Original Article
tsm, Volume: 12( 2)Biodegradation Studies of Cellulose-Based Polyurethane Foams.
- *Correspondence:
- Ekebafe L, Department Polymer Engineering Technology, Auchi Polytechnic, Auchi, Nigeria, Tel: +2348029320114, +2348088785639; E-mail: lawekebafe@gmail.com
Received: April 4, 2017; Accepted: May 24, 2017; Published: May 29, 2017
Citation: Ekebafe LO, Olugbemide AD, Akpa FAO Biodegradation Studies of Cellulose-based Polyurethane Foams. Macromol Ind J. 2017;12(2):106.
Abstract
The extent of biodegradation of polyurethane foams produced using the sugar bagasse from native sugar cane plant as a source for natural cellulose fibers for the production of cellulose based biodegradable polyurethane foams was explored in this study. Cellulose microfibers from sugar bagasse were isolated. The chemical and surface morphological structures of the isolated cellulose were characterized with FTIR, SEM, and Lignin content determination. Polyurethane foams were made from polyols containing as much as 25 ml liquid cellulose. The extent of biodegradability of the foams were evaluated using short-term accelerated laboratory experiments including microbial analysis, and soil burial experiments, to determine the integrity of the foams in the soil. The experimental results have shown that the foams studied biodegraded under anaerobic conditions. Weight loss and change in the tensile strength of the foams after biological exposure were observed. The composition of the foams and the liquid cellulose used in this study could have played a significant role in its yield to microbial attack during the biodegradation experiments.
Keywords
Sugar baggase; Cellulose; Polyurethane; Foams; Biodegradation
Introduction
Polyurethane foams (PUFs) are materials utilized widely in many fields such as furniture, automotive, structural, and shoe industry, building construction and packaging, agriculture and medicine [1-3]. However, due to the recycling complications of the polyurethane foams discarded after use, constituting environmental pollution problems because of their difficult in disintegration when exposed to environmental conditions [3], it has become very necessary to find possible sustainable solution to the environmental nuisance caused by disposed foams. Polyurethane foams (PUFs) are also utilized in structural, cushion, insulation, electrical, and flotation applications. The materials prepared from plant biomass not only open a new and efficient way to use renewable natural resources, but also possess a great potential for bio and photo degradability. The latter advantage is more notable in the urgent need for sustainable environmental protection.
Environmental concerns have created a drive to assess novel methods for producing items using renewable and biodegradable resources as opposed to the synthetic and petrol-based materials usually used. The major disadvantage of present day polyurethane foams is that they are produced from non-renewable, non-recyclable, and non-biodegradable materials, such as polyurethane. The large abundance of natural cellulose from plant biomass, along with its biodegradability properties, makes it an attractive alternative to such environmentally toxic materials. Cellulose however must first be converted into a liquid form by either direct dissolution or by derivatization and subsequent dissolution of the derivative to produce flexible forms [4]. The interest in biodegradation testing is growing rapidly and toxicity testing is now gradually incorporated in environmental legislation in many countries. The main objective of the study is to assess the biodegradation ability of the forms produced in soil and in the laboratory.
Material and Methods
Sugar bagasse for the cellulose was sourced locally from Aviele in Etsako west LGA, Edo State, Nigeria. All reagents used were products of Sigma Aldrich, Germany sourced from a commercial sales outlet in Ondo State, Nigeria.
Preparation and characterization of the polyurethane foam
The cellulose microfiber from the sugar bagasse was isolated and characterized using standard methods. The preparation and characterization of polyurethane foams is as described in literature [5,6]. To measure bulk density, the foams were cut into cubes with 2.00 cm sides, mass being determined with an analytical balance. The mechanical properties were analyzed according to the compression method described by ASTM-D695M [7]. Compression stress at 10% strain. The surface morphology of the polyurethane foam was examined using scanning electron microscope (SEM). Scanning electron microscopic (SEM) studies of foams were carried out on electron microscope.
Biodegradation experiments
During the experiments, American society for testing materials (ASTM) methods designed for testing biodegradation of plastics under simulated environmental conditions [8] and other methods currently used in the published literature were used [9]. Since the burial of dumb-bell shaped pieces in soil followed by tensile strength testing is a standard method for assessing the susceptibility of plastics to biodegradation [10] the foams were buried in Eborieme market, Auchi Polytechnic, Nigeria, waste dump soil to stimulate the relevant environmental conditions in the waste dump.
