Original Article
, Volume: 13( 2)Tyrosinase Inhibitory and Antioxidant Activity by Bromophenols from the Alga Odonthalia corymbifera
- *Correspondence:
- Kurihara H, Faculty of Fisheries Sciences, Hokkaido University, Minato, Hakodate, Hokkaido 041-8611, Japan, Tel: +81 138 40 5561; Fax: +81 138 40 5561; E-mail: kuri@fish.hokudai.ac.jp
Received: August 05, 2017; Accepted: August 31, 2017; Published: September 06, 2017
Citation: Islam MR, Mikami D, Kurihara H. Tyrosinase Inhibitory and Antioxidant Activity by Bromophenols from the Alga Odonthalia corymbifera. Nat Prod Ind J. 2017;13(2):110
Abstract
In the course of our search for tyrosinase inhibitors and antioxidants, six known bromophenol dimers were purified from methanol extract of the red alga Odonthalia corymbifera. The compounds were identified by comparison with published spectroscopic data. These bromophenols were categorized into symmetric and asymmetric dimers. Among them, the tetrabrominated dimers displayed more potent tyrosinase inhibition than the tribrominated ones. Especially, the asymmetric tetrabrominated compound showed strong inhibition. These results suggest that number of bromine substitution and orientation of bromine and phenolic hydroxy groups are important factors of tyrosinase inhibitory potency. The bromophenols were also investigated for antioxidant activities by using DPPH and ABTS radical scavenging, CUPRAC and FRAP metal reducing and copper chelation assays. All dimers showed comparable antioxidant activities to the positive controls examined. Symmetric dimers displayed relatively higher antioxidant activities than asymmetric ones.
Keywords
Bromophenol; Tyrosinase; Inhibition; Antioxidant
Introduction
Marine algae, commonly used as foodstuffs in East Asian countries [1], are also important sources of bioactive secondary metabolites such as terpenoids, polyphenols and halogenated compounds [2]. Red algae of the family Rhodomelaceace are enriched with bromophenols which exhibit radical scavenging [3], enzyme inhibition [4-8], feeding deterrent [9], anti-inflammatory [10], cell protection [11], antimicrobial [12], anticancer [13], anti-diabetic [14], and antiviral activity [15].
Tyrosinase (EC 1.14.18.1), one of polyphenol oxidases, is a key enzyme for melanin biosynthesis. The enzyme mediates both hydroxylation of monophenols and oxidation of o-diphenols in the earlier steps of melanin formation related to mamalian melanogenesis, fruit browning, and prawn and crab blackening [16]. However, its excessive production is led to hyperpigmentation [17]. In addition, free radical or reactive oxygen species (ROS) may induce α-melanocyte-stimulating hormone (α-MSH) resulting abnormal pigmentation such as age spot, freckles, skin aging, melasma [18]. Many natural tyrosinase inhibitors have been reported as polyphenols and lipids [16].
Free radicals are continuously generated in human body and cause to deteriorate lipids, proteins, enzymes, DNA and RNA. They may lead to many diseases such as atherosclerosis, cardiovascular diseases and carcinogenesis [3]. Although synthetic antioxidants are commonly used to prevent these problems, their uses are critical for their toxic and carcinogenic effect [19]. Natural antioxidants without any side effects are desired to radical scavenging activity and protection against oxidative damage [20].
In the course of our search for tyrosinase inhibitor and antioxidant, naturally occurring bromophenols were isolated from the marine red alga Odonthalia corymbifera (S. G. Gmelin) Greville (Rhodomelaceae). The bromophenols were investigated for comparison of tyrosinase inhibitory and antioxidant activities.
