Original Article
, Volume: 16( 2)Selective Leaching of Uranium from Recent Deposits of Wadi El Reddah Stream Sediments, Northeastern Desert, Egypt
- *Correspondence:
- O. A. Desouky Nuclear Materials Authority (NMA), P.O. Box 530, El-Maadi, Cairo, Egypt
Tel: +0201009061678; E-mail: o_a_desouky@hotmail.com
Received Date: May 11, 2018 Accepted Date: June 08, 2018 Published Date: June 15, 2018
Citation: Desouky OA, Mira H, Abed NS. Selective Leaching of Uranium from Recent Deposits of Wadi El Reddah Stream
Sediments, Northeastern Desert, Egypt. Mat Sci Ind J. 2018;16(2):132
Abstract
The leachability of recent uranium deposits from stream sediments at wadi El Reddah area, Northeastern desert, Egypt are investigated as a part of ongoing plan for uranium exploration. Extensive laboratory scale leaching tests were carried out to optimize various process parameters for maximizing the recovery of uranium. Leaching parameters like temperature, agitation time, grinding size, solid/liquid ratio; leaching agents, oxidant, etc. were carried out. The maximum leaching of uranium (91.93%) was found after 2 hours of leaching using sulphuric acid, maintained at solid/liquid ratio of 1:5 at 160°C. Temperature plays a significant odd role in leaching percent of uranium. The present paper provides a new approached of detailed account of optimization of various process parameters.
Keywords
Acid leaching; Uranium; Stream sediments; Wadi El Reddah; Surficial deposits
Introduction
Uranium is widely distributed lithophile metallic element. It may be present as a significant constitute in some minerals (e.g. uraninite, brannerite, uranophane and carnotite) or as an accessory element in others (e.g. zircon, apatite, allanite and monazite). Stream sediments remain the most important medium for geochemical exploration. According to IAEA [1], surficial deposits considered from the most important terms for uranium production. Because of their relatively young age, they are not easily detected by the conventional radiometric method of surveying op. cit. Uranium occurs in stream sediments (surficial deposit) almost exclusively as secondary minerals (hexavalent stage) or adsorbed on other materials. The major controlling factors which affect the world distribution of the surficial chemo genic U deposits particularly the uraniferous calcrete are climate; geomorphology; and provenance, i.e. the weathering terrance from which U and associated substances are derived.
Uranium is a very essential element because of its strategic importance in the energy field [2]. Uranium has become a widely used source for nuclear energy especially for the nuclear electrical generation plants [3]. Also, it is considered as a long- term potential environmental hazard as a result of its long half-life [4-6].
Metal recovery from this kind of low grade resource is frequently applying hydrometallurgical processes: (a) first leaching using in most cases acidic solutions [7-9], followed by (b) a series of separation techniques including precipitation [8], solvent extraction [9,10], ion exchangers [11], ion exchange or chelating resins [12-15]. In addition, the uptake of uranium by human beings can cause irreparable damage e.g. severe liver damage, kidney failure and finally death [16]. Due to its high radiological and biological toxicity, it is so important to decrease its discharge into the environment according to activities of nuclear industry. Environmentally uranium is found in the hexavalent oxidation state, and it has severe chemical toxicity, leading to robustic hard to mammalian reproduction systems in the form of reduced fertility [17-20].
The stream sediments that cover W. El Reddah were mineralogically studied to reveal the heavy mineral content of these sediments, especially those radioactive and uranium and thorium-bearing ones. The identified minerals were mainly thorite, uranothorite, zircon, monazite, xenotime, columbite, fergusonite, REE-bearing minerals as well as cassiterite.
Special emphases on REEs content of thorite, uranothorite, zircon and xenotime has done in order to characterize them and relate them to the increase of uranium contents in these streams. Acid leaching is generally preferred, because it gives high results for uranium dissolution. The choice between an acid and an alkali leaching process depends primarily on the type of uranium mineralization and gangue minerals of the stream sediments under consideration [21]. It is clear from the mineralogy of the stream sediments that it is composed of Thorite, uranothorite, Xenotime, Zircon and REEs minerals…etc. The ore contains no Acid consuming material as carbonate.