Bioavailability studies
A series of batch cultures were set up to evaluate the biodegradability and to monitored degradation of the polyurethane foams. The weighed polyurethane foams were enclosed in a lidless plastic bottle of known weight, and the bottle was buried in the waste dump at an angle of 45°C and it is opening downwards, and then buried with the soil. At an interval of 2 weeks period, the bottle was taken out and weighed for eight weeks.
The degradation was monitored by measuring the weight of the foams before and after incubation in the soil containers. The foams were taken out and washed with distilled water and ethanol to remove debris on the surface. Then, foams were dried to constant weight.
The degradation percentage (DP) of the foams was calculated using the following equation
% DP = [(Wo – W1)/W1] x 100 (i)
Where Wo and W1 were the wet weights of the foams in the bottle before and after degradation.
Laboratory microbial analysis of the polyurethane foams for biodegradation
The methods currently used in testing microbiological degradation and deterioration of a wide range of polyurethane foam materials have been recently reviewed by Gu and Gu [11,12]. Among them the most commonly practiced methods to assess the biodegradability of polyurethane foams in different environmental conditions are tensile strength, weight loss, and bacterial growth. Physical examination of foams for deterioration is considered as an important method for assessing the biodeterioration of the foams [13] because physical changes in the structure of polyurethane foams are more likely to occur before complete degradation of foam takes place. The degree of deterioration of sampled foam was assessed each week by measuring changes in selected physical properties including tensile strength and weight loss after appropriate sample cleaning procedures. The weight loss percentage (WL) of the foams were calculated using the following equation
% WL = [(Wo – W1)/W1] × 100 (ii)
Where Wo and W1 will be the wet weights of the polyurethane foam in the bottle before and after degradation.
Tensile strength measurements
Tensile strength is a measure of the force, generally given in pounds per square inch (psi), required to break the polymer foam. The tensile strength at breakpoint of the foam was measured by ASTM procedure D638 [14] using an Instron Universal Testing Instrument.
Growth assay
The total number of microorganisms in the sample which could have the ability to degrade and deteriorate the foams were determined by direct microbial population density/count and expressed as number of colony forming units (CFU) per ml. 2 ml of sample taken from the collected sample was stained with 0.2 ml of 0.1% Acridine orange. The stained microbial mixture could stand at room temperature for 1 or 2 min for the reaction with the stain. Then, a treated filter paper was placed in a vacuum filter funnel and the sample was filtered to get a distribution of colonies on the filter paper. Then, the damp filter was placed on a glass microscope slide (2 cm × 5 cm) for analysis. The number of microorganisms per millimeter area is estimated from a count of at least 5-6 randomly chosen microscope fields.
Characteristics of waste dump soil
Top soil samples (0 cm to 15 cm, depth) were collected from the site using auger. Core samplers were used to obtain samples for bulk density determinations. Except for core samples, other soil samples were air-dried and sieved using 2 mm sieve preparatory to laboratory analysis.
Sampling and preparation of the soil
A small pit was dug to a depth of 15 cm in a sandy loam soil at the waste dump site and samples were taken from the wall of this by cutting slices out with a spade. The samples were air dried for three days, mixed, lightly crushed with a piece of wood to remove large lumps, plastics and then pass through the 2 mm sieve, and roots were removed. The portion that passed through the sieve was used as the study. Samples of the soil and characterized in terms of their physico-chemical and biological properties using standard methods.
Results and Discussion
Preparation and characterization of the cellulose based polyurethane foams
The isolation, characterization of the cellulose microfiber from sugar bagasse and the preparation and characterization of the polyurethane foams have been reported [15].
