Original Article
, Volume: 14( 1)Synthesis, Structural and Morphological Studies of Pine Cone Powder by Fenton Oxidation and Grafting With Acrylic Acid Using Ammonium Ceric Nitrate as Initiator
- *Correspondence:
- Mabaso N , Biosorption and Water Research Laboratory, Department of Chemistry, Vaal University of Technology, P. Bag X021, Vanderbiljpark 1900, South Africa, Tel: +27 9506695; E-mail: noziphom@vut.ac.za
Received: November 21, 2017; Accepted: January 22, 2018; Published: January 26, 2018
Citation: Mabaso N, Naidoo B, Ofomaja A, et al. Synthesis, Structural and Morphological Studies of Pine Cone Powder by Fenton Oxidation and Grafting With Acrylic Acid Using Ammonium Ceric Nitrate as Initiator. Nat Prod Ind J. 2018;14(1):116
Abstract
Chemical grafting of acrylic acid onto pine cone powder was carried out using free radical initiation. Pre-treatment with Fenton oxidation was used for the removal of plant organic components an enhancement of the accessibility of cellulose for ceric ammonium nitrate initiator and monomer. The effects of temperature, monomer (Acrylic Acid), initiator (CAN), time, acid (HNO3) and mass of pine were investigated. The optimum conditions were 70°C, monomer ratio 7:10, initiator 1 g, Fenton pine cone powder 1 g, 0.9 mol/L HNO3 and time was 60 minutes. Acetone and tetrahydrofuran (THF) was used to remove homopolymer from the graft copolymer. A relatively high percentage of grafting was achieved when optimum reaction conditions were used. The Fenton pre-treatment of the pine cone powder before grafting provided hydroxyl radical, which improved grafting efficiency. Furthermore, the advantages of using CAN provided high grafting efficiency and lower amount of homopolymer formation. The grafted copolymer was characterized by FTIR, TGA, DSC, SEM, XRD and EDS analysis.
Keywords
Grafting; Acrylic acid; Ceric ammonium nitrate; Fenton reagent; Pine cone powder
Introduction
Green chemistry has generated a renewed awareness on novel composites derived from a variety of agricultural waste materials [1]. Agricultural by-products are available in large quantities and have potential as inexpensive sorbents. These natural materials are eco-friendly due to their unique chemical composition [2]. Pine cone is lignocellulose material; it is an abundant natural resource from wood industry. It belongs to the class of Gymnosperma and family of Pinaceae, is a renewable resource and large quantities are produced annually [2]. Pine plantations are grown for the furniture, building and paper industry and the cones are available as waste product. Nurseries grind the cones into mulch or they are sold as crafts, but consumer demand for these cones are small compared to by-products from other industries [3].
These pine cone are composed of mainly cellulose (18.8%), hemicellulose (46.5%) and lignin (37.4%) [4,5]. Hence, the chemical or biological treatment to prevent the leaching of colored organic compounds such as tannins and to depolymerize others such as lignin is necessary [3]. Different modification techniques which include the use of organic acids (sulphuric acid, nitric acid, phosphoric acid, hydrochloric acid, citric acid, tartaric acid), strong bases (calcium hydroxide, sodium hydroxide, sodium carbonate), oxidising agents (Fenton’s reagent, potassium permanganate), organic compounds (ethylenediaminetetraacetic acid, formaldehyde, epichlorohydrin), grafting and solvent extraction have been reported in literature by several authors [2,5-8].