Accordingly acid leaching is the suitable techniques which were used in the present studies. In acid leaching several techniques are used for leaching of the valuable elements. These include agitation leaching, percolation leaching, hot digestion, constant pH leaching, acid curing at high solids (pugging), besides acid leaching under pressure. Each technique has an advantage for certain materials and drawbacks for others. Agitation leaching is the most widely used technique in uranium industry which offers maximum consistency of the solid contents of the pulp with the leached liquor [22]. The main objective of processed uranium from stream sediments is obtaining uranium concentrate under certain conditions: high recovery, low costs of production and concentrate of good quality. Leaching process is the dissolution of uranium from the uranium containing minerals and adsorbed clays. The selection procedure for dissolving uranium minerals is dependant on the physical characteristics of the ore such as: type of mineralization, ease of liberation and the nature of other constituent’s minerals presents [23]. Uranium is a redox-sensitive element with two dominant species (U (VI) and U (IV)) found in nature. The mobility of U is greatly influenced by the redox conditions [24]. Juan Liu et al., [25] confirmed that enrichment levels of U were dispersed not only in the surface sediments but also in deep sediments across the depth profile. Further analysis by SEM-EDS and XRD indicated that U partitioning in the depth profile was possibly controlled by complicated interplay of leaching and precipitation cycles of U-bearing minerals the use of Pb isotopes as a powerful tool for quantitatively fingerprinting the sources of U dispersal in the sediment core, and natural-occurring U contamination that may become a hidden geo environmental health hazard.
Baowei Hu et al., [26] suggested that the distinct structure of modified montmorillonite can be utilized for synthesizing supported NZVI to create properties compatible for enhanced enrichment of radionuclides specially U (VI) in which inhibition effect of 1, 10-phenanthroline demonstrated that surface-adsorbed Fe (II) on corrosion products and clay surfaces played an indispensable role in U (VI) and Se (IV) reduction, which was further confirmed by XPS identification.
During past few decades different types of leaching processes based on acid leaching and alkaline leaching methods have been developed for processing uranium ore of different characteristics [27-29]. Leaching studies are carried out to develop a suitable method for the recovery of uranium.
Wensheng Linghu et al., [30] reported that the adsorption of U(VI) on LDH and LDH/GO composites at pH < 4.0 and pH > 5.0 was cation exchange and inner-sphere surface complexation, respectively. These observations indicated that LDH/GO composites can be a promising adsorbent for the high efficient adsorption of radionuclide from aqueous solutions in environmental cleanup.
The present lab scale study is based on acid leaching by varying different leaching parameters which is the predominant process for recovery of uranium from stream sediments.
Experimental
Sample collection and preparation
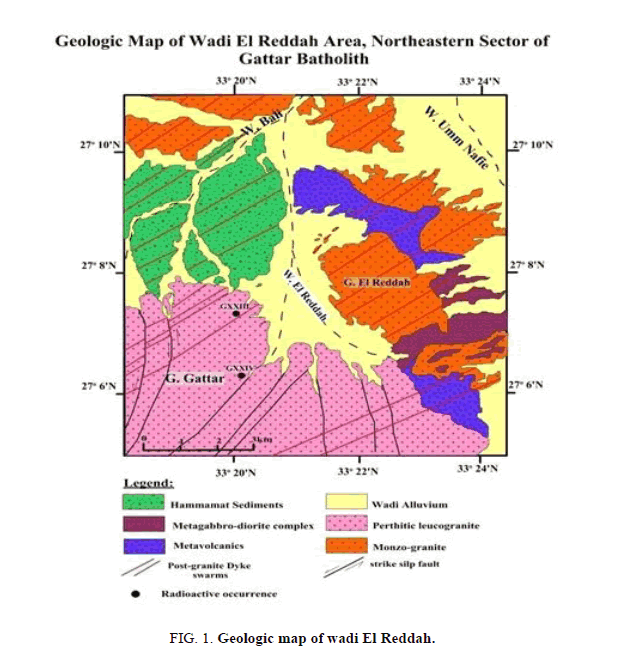
Wadi El Reddah area represents the northeastern peripheral sector of G. Gattar batholiths and is considered as one of the important wadis in northern Gattar batholith (Figure 1). It represents a semi-closed basin as it has only one outlet in its northern tip while the other parts of the wadi collect its flood from internal tributaries from granites and other rock types. So, the importance of the study of this wadi lies in being ideal for the presences of geochemical prospection for uranium in this very topographically rough sector of Gattarian granites and being the only corridor or the path for reaching this important part.
The representative stream sediment sample was collected from different locations of wadi El Reddah of ore grade 380 ppm U3O8 for carrying out the study. The stream sediment sample was ground to desired particle size. After sample preparation, the sample was subjected to analysis and leaching study.
Instrumentation
The analyses of samples generated during the experiments were carried out by a double beam spectrophotometer of high resolution power (UNICAM) UV-V spectrophotometer model UV 2-100, England.