Materials and Methods
General experimental procedures
NMR spectra were recorded on a Bruker AMX-500 (Karlsruhe, Germany) NMR spectrometer at 500 MHz for proton and 125 MHz for carbon in (CD3)2CO or CD3OD. Field desorption-MS (FD-MS) spectra were recorded on a JEOL JMS-T100GCV mass spectrometer (Tokyo, Japan). High performance liquid chromatography (HPLC) was performed using SHIMADZU LC-10ATvp apparatus (Kyoto, Japan) equipped with a diode array detector SHIMADZU SPD-M10Avp and RP HPLC column (Mightysil® RP-18, Kanto Chemical Co. Inc. Tokyo, Japan). Silica gel (Chromatorex®, Fuji Silysia, Japan), reversed-phase (RP-18) silica gel (Nacalai Tesque Inc. Kyoto, Japan) and Sephadex® LH-20 (GE Healthcare, Uppsala, Sweden) were used for column chromatography (CC). Thin layer chromatography (TLC) was performed on glass plate with precoated silica gel (60 F254, RP-18 F254 Merck, Darmstadt, Germany). TLC spots were visualized under UV lamp or by spraying with 5% H2SO4. Mushroom tyrosinase (EC 1.14.18.1), 2, 2´-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), 2, 4, 6-tris (2-pyridyl)-s-triazine (TPTZ), and 2, 2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Kojic acid was available from Tokyo Chemical Industry (Tokyo, Japan). Ethylenediamine-N, N, N’, N’-tetraacetic acid (EDTA) disodium salt was available from Dojindo Laboratories (Kumamoto, Japan). Catechol and 2 (3)-tert-butyl-4-hydroxyanisole (BHA) were purchased from Wako Pure Chemical Industries (Kyoto, Japan).
Alga material
The marine red alga Odonthalia corymbifera (Rhodomelaceae) was collected at the coast of Hakodate city, Japan in May, 2016. The species identification was done by one of the author (H. Kurihara), supervised by Professor H. Mizuta (Faculty of Fisheries Sciences, Hokkaido University, Japan), and voucher specimen was deposited in our laboratory.
Extraction and isolation
Collected alga was washed with tap water and cut into small pieces. O. corymbifera (1.8 kg) was extracted with 95% methanol (MeOH) and concentrated (3 days, twice). The extracts were partitioned into n-hexane, ethyl acetate (EtOAc), n-butanol and water-soluble fractions. The EtOAc soluble fraction (19.5 g) of O. corymbifera was chromatographed over silica gel, RP-18 column chromatography and Sephadex LH-20. Final purification was performed by preparative TLC (developing solvent; toluene/EtOAc/acetic acid, 10:10:1, v/v/v) or RP-18 HPLC (eluted with 70% aqueous MeOH) to obtain compound 1 (10.8 mg), 2 (4.2 mg), 3 (6.3 mg), 4 (10.5 mg), 5 (3.0 mg), and 6 (1.5 mg).
Tyrosinase inhibition assay
Sample solution (15 µl) was added into 50 mM sodium phosphate buffer (780 µl, pH 6.8) in test tube, followed by addition of 0.1 mg/ml L-tyrosine (0.5 ml) as substrate. Enzymatic reaction was started after adding 200 U/ml mushroom tyrosinase solution (205 µl). Reaction solution was incubated at 25°C for 30 min and absorbance was measured at 490 nm. Kojic acid was used as a positive control. The IC50 value was expressed as sample concentration which can inhibit fifty percentage of tyrosinase reaction. Kinetic study was performed as same as tyrosinase inhibition assay except reaction mixture consist of different concentrations of L-tyrosine in buffer and test sample in methanol. Lineweaver-Burk and Dixon plot analysis was used to determine inhibition type and inhibitor constant [21].
DPPH radical scavenging assay
Tested sample solution (50 μl) in MeOH was added to 40 μg/ml DPPH solution (950 μl) in MeOH in a test tube and mixed vigorously. In the dark, the mixture was left for 30 min and measured absorbance at 517 nm. Catechol and BHA were used as positive control. The EC50 value was expressed as sample concentration which can quench fitty percentage of DPPH free radicals [22].
ABTS radical scavenging assay
ABTS radical cation solution was prepared by adding 2.45 mM (final concentration) potassium persulfate to 7 mM ABTS in water and kept overnight in dark place. Before assay, the cation solution was diluted with ethanol to absorbance of 0.70 at 734 nm. Tested sample solution (10 μl) was added to the ABTS radical solution (1.0 ml). The mixture was left at room temperature for 10 min and absorbance was recorded at 734 nm. The EC50 value was expressed as sample concentration which can quench fifty percentage of ABTS radicals [23].
Cupric reducing antioxidant capacity (CUPRAC) assay
Tested sample solution (0.1 ml) was added to the premixed reaction mixture containing 10 mM CuCl2 solution (0.25 ml), 7.5 mM ethanolic neocuproine solution (0.25 ml), and 1 M ammonium acetate buffer solution (0.25 ml, pH 7.0) in each tube. The mixture was incubated for 30 min at room temperature, and then absorbance was measured at 450 nm. ECA0.50 means sample concentration with absorbance of 0.50 (A0.50) [24].