It was reported that chemical treatment solubilized a small fraction of the lignin and soluble organics in the plant waste and improved the penetration of modifying agents [4]. They further reported that chemical grafting using radical initiators is known to form covalent linkages between biosorbent and polymer molecules that are not reversible under low solution pH conditions. Grafting using different monomers onto cellulosic or lignocellulosic materials has been used to enhance their chemical and physical properties. Grafting acrylonitrile onto pineapple leaf fibers improved their thermal properties, acrylamide onto coconut husk produced a selective adsorbent for mercury (II) and acrylamide onto sisal fibers improved their moisture absorption [8]. To improve structural characteristics of the initial material pre-treatment is necessary for removing plant organic components and enhancement for the accessibility of the cellulose by the initiator and monomer [9]. An increased in grafting percentage was reported when pretreated cellulose fiber by passing nitrogen through the aqueous solution agitated in the Fenton reagent initiator [10]. Hence, the type of pre-treatment and initiator has an important effect on the grafting and it determines the grafting percentage depending on the monomer to be grafted [9]. The pre-treatment by Fenton reagent (Iron (II) hydrogen peroxide) is cheap because it involves the addition of hydrogen peroxide to a solution containing cellulose in the presence of ferrous salts, creating and primary hydroxyl [4]. Additionally, the type of initiator has an important effect on the grafting, for example ceric ion offers many advantages because of its high grafting efficiency and lower amount of homopolymer formation. The radical formation on the cellulose backbone may occur either on the carbon (C-6) or oxygen atom of methylol (–CH2OH) group [9]. Therefore, this study seeks to (1) pre-treat the surface properties of pine cone powder by using Fenton reagent and (2) graft with acrylic acid using ceric ammonium nitrate as initiator. The resulting composite will then be characterized using FTIR, TGA, DSC, SEM, XRD and EDS.
Materials and Methods
Sample collection
The cones were collected from pine trees (class Gymnosperma and Pinaceae family) in Vanderbijlpark, Gauteng province in South Africa. Megasporophylls (female pine cone) are a cone scale that was used for this study. They were washed and dried in an oven at 80°C for 48 hours to remove impurities and some volatile organics such as resin acids, without destroying the pine cone matrix. The cone scales were peeled and crushed to a powder using a pulveriser then sieved in order to obtain particles in the size range 45 μm-90 μm for later experimental use.
Reagents
All reagents were purchased from Sigma-Aldrich or Merck and were used without further purification: H2O2 (30%), FeCl2.4H2O (98%), HCl (32%), HNO3 (70%), ammonium ceric nitrate (98.5%), acrylic acid (99%), acetone (99.5%) and tetrahydrofuran (99.5%).
Physical measurements
Infrared Spectra (500 cm-1-4000 cm-1) were recorded on an FTIR/FT-NR Spectrometer Perkin Elmer Spectrum Model. TGA and DSC were recorded using Thermal Analyzer (STA) 6000. The chemical composition and morphology were investigated by a Scanning Electron Microscope (SEM/EDS) at different KV magnifications, using a VEGA 3 TESCAN model. Particle size distribution was measured using a laser diffraction technique (Malvern Mastersizer 2000 Instrument).
Synthesis: The sieved pine cone powder was then divided into two parts. The first part was used untreated and the second was modified with Fenton’s reagent and then grafted with acrylic acid.
Fenton’s reagent: Fenton’s reagent was prepared by measuring 303 mL of 30% H2O2 and 6.993 g of Fe2+ as FeCl2.4H2O, which were separately placed in 1000 mL flasks containing distilled water. The pH of the Fe2+ solution was adjusted to between 3 and 4.5 with 0.1 M HCl. An Adwa AD8000 pH meter was used for the pH measurements. In a 500 mL three-necked round-bottomed flask with a nitrogen inlet and magnetic stirrer, 250 mL of Fe2+ and 50 g of pine cone powder were mixed and heated at 50°C for 30 minutes. To this mixture, 250 mL of the prepared H2O2 was added and the heating was continued for a further 30 minutes. The resulting bleached/oxidised mixture was filtered under suction with a WhatmanTM filter paper and the residue was washed with distilled water until the filtrate was clear and then dried overnight at 80°C.
Grafting: TABLE 1 shows the values of the materials and conditions used for the grafting procedure. Fenton-treated pine cone powder was mixed with 20 mL distilled water, then added nitric acid and placed the mixture in the reaction setup. Ceric ammonium nitrate (CAN) was dissolved in distilled water then added to the mixture. The mixture was heated at the required temperature under vacuum for a few minutes then switched to nitrogen gas for 15 minutes. The required ratio of acrylic acid was added to the mixture gradually over 15 minutes (TABLE 1), with continuous stirring. The mixture was then centrifuged and the solid washed with distilled water to remove trace of initiator and acid, dried at room temperature for 24 hours then washed again with acetone (2 × 30 ml) and (2 × 30 ml) tetrahydrofuran (THF) to remove homopolymer. The grafting and efficiency for the composite were calculated using the formulas below:
| Mass of pine (g) | 1.0, 1.5, 2.0, 2.5, 3.0 |
| Mass of CAN (g) | 1.0, 1.5, 2.0, 2.5, 3.0 |
| Temperature (°C) | 30, 40, 50, 60, 70, 80 |
| Time (minutes) | 15, 30, 45, 60 |
| [HNO3] (M) | 0.3, 0.6, 0.9, 1.1 |
| Acrylic acid ratios | 2:10, 3:10, 4:10, 5:10, 6:10, 7:10, 8:10, 9:10, 10:10, 11:10, 12:10, 13:10, 14:10 |
Table 1: Variables that were used for the grafting procedure.