Chemicals and reagents
All the chemical and reagents i.e. Sulphuric Acid (443.2 g/l), Hydrogen Peroxide (21.6 g/l), solid potassium Permanganate, Hydrochloric Acid (279.3 g/l), Nitric acid (304.6 g/l) used in the leaching experiments were of Analytical grade of Merck (Darmstadt, Germany).
Methodology of leaching process
The lab scale leaching experiments were conducted on Wadi El- Reddah stream sediments sample by taking 10 g stream sample in each experiment. Leaching experiments were carried out by varying one parameter at a time. The different factors that affect the uranium leaching, such as the amount of acid added, mixing time and temperature were studied.
Results and Discussion
Effect of different leaching agents
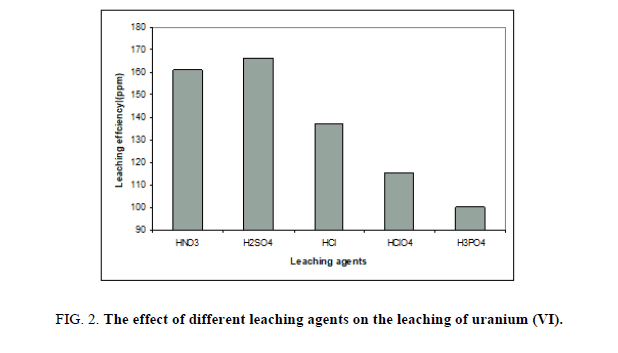
Sufficient free acid concentration is required to attack uranium minerals without dissolving an excessive amount of associated gangue materials. After the uranium is dissolved some excess acid must remain to prevent reprecipitation. This factor has been studied by using different mineral acids like H2SO4, HNO3, HCl, HClO4 and H3PO4. Other factors have been fixed at concentration of 5 M from all, 1/5 solid / liquid ratio, agitation time, 2 hours, room temperature and upon ore ground to -60 mesh size. The obtained results were presented in Figure 2; where it was clear that leaching with sulphuric acid as leaching agent gave high leaching percentage compared to others and its cost comparatively less. However, lowest leachability of uranium was found in phosphoric acid.
Effect of alkaline leaching
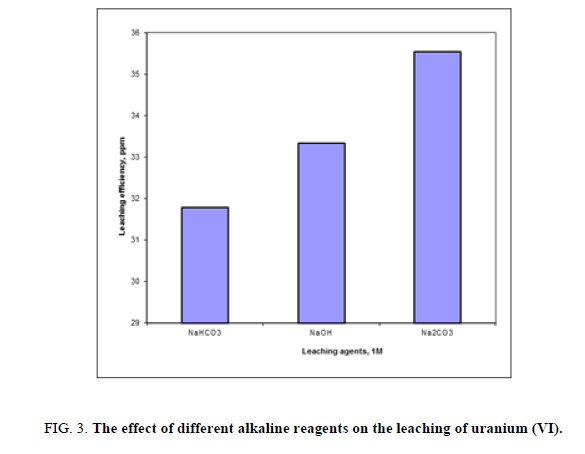
A series of leaching experiments were performed for different alkaline solutions as NaHCO3, NaOH and Na2CO3 at constant concentration of 1 M for all. Other variables were fixed at -60 mesh size, room temperature, alkaline concentration 1M and solid/liquid ratio 1:5 for 2 hours. The obtained uranium leaching results were presented in Figure 3. Form the obtained results it was shown that alkaline leaching was less effective than acid leaching on particularly leaching of uranium.
Effect of different concentrations of leaching agent (H2SO4)
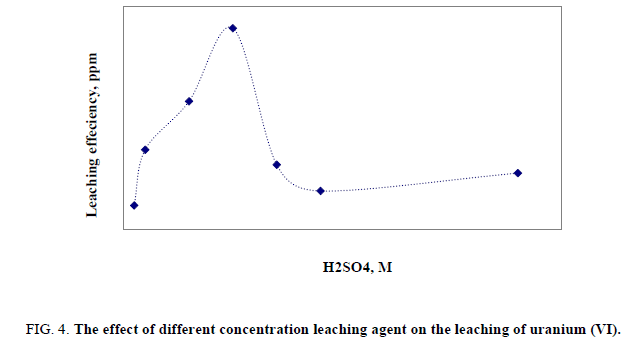
This factor has been studied by applying different acid concentrations, 10 gm of the stream sediment sample, 50 ml of leaching agent (H2SO4) with concentration ranging from 0.5 to 9 M, and then the agitation done for 2 hours at room temperature, where other factors have been fixed. Obviously, it was found that leaching percent of uranium was increased gradually with increasing H2SO4 concentration from 0.5 to 5 M. After that the leaching efficiency was decreased. Probably due to increase in reprecipitation of uranium, so this occurs in sulfate media due to the equilibration between HSO4-, SO42- and H2SO4 due to hydrolysis. The optimum value of 5 M of H2SO4 was chosen as presented in Figure 4.
