Original Article
, Volume: 16( 1)Synthesis, Characterization and Studies on Antimicrobial Properties of Copper Nanocomposite
- *Correspondence:
- Giriraj Tailor, Department of Polymer Science, M.L.S. University, Udaipur, Rajasthan, India, 313001, Tel: +919529597519; E-mail: giriraj.tailor66@gmail.com
Received: March 16, 2018; Accepted: April 04, 2018; Published: April 10, 2018
Citation: Chaudhary J, Tailor G, Tomar N. Synthesis, Characterization and Studies on Antimicrobial Properties of Copper Nanocomposite. Mat Sci Ind J. 2018;16(1):130
Abstract
Copper nanocomposite has been synthesized by simple chemical precipitation method. Firstly, the polymer metal complex was synthesized which was followed by the application of calcination technique. The synthesized polymer metal complex was analyzed by (IR) infra-red spectroscopy and nuclear magnetic resonance (NMR). Thereafter chemical composition and crystallographic structure of nanocomposite was confirmed by XRD measurement, which reveals that crystal system and size of nanoparticles is 19.34. While the scanning electron microscopic (SEM) images were taken for finer details of surface morphology of nanocomposite. These synthesized nanocomposites were also studied for antimicrobial activity.
Keywords
Copper nanocomposite; Polymer; SEM; Morphology; NMR
Introduction
Nanocomposites exhibit different optical, mechanical, thermal, magnetic and antibacterial properties. The antimicrobial properties of metal nanoparticles have been of focus during the last decade. Some of the biological properties of nanoparticles of various metals have worked find out by testing their antimicrobial potential. Nanoparticles of Ag, Cu, Zn and Au show a wide spectrum of antimicrobial activity against different bacterial and fungal species [1-7]. The development of polymer based nanocomposite with antimicrobial activity offer interesting possibilities as the polymer matrix can be multiplied in order to fulfil not only specific technological requirement but nanostructures too with shape and size depending on properties that can be absorbed [8]. Various inorganic nanostructures with antibacterial properties have been used in a wide range of matrix [9,10]. Among the polymer nanocomposites investigated so far, those incorporating ligand nanoparticles have been regarded as particularly useful for applications in various fields, including biomedical equipment and devices, water treatment, and food processing [11]. Ag has been widely investigated for its antimicrobial activity due to its superior effectiveness and strong cytotoxicity towards a broad range of microorganisms [12]. The great interest for Cu based nanocomposite can be easily perceived by the number of polymer matrix investigated in their preparation, both of synthetic and natural origin [13]. These biocompatibility and biodegradability that can be used in a variety of formulations depending on the envisaged functionality. The present study was carried out to find out the antimicrobial potential and the mechanism of interaction of copper nanocomposites with some selected species of bacteria i.e. S. haemolyticus, K. pneumonia, B. cereus and E. faecalistaking the criteria bacterial growth inhibition.
Experimental
All AR grade chemicals were used, namely, aniline, formaldehyde and sodium hydroxide (Central Drug House Pvt. Limited) and hydrochloric acid (Fisher Scientific). Metal ion solutions have been prepared by dissolving suiTable amount of in distilled water.
Synthesis of copper nanocomposites
Copper nanocomposites have been synthesized in two steps: Synthesis of polymer metal complex 9.5 ml of aniline in dilute hydrochloric acid was taken in 250 ml beaker to which 10 ml of formaldehyde solution (40%) was added slowly with stirring. The mixture was then stirred well for 15 minutes to form an orange red solution. This solution is fed into aqueous sodium hydroxides solution (1%) and refluxed for 30-45 minutes. The aniline formaldehyde was precipitated out. Thereafter 15 ml of 1N Metal ion solution was added drop by drop. The reaction mixture was continuously stirred for 30 minutes then heated at 450°C for one hour on heating mental. After heating, polymer metal complex has been formed. The excess metal ion and impurities on the solid sample was purified by the washing with distilled water solution.
Synthesis of nanocomposite: Decomposition of polymer metal complex at 900° in muffle furnace for 45 minutes was done in order to get nanocomposite of the complex.