Biodegradation Analysis
Physico-chemical properties of the waste dump soil
Table 1 shows the some of the physical characteristics and Table 2 the chemical characteristics of the waste dump soil.
| Analytical characteristics | Results |
|---|---|
| Particle size distribution (%) | Clay 16.38 |
| Silt 14.40 | |
| Sand 69.22 | |
| pH | 8.10 ± 0.01 |
| Bulk Density (g/cm3) | 1.33 ± 0.02 |
Table 1. Result of physical characterization of the soil sample.
| Analytical characteristics | Results |
|---|---|
| Soil organic carbon, g/kg | 0.544 ± 0.05 |
| Total Nitrogen, (%) | 0.750 ± 0.008 |
| Cation exchange capacity, mg/kg | 286.02 ± 0.92 |
| Phosphorus, mg/kg | 186.32 ± 0.01 |
| Sodium content, mg/kg | 7.18 ± 0.07 |
| Potassium content, mg/kg | 2.70 ± 0.05 |
| Magnesium content, mg/kg | 67.63 ± 0.25 |
| Calcium content, mg/kg | 208.51 ± 0.55 |
| Lead (mg/Kg) | 0.05 |
| Cadmium (mg/Kg) | ND |
| Arsenic(mg/Kg) | ND |
| Copper (mg/Kg) | 0.002 |
| Total acidity | 0.2 |
ND=Not Detected
Table 2. Result of chemical characterization of the soil sample before planting.
Preliminary visual inspection showed that the soil was dark grey in colour indicating a low amount of humus. Colour is one of the characteristics of soil, which tells much about the origin of the soil and its composition [16]. Textural analysis showed the preponderance of sand fraction (69.22%), followed by clay (16.38%), then silt (14.40%), thus classifying the soil as sandy loam soil. The slightly alkaline pH 8.10 recorded for the soil is within the range of agricultural soils. Soil pH plays a major function in the sorption of heavy metals as it directly controls the solubility and hydrolysis of metal hydroxides, carbonates and phosphates. It also influences ion-pair formation, solubility of organic matter, as well as surface charge of Fe, Mn and Al-oxides, organic matter and clay edges [17]. The soil pH is an indication of the soil’s acidity or alkalinity. The soil pH (8.1) can also be interpreted in terms of the very low level of total acidity (0.2). Which means low presence of hydrogen ions in the soil.
The soil bulk density (1.33 g/cm3) is an indication of the textural nature of the soil. Bulk Density of soil is defined as the mass (weight) of a unit volume of dry soil. It is useful in estimating the differences in compaction of a given soil.
The soil had an average cations exchange capacity (CEC) of 286.02 mg/kg. The CEC parameter particularly measures the ability of soils to allow for easy exchange of cations between its surface and the solution. The soil organic carbon recorded as 0.544 g/kg, is an indication of the soil organic matter or the amount of humus present in the soil.
However, the rate of decomposition of organic matter in soil usually depends on the amount of nitrogen present [17]. The total nitrogen recorded in the soil is given as 0.750%, indicating the low level of humus in the soil assessed by the carbon, nitrogen ratio and the high rate of organic matter decomposition in the soil. The mean aerobic bacterial count for the soil sample is 1.94 × 104 cfu/g for the soil sourced from the dump site. The mean heterotrophic fungal counts is 3.8 × 103 cfu/g. Six bacterial and four fungal isolates were characterized and identified from the dumpsite soils. The bacterial isolates were; Bacillus sp., Pseudomonas sp., Aeromonas sp., Enterobacter sp., Klebsiella sp. and Staphylococcus sp. While the fungal isolates were; Aspergillus sp., Mucor sp., Saccharomyces sp. and Fusarium sp. respectively. Both Bacillus sp. and Pseudomonas sp. were the most dominant amongst the bacterial isolates whilst Staphylococcus sp. was the least occurring bacterial isolate. Aspergillus sp. had the highest frequency of isolation while the least isolated fungal culture was Saccharomyces sp. This phenomenon might be the result of the increased availability of biodegradable organic and inorganic substrates from the wastes continuously being dumped at the site. The isolation of Bacillus sp., Pseudomonas sp., Klebsiella sp., Staphylococcus sp., Aspergillus sp., Mucor sp., Fusarium sp. and Saccharomyces sp. from the soil samples, was similar to the report of Obire et al. [18]. Aspergillus, Fusarium, Mucor, Penicillium, Rhizopus and a variety of yeasts have also been reported to be associated with waste biodegradation [19].