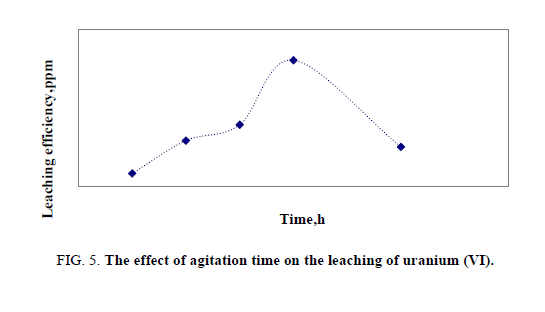
Effect of mixing time
Leaching (contact) time experimentally chosen based on the characteristics of the ore e.g. type of mineralization, particle size and leaching conditions. In this case, the time of leaching was changed from 0.5 to 3 hours in a series of experiments under the same conditions. The optimum value of agitation time was found to be two hours (Figure 5).
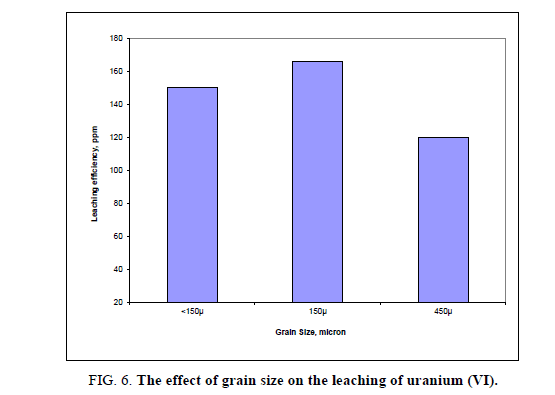
Effect of different grain size
The influence of particle size on the efficiency of uranium leaching process was studied using 5 M H2SO4, at room temperature with a solid/liquid ratio 1:5 for 2 hours. Three fractions with different granulations were tested: -150 μ, -450μ and <150 μ. Under above conditions, no evidence influence of particle size on the uranium leaching efficiencies was observed. Usually, the literature shows that the decrease in particle size enhanced metals dissolution. The observed small differences can be likely ascribed to the mineralogical and elemental distribution within the sizes and interaction of the minerals/phases within the ore [29]. The obtained uranium leaching results were presented in Figure 6. Form the obtained results it was shown that -150 μ grain size particle gave the highest leaching of uranium. From these data, the best leachability was obtained at the finer particle size. This might be attributed to the larger surface area which increasing the exposure of uranium particles to the leaching solution leading to increase the leachability.
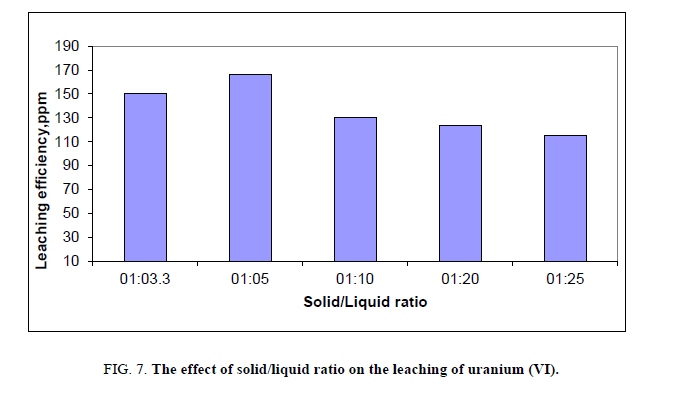
Effect of solid to liquid ratio
The selection of proper solid to liquid ratio (the weight of sample to volume of acid) is important for optimization of the leaching process. The effect of solid to liquid ratio depends on e.g. grain distribution and free surface area [31]. For this reason it was necessary to test the effect of solid to liquid ratio on leaching operations of stream sediments. This factor has been studied by applying different solid/liquid ratio ranging from 1:3.3 to 1:25, and then the agitation done for 2 hours at room temperature where other factors have been fixed. The optimum solid to liquid ratio was found to be 1:5 (Figure 7).
