazole was synthesized in the 1800’s and its chemistrywas extended during World War II as part of the penicillin endeavor, which was expected to include an oxazole core. The parentchemical, which is a stable liquid at room temperature and has a boiling point of 69 degrees Celsius, was first created in 1947.
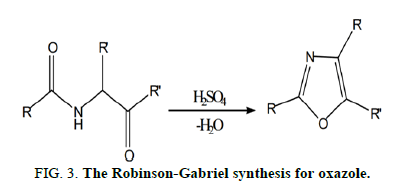
The Robinson-Gabriel synthesis, in which a 2-acylamino-ketone interacts intramolecularly followed by dehydration to yield an oxazole, is one of numerous described processes for the production of oxazole. A cyclodehydrating agent is present to catalyze the reaction (Figure 3).
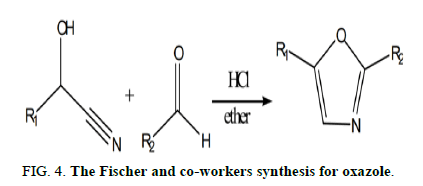
Fischer and colleagues presented an excellent synthesis of oxazole derivatives first published in 1896 by reacting a cyanohydrin with an aldehyde in the presence of anhydrous hydrochloric acid to produce oxazole (Figure 4).
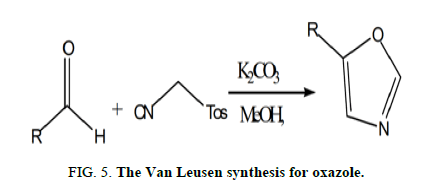
Finally, there was the Van Leusen response, which was initially reported by Van Leusen and colleagues in 1977 [8]. Ketones interacted with TosMIC (Toluenesulfonylmethyl Isocyanide), forming a nitrile. When aldehydes are used, the Van Leusen reaction is very useful for forming oxazole (Figure 5).
Results
Oxazole antifungal activity
Oxazole derivatives are among the most valuable heterocyclic molecules in terms of both synthetic and medicinal chemistry and we have emphasized here the most recent breakthroughs in oxazole synthesis as well as their antifungal activity profile.
Antifungal activity: Fungi are eukaryotic microorganisms that are more closely related to humans at the cellular level than bacteria and can cause a variety of human illnesses.
Despite the recent release of promising antifungal medicines, the frequency of invasive fungal infections has increased considerably during the last two decades. There is a growing demand for new antifungal medicines with unique chemical structures and mechanisms of action.
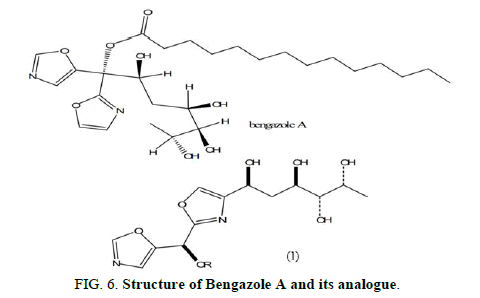
Mulder, et al. synthesized analogues of the powerful antifungal drug bengazole A and tested them against Candida spp. Using microbroth dilution and disc diffusion experiments. Some bengazole A analogues showed good efficacy, such as compound, which included one or two 1,3-oxazole rings, but none were more effective than bengazole A (Figure 6).
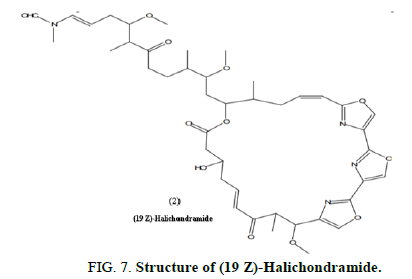
Chung, et al. reported a series of unique oxazole containing macrolides discovered from the marine sponge Chondrosia corticata in 2011 and tested them for actin depolymerizing activity by measuring the fluorescence intensity of pyrene F-actin. These investigations led to the discovery of (19Z)-halichondramide (2) as a novel actin depolymerizing agent. The activity of (19Z)-halichondramide (2) in depolymerizing actin was four times that of halichondramide. Both compounds show significant antifungal activity and early structure activity relationships of these compounds were investigated to determine the important structural requirements (Figure 7).
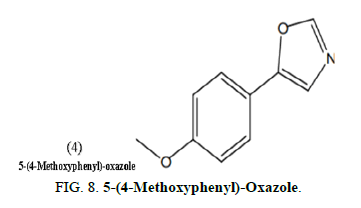
Yamamuro, et al. synthesized and evaluated nineteen 5-(4-Methoxyphenyl)-Oxazole (MPO) variants as a caenorhabditis elegans hatch and growth inhibitor. The structure activity relationship research of the derivatives revealed that the whole structure of MPO (4) is required for anti C. Elegans action, which must be considered while designing antifungal agents (Figure 8).