Ferric reducing antioxidant power (FRAP) assay
Each tube contained freshly prepared FRAP reagent by mixing 300 mM sodium acetate buffer (750 μl, pH 3.6), 10 mM TPTZ in 40 mM HCl (75 μl) and of 20 mM FeCl3.6H2O (75 μl). Then sample solution (30 μl) was added along with water (100 μl) to the premixed FRAP reagent. The mixture was left for 4 min at room temperature and absorbance was measured at 593 nm. ECA0.50 means sample concentration with absorbance of 0.50 (A0.50) [25].
Cupric ion chelation assay
Cupric ion chelation ability was assessed according to the method of Santos et al. [26] with slight modification. Sample solution (120 μl) was mixed with 50 mM sodium acetate buffer (800 μl, pH 6.0). Then, 100 mg/l CuSO4.5H2O solution (120 μl), 2 mM pyrocatechol violet solution (34 μl) were added to reaction mixture and kept for 2 min. The mixture was shaken for 10 min and further incubated at 25°C for another 10 min. Absorbance was measured at 632 nm and EDTA-Na2 was used as positive control. The EC50 value was expressed as sample concentration which can chelate fitty percentage of Cu2+ ion [26].
Results and Discussion
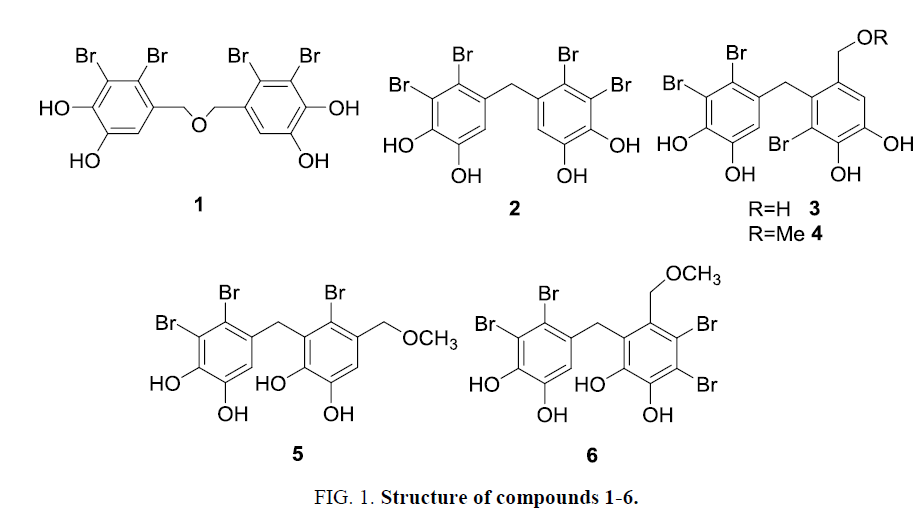
Six known bromophenols 1-6 were isolated from O. corymbifera and elucidated their structures on the basis of MS and NMR data (Figure 1). They were identified with comparison of reported spectroscopic data [4,9,13,27-29]. These bromophenols divided into symmetric (1 and 2) and asymmetric dimers (3-6). The isolated bromophenols were investigated for various activities such as antioxidant and enzyme inhibition.
In tyrosinase inhibition assay, compound 6 showed the most potent inhibitor (IC50=1.0 µM) among the bromophenols examined and the positive control kojic acid. Other two potent inhibitors were compounds 1 and 2 with IC50 values of 5.2 µM and 11.0 µM, respectively. These compounds possess two 2, 3-dibromo-4, 5-dihydroxylbenzyl moieties in their structures. All the bromophenols 1-6 possess the catechol moiety that behaves as a copper chelator of tyrosinase [18]. However compound 6 showed weak Cu-chelating potency (Table 1) than compounds 1 and 2. Thus tyrosinase inhibition by bromophenol might be influenced by other mechanism different from Cu-chelation. Multibrominated compounds displayed higher protein tyrosine phosphatase inhibitory activity than monobrominated compounds [30,31]. Compounds 1, 2 and 6 also displayed inhibitory activity against protein tyrosine kinase and significant cytotoxicity against human cancer cell [13,32]. Thus the two 2, 3-dibromo-4, 5-dihydroxylbenzyl moieties are seemed to be important structures for tyrosinase inhibition, compared with tribrominated ones. Although the reason why the asymmetric tetrabrominated compound 6 showed strong inhibition is still unclear. These results suggest that both number of bromine substitution and orientation of bromine and phenolic hydroxy groups are important factors of tyrosinase inhibitory potency. Among the bromophenols investigated, only compound 1 was examined for kinetic analysis to be non-competitive inhibition and a Ki value of 2.4 µM. This suggests that inhibitor 1 at least may bind other site of active site and combine with either free enzyme or enzyme-substrate complex [33,34] like prenylated flavonoids as tyrosinase inhibitors [18].