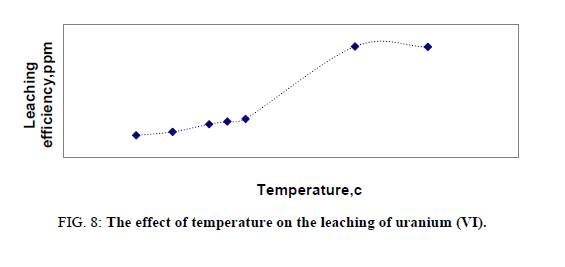
Effect of temperature on leaching of uranium
Temperature is an important factor playing a significant role in the leaching process. The effect of temperature of leaching was studied in a series of experiments in which other factors were fixed at -150 μ size, solid/liquid ratio 1:5, time 2 hours and acid concentration of 5 M. Effect of temperature covered the range from room temperature up to 200ºC. This effect was significant as it shown in Figure 8. The optimum temperature was found to be 160ºC. This sudden change in leaching efficiency probably was due to the high sulphur content in the stream sediment sample. So that, by increasing temperature acidity increased due to sulfur content in the sample and these in terms achieving conditions of heap leaching, so the sample under study was subjected at the same time to both agitation leaching and heap leaching so that leaching efficiency of uranium was more increased than other. At low temperature uranium may liberate into solution by increasing temperature also, uranium inside lattice dissolve or liberate causing increasing in U (VI) concentration. It can be concluded that the refractory minerals such as zircon, thorite and uranothorite were dissolved beside secondary minerals at elevated temperature.
Effect of different amounts of oxidant with different temperatures on leaching of uranium
A number of leaching experiments were performed over a range of concentration of potassium dichromate (K2Cr2O7) from 0.05 to 0.3 gm with different temperature 80,100 and 160ºC. Other variables were fixed at 150 μ grain size, acid concentration (5 M) and solid/ liquid ratio 1:5 for 2 hours. The obtained uranium leaching results were reported in Table 1 from the obtained results it was obvious that by increasing temperature, no need to increase amount of oxidant because the effect of oxidant was negligible.
| Temperature (°c) | Oxidant, gm | Leaching Concentrations (ppm) |
|---|---|---|
| 80 | 0.1 | 169.367 |
| 100 | 0.05 | 153.184 |
| 160 | 0.3 | 417.75 |
Table 1. Effect of different amount of oxidant with different temperature on the leaching of uranium (VI).
Effect of different amounts of oxidant on leaching of uranium at constant temperature
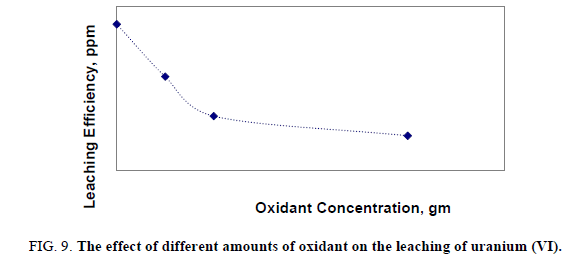
A number of leaching experiments were performed over a range of concentration of potassium dichromate (K2Cr2O7) from 0.05 to 0.3 gm at temperature160ºC. Other variables were fixed at 150 μ grain size, acid concentration 5 M and solid/ liquid ratio 1:5 for 2 hours at constant temperature (160°C). The obtained uranium leaching results were shown in Figure 9. From the obtained results it was obvious that by increasing amount of oxidant, leaching efficiency of uranium was decreased. Probably due to uranium was found to be in U (VI) rather than any other oxidation state especially, U (IV) state.
Application of optimum conditions for leaching uranium from the present stream sample.
From the previous experimental results obtained on studying the present stream sediment sample, the following optimum leaching conditions could be deduced to obtain complete uranium dissolution in real sample: sample mesh size should be 150 μ, the best concentration of sulphuric acid could be taken as 5 M, the leaching was more effective without adding any oxidant, solid/liquid ratio was 1:5, agitation time was two hours and the temperature is 160ºC.
A stock pregnant leaching solution (1liter) was prepared under the above mentioned conditions, for performing the experiments of ion exchange or solvent extraction to obtain more purified yield. The measured oxidation reduction potentional was found to be -480 mv, uranium assays 420 ppm in this pregnant leached liquor. Solid /liquid ratio was fixed at 1:5.
It is customary to regard the form of the resulting uranium in solution as being UO2SO4. In practice, however high proportions of the anionic complexes UO2 (SO4)22- and UO2 (SO4)34- are also present. As a matter of fact the dissolved uranium occurs in all three forms simultaneously, the abundance of which being controlled by complex equilibria involving the concentrations of H+, SO42- and HSO4- ions besides that of the various forms of dissolved uranium [32]. These complex anionic forms take a major role in the subsequent purification and recovery of uranium from the pregnant acid liquor that is quite rich in co-dissolved impurities incorporated in the ore. That is why these complex forms are quite responsible for the importance of sulphuric acid in uranium leaching.