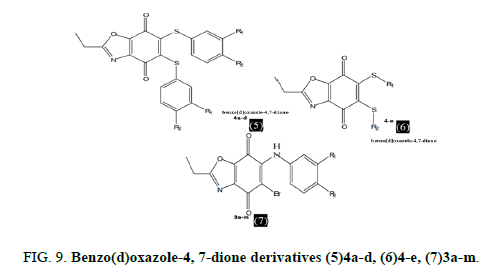
Ryu, et al. produced and evaluated benzo(d)oxazole-4,7-dione derivatives (5(4a-d), 6(4-e) and 7(3a-m) for in vitro antifungal activity. Some compounds showed remarkable antifungal inhibition (MIC 0.8 g/ml) against C. albicans and A. niger. The biological data indicated that benzo(d)oxazole-4,7-diones might be effective antifungal drugs (Figure 9).
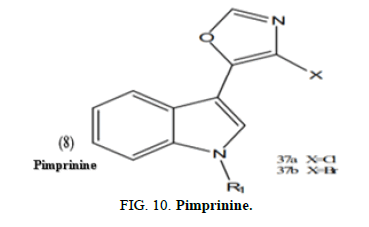
Zhang, et al. created a variety of new analogues of the natural substance pimprinine. A bioassay revealed that some of the produced compounds have fungicidal efficacy. Compounds (8a,b) in particular shown antimicrobial action against the four pathogens tested in artificial medium, namely Pythium dissimile, Alternaria solani, Botryotinia fuckeliana and Gibberella zeae (Figure 10).
Discussion
1,3-Oxazole accompanying anti-fungal activity
Thermotolerant and psychrotolerant fungi: The majority of fungi are mesophiles, meaning they thrive at temperatures ranging from 5°C to 35°C, with optimal growth temperatures between 25°C and 30°C. However, there are fungi that thrive outside of the mesophilic zone. Temperature range; some are cold tolerant (psychrotolerant) and are others are heat tolerant and capable of growing at or below 0°C. Thermotolerant and grow over 40°C. Many thermotolerant funguses thrive. Spanning the majority of the mesophiles spectrum, but some are so well suited to growth in hot environments because they can only develop at quite high temperatures temps. These are the so called thermophiles (heat lovers). Cooney and Emerson established optimal temperatures for growth and in 1964 defined those fungi that cannot grow below 20°C. Similarly, psychrophilic exist among psychrotolerant organisms with low maximum and optimal development temperatures (Table 1).
While dividing fungi into groups based on tolerance of temperature extremes is useful for distinguishing between the physiological behavior of different species, these divisions are rather arbitrary and of course, quite artificial because psychrophilic, mesophiles and so on are all part of a temperature response continuum by fungi.
Fungi that are thermo tolerant and thermophilic: Thermo tolerant and thermophilic fungi are abundant in big, well insulated, wet heaps of plant materials where waste heat from microbial metabolism becomes trapped, leading interior heap temperatures to rise in a self-heating (thermogenesis) process. Garden compost heaps is a popular environment in which heat tolerant fungus grows. Plant waste piled into piles is a simple yet efficient method of increasing temperature is used to hasten degradation and if compost heaps are properly constructed and large enough, peak heating temperatures may easily approach 60°C, giving excellent circumstances for the development of heat tolerant fungus.
| Species | Minimum | Optimum | Maximum |
|---|---|---|---|
| Thermophiles | |||
| Rhizomucor (Mucor) michei | 25 | 42-45 | 57 |
| Rhizomucor (Mucor) pusillus | 20 | 40-45 | 55 |
| Chaetomium thermophile | 27 | 50-52 | 58 |
| Talaromyces thermophiles | 27 | 47.5 | 59 |
| Humicola insolens | 23 | 45-47 | 55 |
| Thermomyces lanuginosus | 30 | 47.5-52 | 60 |
| Thermotolerant | |||
| Aspergillus fumigatus | 12 | 40 | 52 |
| Coprinus cinereus | 13 | - | 45 |
| Neurospora sitophila | 4 | 36 | 44 |
| Mesophiles | |||
| Mucor mucedo | 8 | 20 | 30 |
| Mucor hiemalis | 8 | 20-25 | 35 |
| Mortierella ramanniana | 10 | 23-25 | 35 |
| Penicillium chrysogenum | 4 | 23 | 36 |
| Psychrophilic | |||
| Cylindrocarpon magnusianum | 0 | c.5 | c.15 |
| Mucor strictus | 0 | c.10 | 20 |
| Mucor oblongisporus | 0 | c.10 | 20 |
| Candida scottii | 0 | c.10 | 15-16 |
| Psychrotolerants | |||
| Phacidium infestans | -3 | 15 | 27 |
| Phytophthora infestans | 2 | 18-21 | 26 |
| Cryptococcus sp. | 3 | 20-22 | 25 |
| Chrysosporium pannorum | -5 | 18 | 28 |
| Source: Data mainly from Lilly and Barnett; Di Menna; Hagan and Rose; sumner, Morgan and Evan; Flanagan and Scarborough; Rosenberg; Milivec and Tuite; Hurst and pugh. | |||
TABLE 1. Cardinal temperatures (°C) for growth of some mesophilic, heat tolerant and cold tolerant fungi.