Laboratory Biodegradation Analysis
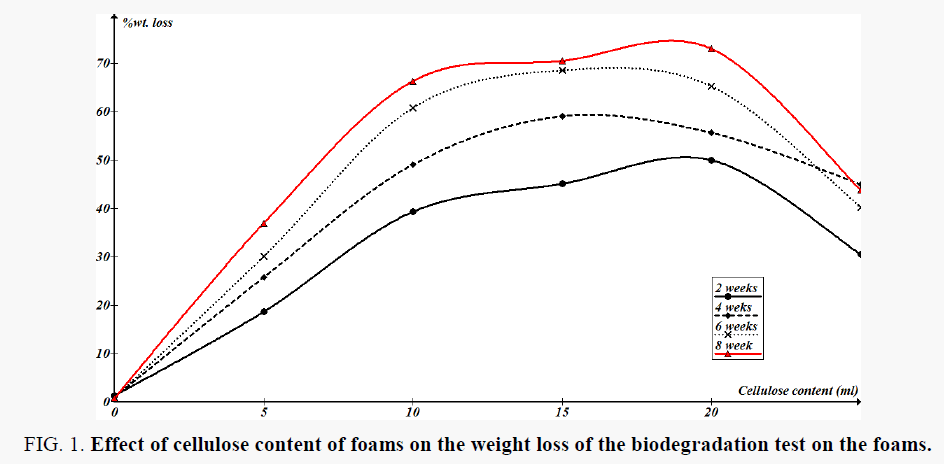
Table 3 shows the bacterial count and fungal counts in the foams culture in the laboratory; Figure 1 shows the weight losses in foams as function of treatment time in the shake-flasks. The obtained weight loss of foams ranging from 0.76% to 72.99% can be considered as statistically significant based on the weight loss of control samples during the experiments. Tensile strength of the treated foams at break can be considered as indicative of microbial deterioration under anaerobic conditions [20].
Figure 1. Effect of cellulose content of foams on the weight loss of the biodegradation test on the foams.
| Samples Foam with cellulose content (ml) |
Mean aerobic bacterial count (cfu/g) | Mean fungal count (cfu/g) |
|---|---|---|
| 0 | 8.9 × 103 | 2.9 ×103 |
| 5 | 5.2 × 103 | 7.8 ×102 |
| 10 | 1.3 × 104 | 2.4 ×104 |
| 15 | 1.9 ×104 | 4.9 ×104 |
| 20 | 9.7 ×103 | 1.4 ×104 |
| 25 | 4.8 ×103 | 8.1 ×103 |
Table 3. Total bacterial and heterotrophic fungal counts in the foams culture.
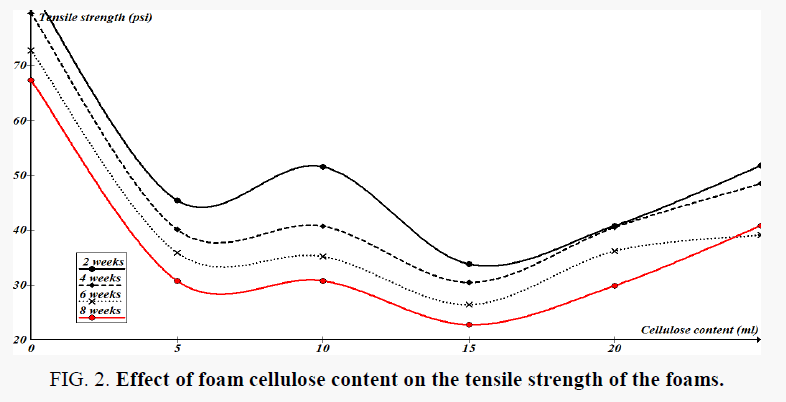
Degradation was assessed quantitatively by measuring changes in tensile strength at failure of dumb-bell-shaped foams prepared according to ASTM D 638 method. From tensile strength analysis of the foams, it can be concluded that there is deterioration on the surface of the foams during these bioassays. The tensile strength results are important because it has been reported that mechanical failure of the foams occurred before the weight loss [20,21]. During initial degradation of the foam material with 0.76% weight loss, the tensile strength loss was 66%, (Figure 2) hence foam material lost affects its mechanical properties significantly [22]. These observations suggest that weight loss measurement alone is not a reliable end point to assess the foam biodeterioration. The results from this study show that there is significant biodeterioration of the foams based on both weight loss and tensile strength measurements.