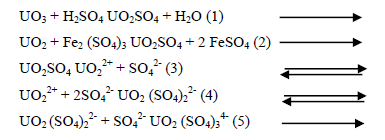
It was shown from the effect of temperature on the leachability of uranium from the present stream sediment that there was an increase in uranium leachability with temperature and the stream sediment minerals should be subjected to 160ºC in order to be amenable to leaching, this was quite logic since the rate of most chemical reactions various exponentially with the reciprocal of the absolute temperature according to Arrhenius equation [33].
dc/dt = - K exp (- Ea/RT)
Where: dc/dt: rate of leaching reaction K is pre-exponential factor, Ea is the energy of activation, R is the gas constant and T is the absolute temperature.
Since our study showed that the addition of oxidant has no effect in leaching percent and this could be refers to that all uranium content in this sample was already in hexavalent state which is easily soluble in dilute sulphuric acid.
Maximum uranium leaching could be obtained using sulphuric acid concentration of 5 M for two hours. Higher free acidity was not recommended since the silica gangue in the sample would form silicic acid and gelation of this cause poor phase separation in subsequent uranium recovery stage.
Precipitation of uranium as yellow cake
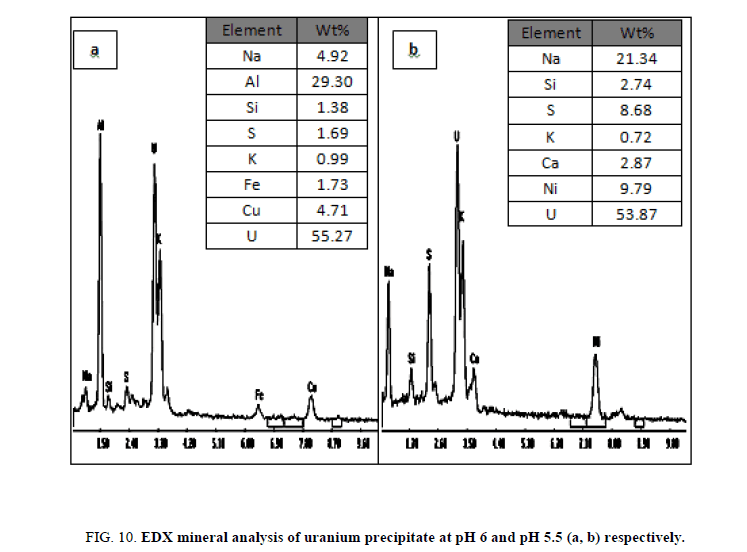
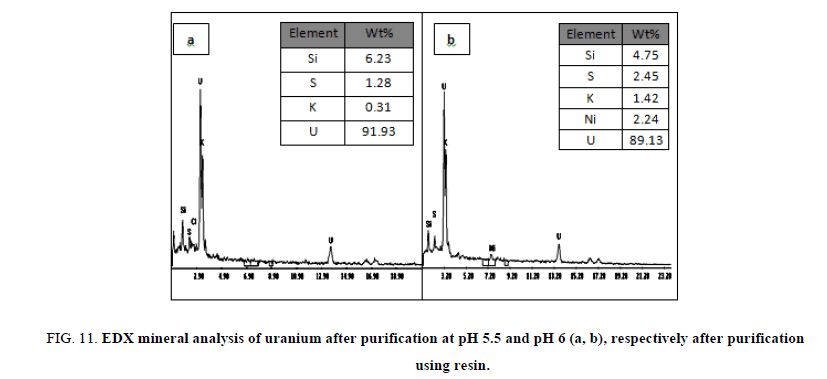
Preliminary experiments showed that cold precipitation results in very fine highly fluctuated precipitate, with a very slow settling rate and sluggish filtration. Precipitation was affected therefore at room temperature with continuous mechanical agitation and at pH 6. This pH value was achieved by gradually addition of 1 M sodium hydroxide from a burette. Neutralization of the solution was continued where granular deep yellow uranium compound (yellow cake), precipitated and settled rapidly. The precipitate was filtered using Whitman filter paper no. 42 then washed several times with equal volumes of distilled water and then dried at 110ºC. The content of uranium in the yellow cake was found to be 64.35% U3O8 as determined by spectrophotometry and also by an Environmental Scanning Electron Microscope (ESEM) model XL30. In which uranium was precipitated at pH 5.5 and after precipitation it was measured by (ESEM) and its results about 55.27% and represented in Figure 10a and at pH 6, the results was about 53.87% and represented in Figure 10b after precipitation several cycle purifications were performed by ion exchange resin (1×2 Cl- form) and the results of purifications were represented in Figure 11a,b in which purification percent reached about 91.93% and 89.13% respectively.