1,3-oxazole is a heterocyclic compound and exhibits a wide variety of pharmacological activities such as analgesics, anti-inflammatory, anti-microbial, antc-ancer, anti-depressants, anti-diabetic and anti-obesity, anti-convulsant, diuretics and anti-cancer.
With the increasing widespread medical usage of oxazole and its derivatives, stated 1,3-oxazole has supplied a beneficial conclusion to lessen the adverse effects and its types. The treatment of oxazole and derivatives was theoretically described in the analysis, with 1,3-oxazole interacting with the fungus and exhibiting an estimative consequence to undertake. When the substance interacts with the fungus, the temperature ranges from 5°C to 35°C, with ideal growth temperatures between 25°C and 30°C.
At specific quantities bengazole A, pyrene F-actin, 5-(4-Methoxyphenyl)-oxazole and benzo(d)oxazole-4,7-dione, pimprinine, and streptochlorine have well included 1,3-oxazole compounds and target fungal activity and ensure it to be used as an anti-fungal medication.
Conclusion
The theoretical establishment of 1,3-oxazole interaction as an anti-fungal agent is well undertaken along its derivatives such as bengazole A, pyrene F-actin, 5-(4-Methoxyphenyl)-oxazole and benzo(d)oxazole-4,7-dione, pimprinine and streptochlorine have acquired astonishing results for this analysis at ambient temperature with effective development and correspondence. With the emergence of new and serious infections, it is necessary to search for novel therapeutics and minimize the impacts of related fungal activity.
Availability of Data and Materials
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Acknowledgements
Special thanks to my parents and Hazarimal Somani college along university of Mumbai.
References
- Kakkar S, Narasimhan B. A comprehensive review on biological activities of oxazole derivatives. BMC Chem. 2019;13(1):1-24.
[Crossref] [Google Scholar] [PubMed]
- Wiley Rh. The chemistry of the oxazole. Chem Rev. 1945;37(3):401-442.
[Crossref] [Google Scholar] [PubMed]
- Bazukyan I, Matevosyan L, Toplaghaltsyan A, et al. Antifungal activity of lactobacilli isolated from Armenian dairy products: An effective strain and its probable nature. AMB Express. 2018;8(1):1-8.
[Crossref] [Google Scholar] [PubMed]
- Yan Z, Liu A, Huang M, et al. Design, synthesis, DFT study and antifungal activity of the derivatives of pyrazolecarboxamide containing thiazole or oxazole ring. Eur J Med Chem. 2018;149:170-181.
[Crossref] [Google Scholar] [PubMed]
- Wiley Rh. The chemistry of the oxazole. Chem Rev. 1945;37(3):401-442.
[Crossref] [Google Scholar] [PubMed]
- Kim SH, Markovitz B, Trovato R, et al. Discovery of a new HIV-1 inhibitor scaffold and synthesis of potential prodrugs of indazoles. Bioorg Med Chem Lett. 2013;23(10):2888-2892.
[Crossref] [Google Scholar] [PubMed]
- Stokes NR, Baker N, Bennett JM, et al. Design, synthesis and structure activity relationships of substituted oxazole benzamide antibacterial inhibitors of FtsZ. Bioorg Med Chem Lett. 2014;24(1):353-359.
[Crossref] [Google Scholar] [PubMed]
- Liu XH, Zhu J, Zhou AN, et al. Synthesis, structure and antibacterial activity of new 2-(1-(2-(substituted-phenyl)-5-methyloxazol-4-Yl)-3-(2-substitued-phenyl)-4,5-dihydro-1h-pyrazol-5-Yl)-7-substitued-1,2,3,4-tetrahydroisoquinoline derivatives. Bioorg Med Chem. 2009;17(3):1207-1213.
[Crossref] [Google Scholar] [PubMed]