Soil burial biodegradation test
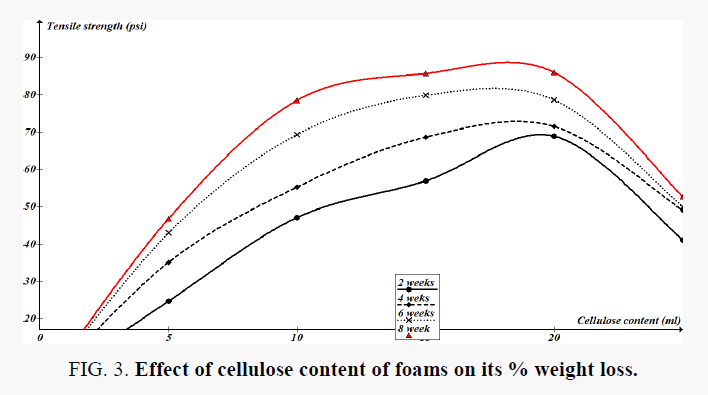
Three sets of three replicate of the foams were buried for 8 weeks in containers in the waste dump site soil to get statistically significant average data on degradation of the foams under anaerobic conditions in soil (Figure 3).
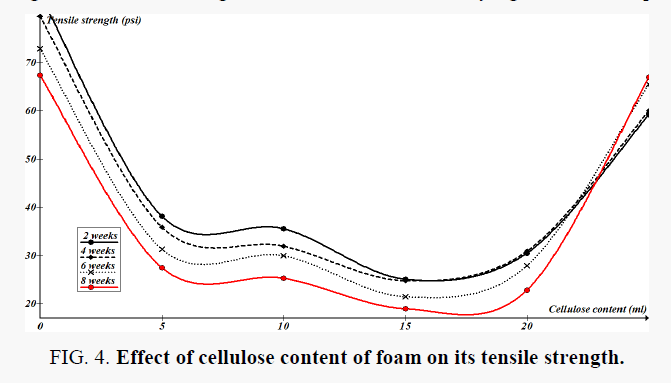
Figure 3 shows the cumulative weight loss in test foams during soil burial experiments. Dry weight analysis of 8 weeks treated samples showed an average of 58% weight change. The results indicate statistically significant weight loss in the foams, within the 5% probability level. It can also be seen that there is distinct trend in the weight loss of the forms with time and hence it can be concluded that the foams are not affected by potential biological activity in the soil. Tensile strength measurements were performed on biweekly removed test of the foams and the results for the three replicate averages are shown in Figure 4. The changes in the tensile strength of the foams are statistically significant at 5% probability level.
Kim and Kim [23] assessed the degradation of several types of polyurethane foams under accelerated composting conditions for 45 days to determine the biodegradability of the foams in the environment by natural degradation process. Bentham et al. [24] also performed soil burial experiments to evaluate the susceptibility of different polyurethane materials to microbial attack for a period of 28 days incubation. The biodegradability studies during this research were carried out under similar accelerated conditions. The foams were exposed to inoculum taking from anaerobic digester sludge which contains large and diverse group of biologically active anaerobic bacteria.
Humidity and nutrient rich environment are other important parameters affecting the rate and extent of decomposition of organic materials including polyurethane foams. Baldwin et al. [25] reported that moisture content was a major factor affecting sanitary materials in the landfills after 6 years of burial because the wetter the samples, the quicker is the decomposition of materials. It has been also reported in another study that the impact of low relative humidity to microbial growth is much more than that of decreasing temperature from 30°C to 22°C [26,27].
Conclusion
Based on the results of the weight loss measurements, and tensile strength measurements, buried in the soil and the control samples, it can be concluded that there is significant biodeterioration of the foams due to potential microbial activity in the field soil under anaerobic conditions during these short-term experiments.
References
- Zulkali M, Ahmad L, Norulakmal H, et al. As heavy metal adsorbent: Optimization with lead as model solution. Bioresoue technol. 2006;31;97(1):21-5.
- Salamatinia B, Zinatizadeh A, Kamaruddin H, et al. Application of response surface methodology for the optimization of Cu and Zn removals by sorption on pre-treated oil palm frond (OPF). Iran J Chem Eng. 2006;3(2):73-84.
- Jang A, Seo Y, Bishop PL. The removal of heavy metals in urban runoff by sorption on mulch. Environ pollut. 2005;31;133(1):117-27.
- Basci N, Kocadagistan E, Kocadagistan B. Biosorption of copper (II) from aqueous solutions by wheat shell. Desalination. 2004;164(2):135-40.