Figure 11: EDX mineral analysis of uranium after purification at pH 5.5 and pH 6 (a, b), respectively after purification using resin.
Conclusion
The leaching experiments of uranium from stream sediment at wadi El Reddah was found to give maximum uranium extraction at 160°C with ore grind size of -150μ passing through 60 mesh maintained at leaching agent 5M sulphuric acid with solid/ liquid ratio 1:5. A significance rule of temperature was observed in leachability of uranium in this stream sediment, in which a sudden increase in leaching of uranium at temperature 160°C was observed for 2 hours. In acid leaching the significance difference in effectiveness of oxidizing agent (K2Cr2O7) was not observed.
Appropriate selection of the parameters allows controlling the process efficiency. A part from uranium such components of the ores like rare earth elements are considered for recovery. The recovery of not only uranium but also other valuable metals could to be considered in the technological scheme to improve economy of such a project. After leaching experiment, purification experiments using ion exchange resins followed by precipitation to obtain final product, yellow cake U3O8.
References
- IAEA. Steps for preparing uranium production feasibility studies: guidebook IAEA-1996, TECDOC-88 5 ISS N1011-428 9.
- Kumar A, Singhal RK, Rout S, Narayanan U, Karpe R, Ravi PM. Adsorption and kinetic behavior of uranium and thorium in seawater-sediment system. Journal of Radioanalytical and Nuclear Chemistry. 2013 Jan 1;295(1):649-56.
- Amin, A. E.; Kamal, H. M.; Ghazala, R. A.; Awad, L. S. Recovery of uranium from the mineralized shear zone of Gabal El Missikat, Eastern Desert, Egypt, J. of research in environmental and earth science. 2017, 3(5): 8-19.
- Elsalamouny AR, Desouky OA, Mohamed SA, Galhoum AA, Guibal E. Uranium and neodymium biosorption using novel chelating polysaccharide. International journal of biological macromolecules. 2017 Nov 1;104:963-8.
- Zhang XF, Ding CL, Liu H, Liu LH, Zhao CQ. Protective effects of ion-imprinted chitooligosaccharides as uranium-specific chelating agents against the cytotoxicity of depleted uranium in human kidney cells. Toxicology. 2011 Aug 15;286(1-3):75-84.
- Zou Y, Wang X, Wu F, Yu S, Hu Y, Song W, Liu Y, Wang H, Hayat T, Wang X. Controllable synthesis of Ca-Mg-Al layered double hydroxides and calcined layered double oxides for the efficient removal of U (VI) from wastewater solutions. ACS Sustainable Chemistry & Engineering. 2016 Nov 15;5(1):1173-85.
- Behera SS, Parhi PK. Leaching kinetics study of neodymium from the scrap magnet using acetic acid. Separation and Purification Technology. 2016 Feb 29;160:59-66.
- Yoon HS, Kim CJ, Chung KW, Kim SD, Kumar JR. Recovery process development for the rare earths from permanent magnet scraps leach liquors. Journal of the Brazilian Chemical Society. 2015 Jun;26(6):1143-51.
- Yoon HS, Kim CJ, Chung KW, Lee JY, Shin SM, Lee SJ, Joe AR, Lee SI, Yoo SJ. Leaching kinetics of neodymium in sulfuric acid of rare earth elements (REE) slag concentrated by pyrometallurgy from magnetite ore. Korean Journal of Chemical Engineering. 2014 Oct 1;31(10):1766-72.
- Rabatho JP, Tongamp W, Takasaki Y, Haga K, Shibayama A. Recovery of Nd and Dy from rare earth magnetic waste sludge by hydrometallurgical process. Journal of Material Cycles and Waste Management. 2013 Apr 1;15(2):171-8.
- Chen Y, Wang H, Pei Y, Ren J, Wang J. pH-controlled selective separation of neodymium (III) and samarium (III) from transition metals with carboxyl-functionalized ionic liquids. ACS Sustainable Chemistry & Engineering. 2015 Oct 27;3(12):3167-74.
- Panda N, Devi N, Mishra S. Solvent extraction of neodymium (III) from acidic nitrate medium using Cyanex 921 in kerosene. Journal of Rare Earths. 2012 Aug 1;30(8):794-7.
- Na P, Liu JF, Zhang HY, Wang RS. Investigation and preparation of novel ionic sieve for extracting neodymium. JOURNAL OF RARE EARTHS. 2005 Dec 1;23:97-100.
- Dave SR, Kaur H, Menon SK. Selective solid-phase extraction of rare earth elements by the chemically modified Amberlite XAD-4 resin with azacrown ether. Reactive and Functional Polymers. 2010 Sep 1;70(9):692-8.