| -- | Tyrosinase inhibition | DPPH | ABTS | CUPRAC | FRAP | Cu2+-chelation |
|---|---|---|---|---|---|---|
| Compound | IC50 b (mM) | EC50c (mM) | EC50 c (mM) | ECA0.50 d (mM) | ECA0.50 d (mM) | EC50 c (mM) |
| 1 | 5.2 ± 0.0 | 8.7 ± 0.1 | 3.4 ± 0.2 | 5.5 ± 0.2 | 7.1 ± 0.1 | 24.0 ± 0.1 |
| 2 | 11.0 ± 0.1 | 7.3 ± 0.1 | 3.3 ± 0.1 | 5.1 ± 0.1 | 7.3 ± 0.1 | 25.4 ± 0.1 |
| 3 | 50.0 ± 0.1 | 5.0 ± 0.1 | 2.0 ± 0.1 | 5.8 ± 0.1 | 7.2 ± 0.1 | 26.2 ± 0.0 |
| 4 | 39.0 ± 0.0 | 9.8 ± 0.2 | 7.7 ± 0.0 | 6.4 ± 0.1 | 8.0 ± 0.1 | 29.6 ± 0.1 |
| 5 | 39.2 ± 0.0 | 19.6 ± 0.1 | 9.8 ± 0.0 | 10.9 ± 0.1 | 7.8 ± 0.1 | 107.8 ± 0.1 |
| 6 | 1.0 ± 0.1 | 17.0 ± 0.1 | 20.4 ± 0.2 | 9.8 ± 0.1 | 12.9 ± 0.1 | 45.9 ± 0.2 |
| Kojic acid | 35.0 ± 0.0 | -- | -- | -- | -- | -- |
| BHA | -- | 34.0 ± 0.1 | 10.4 ± 0.2 | 16.0 ± 0.1 | 8.3 ± 0.1 | -- |
| Catechol | -- | 16.9 ± 0.1 | 7.3 ± 0.0 | 25.4 ± 0.1 | 9.1 ± 0.1 | -- |
| EDTA | -- | -- | -- | -- | -- | 31.6 ± 0.1 |
aAll values are represented as mean ± SE of triple mesurements
bThe half maximal inhibitory concentration
cThe half maximal effect concentration
dThe effective concentration of absorbance of 0.50
Table 1: Various activitya of isolated bromophenols 1-6.
Free radical scavenging, metal reducing and metal chelation assays were employed to determine antioxidant properties of naturally occurring bromophenols. All the bromophenols examined showed radical scavenging properties. Compounds 1-4 exhibited potent DPPH radical scavenging activities with EC50 values of 5.0 to 10.0 µM while 5, 6 exhibited relatively low activities with EC50 values around 20.0 µM (Table 1). Radical scavenging activity depends on hydrogen-donating ability. Many researchers [3,35,36] have reported that increasing in the number of phenolic hydroxy groups led to higher radical scavenging activity. However, in the present study, all the componds 1-6 possessed same four phenolic hydroxy group. In this way, antioxidant potency of bromophenols would be influenced not only by number of phenolic hydroxy groups but also by bromine and alkyl substitution. Compounds 4 and 5 were structural isomers, nevertheless, 4 showed two-fold stronger scavenging activities than that of 5. This difference would rely on different positions of bromine atoms and methoxymethyl group [1]. Alternatively the different activity could depend on different orientation of ortho-dihydroxy groups to methylene or ether bridge, that is, positions of dihydroxy groups to bridge are meta and para in 1-4, and ortho and meta in 5 and 6. In ABTS assay, scavenging trend showed similar patterns of DPPH assay results. CUPRAC and FRAP assays are an indication of reducing power to donate electron to transition metals with a compound [8]. Compounds 1-6 indicated relatively same reducing power compared with 2 or 3-tert-butyl-4-hydroxyanisole (BHA) and catechol as positive controls. Radical scavenging results were also supported compound reducing ability [37]. Compounds 1-3 displayed the highest reducing potentiality in the bromophenols examined due to presence of catechol moieties and structural symmetry [2,38]. In Cu2+-chelation assay, compounds 1-4 exhibited relatively high Cu2+-chelating activity similar to the activity of ethylenediamine-N, N, N’, N’-tetraacetic acid (EDTA) as a positive control. However compound5showed the weakest chelating power. Phenoliccompound with catechol moieties can bind transition metal [26]. It is unclear the reason why compound 5 showed weak chelating power.