Purification of copper nanoparticles
1st Step: Removal of volatile impurity: Several volatile impurities were separated during decomposition. Thus, nanocomposite becomes free from these impurities.
2nd Step: Removal of metallic impurities: For removal of metallic ions, nanocomposites were kept in 12N hydrochloric acid solution for 24 hours. Then they were centrifuged and washed with distilled water till hydrochloric acid was fully removed.
Antimicrobial activity of nanocomposite
The MIC of nanocomposites was determined following method suggested by Chitwood (1969). The sample was dissolved in DMSO for making different concentrations ranging from 100 μg/ml to 2000 μg/ml.100 μl of each concentration was added to tube containing Muller Hinton broth medium. 100 μl of microbial culture of Gram positive bacteria (Klebsiella pneumonia, Staphyllococcushaemolyticus) and Gram negative bacteria (Enterococcus faecalis, Bacillus cereus) with 10-5 CFU was inoculated in each tube with different concentrations of the sample. 200 μg/ml, 500 μg/ml, 1000 μg/ml and 1500 μg/ml concentration was also tested for the zone of inhibition using agar well diffusion method (Sen and Batra, 2012) against the above four microorganisms.
Results and Discussion
Infra-red spectroscopy
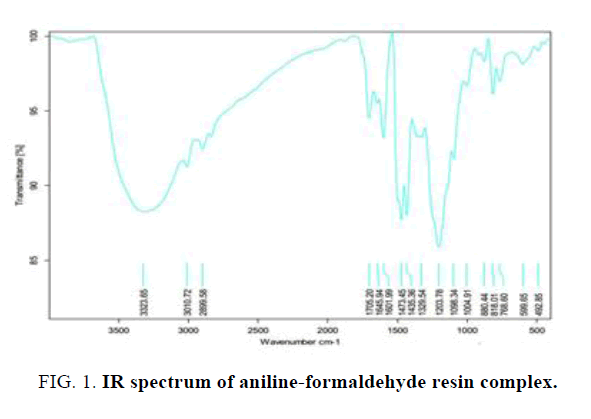
The IR spectra of polymer metal complex are given in Table 1 and Figure 1.
| Prominent absorption band cm | Functional group |
|---|---|
| 3323 cm | N-H stretching |
| 3010-2899 cm | C-H stretching |
| 1645-1601 cm | C=C stretching |
| 1309 cm | C-N aromatic stretching |
Table 1: Prominent absorption band cm-1 and functional groups of aniline-formaldehyde resin complex.
The 1H NMR spectrum of copper doped complex of aniline-formaldehyde polymer metal complex showed a signal at 6.4–7.7 ppm for the aromatic protons of aniline. The methylene groups of the polymer showed signals at 3.6 and 4.2 ppm. All the experiments were carried out at 300 K over a spectral width of 15 ppm. Chemical shifts were referenced to the solvent signal.
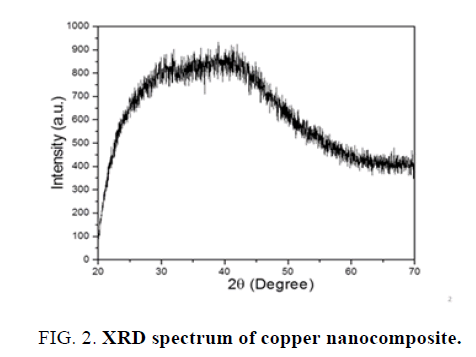
X-Ray diffraction-Orthorhombic copper nanocomposite has been determined using XRD technique. Different copper nanostructure size was obtained using wet chemical precipitation method. Other use of XRD technique is to determine the particle size is using Scherrer equation.
D=Kλ/(B cosө)
Where,
D=The mean size of crystallites (nm), B=Full width at half the maximum (FWHM)
K=Crystallite shape factor, ө=bregg angle, λ=X-ray wave length
Applying Scherrer equation to the obtained XRD pattern of copper nanoparticles, crystalline size was found to be 19.34 nm and crystal system was orthorhombic. The Bravais lattice is base centered and 2ө=41.960. The XRD spectra given in Figure 2.