- Sreedhar B, Hobbs DT, Kawajiri Y. Simulated moving bed chromatography designs for lanthanide and actinide separations using Reillex HPQ™ resin. Separation and Purification Technology. 2014 Nov 5;136:50-7.
- Xiong C, He R, Pi L, Li J, Yao C, Jiang J, Zheng X. Adsorption of neodymium (III) on acrylic resin (110 resin) from aqueous solutions. Separation Science and Technology. 2015 Mar 4;50(4):564-72.
- Kaur H, Agrawal YK. Functionalization of XAD-4 resin for the separation of lanthanides using chelation ion exchange liquid chromatography. Reactive and Functional Polymers. 2005 Dec 1;65(3):277-83.
- Bayramoglu G, Arica MY. MCM-41 silica particles grafted with polyacrylonitrile: Modification in to amidoxime and carboxyl groups for enhanced uranium removal from aqueous medium. Microporous and Mesoporous Materials. 2016 May 15;226:117-24.
- Arica MY, Bayramoglu G. Polyaniline coated magnetic carboxymethylcellulose beads for selective removal of uranium ions from aqueous solution. Journal of Radioanalytical and Nuclear Chemistry. 2016 Nov 1;310(2):711-24.
- Meinrath, G. Aquatic chemistry of uranium. Geoscience. 1998, 1:1-101.
- Rao TP, Metilda P, Gladis JM. Preconcentration techniques for uranium (VI) and thorium (IV) prior to analytical determination—an overview. Talanta. 2006 Feb 15;68(4):1047-64.
- Zou Y, Liu Y, Wang X, Sheng G, Wang S, Ai Y, Ji Y, Liu Y, Hayat T, Wang X. Glycerol-modified binary layered double hydroxide nanocomposites for uranium immobilization via extended X-ray absorption fine structure technique and density functional theory calculation. ACS Sustainable Chemistry & Engineering. 2017 Mar 21;5(4):3583-95.
- Bayramoglu G, Arica MY. Polyethylenimine and tris (2-aminoethyl) amine modified p (GA–EGMA) microbeads for sorption of uranium ions: equilibrium, kinetic and thermodynamic studies. Journal of Radioanalytical and Nuclear Chemistry. 2017 May 1;312(2):293-303.
- Liu J, Luo X, Wang J, Xiao T, Yin M, Belshaw NS, Lippold H, Kong L, Xiao E, Bao ZA, Li N. Provenance of uranium in a sediment core from a natural reservoir, South China: Application of Pb stable isotope analysis. Chemosphere. 2018 Feb 1;193:1172-80.
- Liu J, Luo X, Wang J, Xiao T, Yin M, Belshaw NS, Lippold H, Kong L, Xiao E, Bao ZA, Li N. Provenance of uranium in a sediment core from a natural reservoir, South China: Application of Pb stable isotope analysis. Chemosphere. 2018 Feb 1;193:1172-80.
- Hu B, Ye F, Ren X, Zhao D, Sheng G, Li H, Ma J, Wang X, Huang Y. X-ray absorption fine structure study of enhanced sequestration of U (VI) and Se (IV) by montmorillonite decorated with zero-valent iron nanoparticles. Environmental Science: Nano. 2016;3(6):1460-72.
- Marvin, G. Recovery of uranium from its ores, Proc. Int Conf peac uses of Atomic Energy. Geneva. 1956, 8.
- Nagwa, H. E. Recovery of uranium from El-Eradiya ore central Eastern Desert, Egypt. Ph D. University College for Woman. Ain Shams University.1988, 150 .
- Merritt, R. C. The Extractive Metallurgy of Uranium, Colorado, School of Mines Res Inst. Golden, Colorado. 1971, 83.
- Linghu W, Yang H, Sun Y, Sheng G, Huang Y. One-Pot Synthesis of LDH/GO Composites as Highly Effective Adsorbents for Decontamination of U (VI). ACS Sustainable Chemistry & Engineering. 2017 May 17;5(6):5608-16.
- Nesmeyanova GM, Chernushevich NK. Investigation of the behavior of minerals accompanying uranium in the acid leaching of ores. The Soviet Journal of Atomic Energy. 1961 Aug 1;9(2):646-8.
- Phillips, C. R.; Sears, P. L.; Poon, Y. C. Energy Sources. Part A: Recovery, Utilization, and Environmental Effects. 1982, 6(3), p. 215.
- Gupta, C. K.; Mukherjee, T. K. Hydrometallurgy in Extraction Processes, CRC Press. Boca Raton. 1990, I, 70.