Conclusion
This study reveals possiblity of different mechanism, other than Cu-chelation by catechol moiety, of naturally occurring bromophenols for tyrosinase inhibtion. Both symmetric and asymmetric bromophenols showed tyrosinase inhibitory and antioxidant activities which were comparable to positive controls. Among the bromophenols, assymmetric dimer 6 and symmetric dimers 1 and 2 displayed considerably strong tyrosinase inhibition. This marine alga has the potentiality for possible application in pharmaceutical and cosmetic industry.
Acknowledgment
The authors express humble gratitude to Dr. E. Fukushi and Mr. Y. Takata, GC-MS and NMR Laboratory, Faculty of Agriculture, Hokkaido University for measuring mass and NMR spectra.
References
- Li K, Li XM, Gloer JB, et al. Isolation, characterization and antioxidantactivity of bromophenols from the marine red alga Rhodomela confervoides. J Agric Food Chem. 2011;59:9916-21.
- Mikami D, Kurihara H, Ono M, et al. Inhibition of algal bromophenols and their related phenols against glucose 6-phosphate dehydrogenase. Fitoterapia. 2016;108:20-25.
- Li K, Li XM, Gloer JB, et al. New nitrogen-containing bromophenols from the marine red alga Rhodomela confervoides and their radical scavenging activity.Food Chem. 2012;135:868-72.
- Kurihara H, Mitani T, Kawabata J, et al. Two new bromophenols from the red alga Odonthalia corymbifera. J Nat Prod. 1999;62:882-84.
- Wang W, Okada Y, Shi H, et al. Structure and aldose reductase inhibitory effects of bromophenols from the red alga Symphyocladia latiuscula. J Nat Prod. 2005;68:620-22.
- Kim KY, Choi KS, Kurihara H, et al. β-glucuronidase inhibitory activity purified from Grateloupia elliptica. Food Sci Biotechnol. 2008;17:1110-4.
- Mikami D, Kurihara H, Kim SM, et al. Red algal bromophenols as glucose 6-phosphate dehydrogenase inhibitors.Mar Drugs. 2013; 11: 4050-57.
- Öztaskin N, Çetinkaya Y, Taslimi P, et al. Antioxidant and acetylcholinesterase inhibition properties of novel bromophenol derivatives. Bioorg Chem. 2015;60:49-57.
- Kurata K, Taniguchi K, Takashima K, et al. Feeding-deterrent bromophenols from Odonthalia corymbifera. Phytochemistry. 1997;45:485-87.
- Wiemer DF, Idler DD, Fenical W. Vidalols A and B, new anti-inflammatory bromophenols from the Caribbean marine red alga Vidalia obtusaloba. Experientia. 1991;47:851-53.
- Olsen EK, Hansen E, Isaksson J, et al. Cellular antioxidant effect of four bromophenols from the red algae, Vertebrata lanosa. Mar Drugs. 2013;11:2769-84.
- Oh KB, Lee JH, Chung SC, et al. Antimicrobial activities of the bromophenols from the red alga Odonthalia corymbifera and some synthetic derivatives. Bioorg Med Chem. Lett. 2008;18:104-8.
- Xu X, Song F, Wang S, et al. Dibenzyl bromophenols with diverse dimerization patterns from the brown alga Leathesia nana. J Nat Prod. 2004;67:1661-66.
- Shi D, Guo S, Jiang B, et al. HPN, a synthetic analogue of bromophenol from red alga Rhodomela confervoides: synthesis and anti-diabetic effects in C57BL/Ksj-db/db mice. Mar Drugs. 2013;11:350-62.
- Park SH, Song JH, Kim T, et al. Anti-human rhinoviral activity of polybromocatechol compounds isolated from the rhodophyta, Neorhodomela aculeate. Mar Drugs. 2012;10:2222-33.
- Chang TS. An updated review of tyrosine inhibitors. Int J Mol Sci. 2009;10:2440-75.
- Tan X, Song YH, Park C, et al. Highly potent tyrosinase inhibitor, neorauflavane from Campylotropis hirtella and inhibitory mechanism with molecular docking.Bioorg Med Chem. 2016;24:153-9.