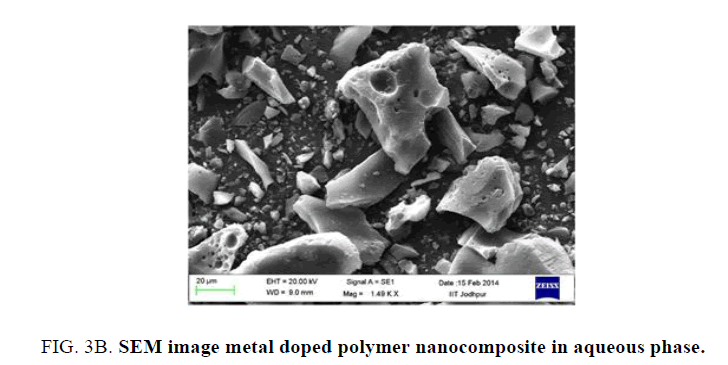
Finer details of the surface morphology of the resulting powder were examined by scanning electron microscope. Figure 3A shows that spherical pots are present on the surface and bland like are visible Spherical dots were assigned as the metal ion present on the surface of the nanomaterial The spherical metal ion present on the surface of composite material plays an important role in the one dimensional electron transportation. Figure 3B shows clear picture of the polymer composite.
Antimicrobial activity:
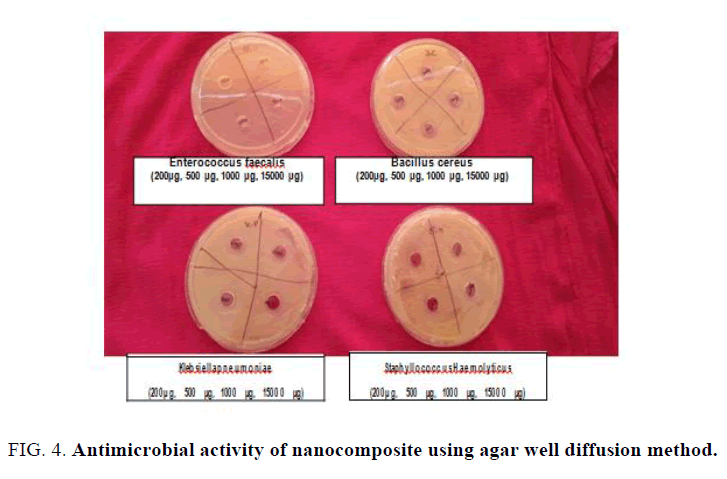
The growth of each microorganism under the regime of different concentrations of copper nanocomposite was assessed at optical density of 600 nm (Table 2). The OD ranged from 0.04 to 0.50 for S. haemolyticus, 1.05 to 1.48 for K. pneumonia, 0.22 to 0.91 for B. cereus and 0.17 to 1.02 for E. faecalis which shows no significant inhibition in the growth of microorganisms. However, growth was reduced with the increased concentration of the compound. Upon testing with well diffusion agar plate method, no zone of inhibition was observed indicating that the compound does not have the antimicrobial activity against the above mentioned four microorganisms (Figure 4). The antimicrobial activity of copper nano-composite has been reported by Singhal et al. and Pinto et al. The biological activities of chemically synthesized nano-composites depend on the pore size of copper nano-particle which increases with the increase pore size [14-16].
Figure 4: Antimicrobial activity of nanocomposite using agar well diffusion method.
| Concentration | Staphylococcus haemolyticus | Klebsiella pneumoniae | Bacillus cereus | Enterococcus faecalis |
|---|---|---|---|---|
| 100 µg/ml | 0.5 | 1.48 | 0.91 | 1.02 |
| 200 µg/ml | 0.25 | 1.48 | 0.8 | 0.92 |
| 300 µg/ml | 0.25 | 1.44 | 0.46 | 0.92 |
| 400 µg/ml | 0.23 | 1.43 | 0.36 | 0.81 |
| 500 µg/ml | 0.23 | 1.42 | 0.35 | 0.67 |
| 600 µg/ml | 0.22 | 1.41 | 0.35 | 0.51 |
| 700 µg/ml | 0.22 | 1.15 | 0.32 | 0.31 |
| 800 µg/ml | 0.2 | 1.14 | 0.32 | 0.28 |
| 900 µg/ml | 0.18 | 1.1 | 0.29 | 0.21 |
| 1000 µg/ml | 0.16 | 1.09 | 0.23 | 0.2 |
| 1500 µg/ml | 0.1 | 1.08 | 0.23 | 0.19 |
| 2000 µg/ml | 0.04 | 1.05 | 0.22 | 0.17 |
Table 2: Optical density at 600 nm of microbial growth against different concentration of nanocomposites dissolved in DMSO.