- Gratuito K, Panyathanmaporn T, Chumnanklang A, et al. Production of activated carbon from coconut shell: Optimization using response surface methodology. Bioresou Technol. 2008;99(11):4887-95.
- Box E, Draper R. Empirical model-building and response surfaces. 1987 Wiley, New York.
- Montgomery C. Design and analysis of experiments, 6th edition. John Wiley & Sons, Inc. New York 2005;67-99.
- Karacan C. Evaluation of the relative importance of coalbed reservoir parameters for prediction of methane inflow rates during mining of longwall development entries. Comput Geosci. 2008;34(9):1093-114.
- Okieimen E, Nkumah E, Egharevba F. Studies on the grafting of acrylic acid on starch. Eur polym J. 1989;25(4):423-6.
- Ekebafe LO, Ogbeifun DE, Okieimen FE. Removal of heavy metals from aqueous media using native cassava starch hydrogel. Afr J Environ Sci Technol. 2012;6(7):275-82.
- Ekebafe LO, Ogbeifun DE, Okieimen FE. Effect of native cassava starch-poly (sodium acrylate-co-acrylamide) hydrogel on the growth performance of maize (Zea may) seedlings. Am J Polym Sci. 2011;1(1):6-11.
- Ko DC, Cheung CW, Choy KK, et al. Sorption equilibria of metal ions on bone char. Chemosphere. 2004;54(3):273-81.
- Jadhav DN, Vanjara AK. Removal of phenol from wastewater using sawdust, polymerized sawdust and sawdust carbon. In J Chem Technol. 2004; 11:45.
- Aisien FA, Amenaghawon NA, Adeboyejo AR, et al. Application of Recycled Rubber from Scrap Tire in the Removal of Phenol from Aqueous Solution. Pac J Sci Technol. 2013;14(2):330-41
- Zheng Y, Hua S, Wang A. Adsorption behaviour of Cu2+ from aqueous solutions onto starch-g-poly (acrylic acid)/sodium humate hydrogels. Desalination 263: 117.
- Chauhan GS, Singh B, Sharma RK, et al. Use of biopolymers and acrylamide-based hydrogels for sorption of Cu2+, Fe2+ and Cr6+ ions from their aqueous solutions. Desalination. 2006;197(1-3):75-81.
- Al-Abachi Q, Al-Awady S, Al-Anbakey M. Removal of Iron (Iii) Ion from aqueous solution using polyacrylic acid hydorgel beads as adsorbent. Iraqi J Sci. 2013;54:775-81.
- Gundogan R, Acemioglu B, Alma MH. Copper (II) adsorption from aqueous solution by herbaceous peat. J Colloid Interface Sci. 2004;269(2):303-9.
- Ho YS, Mckay G. The sorption of lead (II) ions on peat. Wat. Res, 1999;33:585.
- Gupta SS, Bhattacharyya KG. Removal of Cd (II) from aqueous solution by kaolinite, montmorillonite and their poly (oxo zirconium) and tetrabutylammonium derivatives. J Hazard Mater. 2006;128(2):247-57.
- Jiang MQ, Wang QP, Jin XY, et al. Removal of Pb (II) from aqueous solution using modified and unmodified kaolinite clay. J Hazard Mater. 2009;170(1):332-9.
- Ayd?n H, Bulut Y, Yerlikaya C. Removal of copper (II) from aqueous solution by adsorption onto low-cost adsorbents. J Environ Manage. 2008;87(1):37-45.
- Vijaya Y, Popuri SR, Boddu VM, et al. Modified chitosan and calcium alginate biopolymer sorbents for removal of nickel (II) through adsorption. Carbohydr polym. 2008;72(2):261-71.
- Borah D, Senapati K. Adsorption of Cd (II) from aqueous solution onto pyrite. Fuel. 2006;85(12):1929-34.
- Kula I, Ugurlu M, Karaoglu H, et al. Adsorption of Cd (II) ions from aqueous solutions using activated carbon prepared from olive stone by ZnCl 2 activation. Bioresou Technol. 2008 ;99(3):492-501.
- Romero I, Ruiz E, Castro E, et al. Acid hydrolysis of olive tree biomass. Chem Eng Res Des. 2010;88(5):633-40.
- Eren Z, Acar FN. Adsorption of Reactive Black 5 from an aqueous solution: equilibrium and kinetic studies. Desalination. 2006;194(1-3):1-0.