- Lan WC, Tzeng CW, Lin CC, et al. Prenylated flavonoids from Artocarpus altilis: Antioxidant activities and inhibitory effects on melanin production. Phytochemistry. 2013;89:78-88.
- Javan AJ, Javan MJ, Tehrani ZA. Theoretical investigation on antioxidant activity of bromophenols from the marine red alga Rhodomela confervoides: H-atom vs electron transfer mechanism. J Agric Food Chem. 2013;61:1534-41.
- Reyes CDL, Zbakh H, Motilva V, et al. Antioxidant and anti-inflammatory meteroterpenoids from the brown alga Cystoseria usneoides. J Nat Prod. 2013;76:621-29.
- Zheng ZP, Cheng KW, Zhu Q, et al. Tyrosinase inhibition constituents in roots of Morus nigra: A structure-activity relationship study. J Agric Food Chem. 2010;58:5368-73.
- Guo Z, Li P, Hung W, et al. Antioxidant and anti-inflammatory caffeoyl phenylpropanoid and secoiridoid glycosides from Jasminum nervosum stems, a Chinese folk medicine. Phytochemistry. 2014;106:124-133.
- Re R, Pellegrini N, Proteggente A, et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med. 1999;26:1231-37.
- Sabudak T, Demirkiran O, Ozturk M, et al. Phenolic compounds from Trifolium echinatum Bieb and investigation of their tyrosinase inhibitory and antioxidant activities. Phytochemistry. 2013;96:305-11.
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Analytical Biochemistry. 1996;239:70-6.
- Santos JS, Brizola VRA, Granato D. High-throughput assay comparison and standardization for metal chelating capacity screening: A proposal and application. Food Chem. 2017;214:515-22.
- Katsui N, Suzuki Y, Kitamura S, et al. 5,6-Dibromoprotocatechualdehyde and 2, 3-dibromo-4, 5-dihydroxybenzyl methyl ether: New dibromophenols from Rhodomela larix. Tetrahedron. 1967;23:1185-88.
- Kurata K, Amiya T. Two new bromophenols from red alga Rhodomela larix. Chem Lett. 1977;6:1435-38.
- Fan X, Xu NJ, Shi JG. Bromophenols from the red alga Rhodomela confervoides. J Nat Prod. 2003;66:455-58.
- Shi D, Li J, Jiang B, et al. Bromophenols as inhibitors of protein tyrosine phosphatase 1B with antidiabetic properties. Bioorg Med Chem Lett. 2012;22:2827-32.
- Jiang B, Guo S, Shi D, et al. Discovery of novel bromophenol 3, 4-dibromo-5-(2-bromo-3,4-dihydroxy-6-(isobutoxymethyl)benzyl)benzene-1, 2-diol as protein tyrosine phosphatase 1B inhibitor and its anti-diabetic properties in C57BL/KsJ-db/db mice. European Journal of Medicinal Chemistry. 2013;64:129-136.
- Dayong S, Jing L, Shuju G, et al. The antitumor effect of bromophenol derivatives in vitro and Leathesia nana extract in vivo. Chin J OCEANOL LIMNOL. 2009;27:277-282.
- Chu YH, Chen CJ, Wu SH, et al. Inhibition of xanthine oxidase by Rhodiola crenulata extracts and their phytochemicals. J Agric Food Chem. 2014;62:3742-49.
- Jeon HJ, Noda M, Maruyama M, et al. Identification and kinetic study of tyrosinase inibitors found in sakee lees. J. Agric. Food Chem. 2006; 54: 9827-33.
- Al-Qudah MA, Al-Jaber HL, Zarga MHA, et al. Flavonoid and phenolic compounds from Salvia palaestina L. growing wild in Jordan and their antioxidant activities. Phytochemistry, 2014; 99: 115-20.
- Son S, Lewis BA. Free radical scavenging and antioxidative activity of caffeic acid amide and ester analogues: structure-activity relationship. J. Agric. Food Chem. 2002; 50: 468-72.
- Demirkiran O, Sabudak T, Ozturk M, et al. Antioxidant and tyrosinase inhibitory activities of flavonoids from Trifolium nigrescens subsp. petrisavi. J Agric Food Chem. 2013; 61: 12598-603.
- Hung AC, Wilde A, Ebmeyer J, et al. Examination of the phenolic profile and antioxidant activity of the leaves of the Australian native plant Smilax glyciphylla. J Nat Prod. 2013; 76: 1930-36.