Conclusion
The present paper reports a simple, fast, effective polymer based technique to synthesize copper nanocomposite using thermosetting polymer and copper metal salt as a precursor. Nano composite synthesized by the simple chemical precipitation method. The IR spectroscopy confirmed the formation of polymer composite. XRD analysis proves the nanostructure of the chromium nanoparticles through its crystal analysis and spacing pattern. The antimicrobial activity has also been observed in the present nanocomposite.
Acknowledgement
Authors are grateful to Ms. Shefali Nehriya, Mrs. Swati Purohit and Mrs. Suman Jinger, Departments of Polymer Science, Mohan Lal Sukhadia University Udaipur, Rajasthan, India for fruitful discussions and support during the preparation of this manuscript. Experimental assistance provided by the technician of the Instrumental lab, University of Rajasthan, Jaipur and IIT, Jodhpur during this work is thankfully acknowledged.
References
- Duran N, Marcato PD, De Souza GI, et al. Antibacterial effect of silver nanoparticles produced by fungal process on textile fabrics and their effluent treatment. J Biomed Nanotechnol. 2007;3:203-8.
- Anyaogu KC, Fedorov AV, Neckers DC. Synthesis, characterization and antifouling potential of functionalized copper nanoparticles. Langmuir. 2008;24(8):4340-46.
- Chatterjee AK, Sarkar RK, Chattopadhyay AP, et al. A simple robust method for synthesis of metallic copper nanoparticles of high antibacterial potency against E. coli. Nanotechnology. 2012;23(8):085103.
- Jung JH, Kim SW, Min JS, et al. The effect of nano-silver liquid against the white rot of the green onion caused by Sclerotium cepivorum. Mycobiology. 2010;38(1):39-45.
- Lamsal K, Kim SW, Jung JH, et al. Application of silver nanoparticles for the control of colletotrichum species in vitro and pepper anthracnose disease in field. Mycobiology.2011;39:26-32.
- Kim SW, Jung JH, Lamsal K, et al. Antifungal effects of silver nanoparticles (AgNPs) against various plants pathogenic fungi. Mycobiol. 2012;40:53-8.
- Sahar M, Ouda. Antifungal activity of silver and copper nanoparticles on two plant pathogens, Alternaria alternata and Botrytis cinerea. Research Journal of Microbiology. 2014;9:34-42.
- Pinto RJB, Neves MC, Pascoalneto C, et al. Antibacterial activity of nanocomposites of copper and cellulose. Bio Med Res Int. 2013:1-6.
- Ruparelia JP, Chatterjee AK, Duttagupta SP, et al. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Bio Mater. 2008;4:707-16.
- Llorens A, Lloret E, Picouet PA, et al. Metallic-based micro and nanocomposites in food contact materials and active food packaging. Trends in Food Science & Technology. 2017;24(1):19-29.
- Hajipour MJ, Fromm KM, Ashkarranetal AA. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012;30:499-511.
- Morones JR, Elechiguerra JL, Camacho A. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346-53.
- Anyaogu KC, Fedorov AV, Neckers DC. Synthesis characterization and antifouling potential of functionalized copper nanoparticles. Langmuir. 2008;24:4340-46.
- Palza H. Antimicrobial polymers with metal nanoparticles. Int J Mol Sci. 2015:16;2099-2116.
- Sen A, Batra A. Evaluation of antimicrobial activity of different solvent extracts of Medicinal plant: Melia azedarach l. Int J Curr Pharm Res. 2012;4:67-73.
- Chitwood L. Tube dilution antimicrobial susceptibility testing: Efficacy of a microtechnique applicable to diagnostic laboratories. Appl Microbiol. 1969;17:707-9.