Grafting percentage (%)=[(W1–W0)\W0)] × 100 (1)
Where, W1 and W0 are the weights of the grafted copolymer and the original Fenton treated pine cone powder (FPCP), respectively [9].
Grafting efficiency (GE) shows the fraction of monomer grafted onto FPCP among the amount of monomer converted to graft polymer plus the remaining homopolymer, which means the fraction of polymer which is grafted to FPCP in total polymer and it is calculated by the equation given below:
Grafting efficiency (%)=[(W1– W0)\ (W1–W0 +W2)] × 100 (2)
Where, W1, W0 and W2 are the weights of the graft copolymer, the original FPCP and the homopolymer, respectively [9].
Results and Discussion
Fourier transform infrared (FTIR) analysis
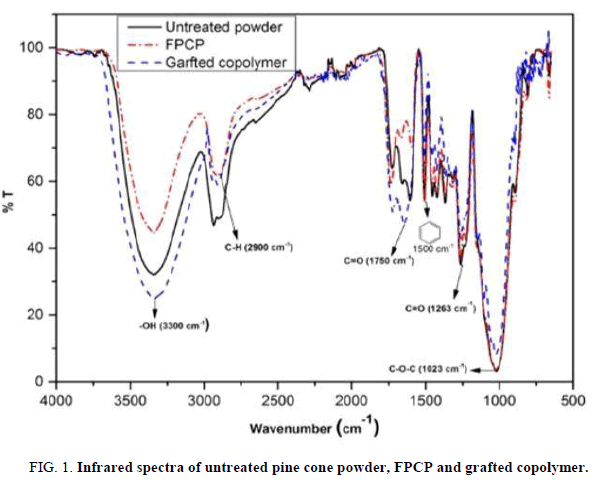
FIG. 1 shows the infrared spectra for raw powder, FPCP and grafted copolymer. The major peak at 3300 cm-1 corresponds to the –OH band and that at 2900 cm-1 to the C–H band; the main difference between them is their intensities. The –OH band for FPCP declined relative to that the raw powder, which indicated the possibility that hydroxyl groups were removed during the Fenton treatment [7].
The increase in intensity of the –OH band for the grafted copolymer is evidence that grafting with acrylic acid was achieved [4]. The C=O band at 1750 cm-1 indicates the asymmetric and symmetric stretching vibrations of the ionic carboxylic acid group.
The grafted copolymer signal increased in intensity, indicating that the carboxylic functions had been grafted onto the surface of the FPCP material. The weakening of the peak at 1263 cm-1 of grafted copolymer due to deformation vibration of C=O and stretching formation of OH for carboxylic acid. The peak between 2750 cm-1 and 2900 cm-1, which is attributed to aliphatic C–H stretching, decreased for both FPCP and grafted copolymer. This may have been caused by the aliphatic fraction of waxes, which decreased during the Fenton treatment [7].
The peak at 1500 cm-1 was due to the benzene ring [4,7] from lignin [11]. The lignin finger–print region (1830 cm-1–730 cm-1) might be rich in methoxyl –O– CH, C–O–C stretching and C=C (aromatic ring) containing compounds [12].
FTIR has revealed the functional groups that were present in the three materials (such as hydroxyl, carboxylic, methoxyl and benzene groups). The band intensity of the –OH group for grafted copolymer increased compared to the FPCP result, which shows that the oxidizing agent did oxidize some functions on the lignocellulosic material and that radical grafting was achieved [13]. The decrease in intensity of the C–H absorption band for FPCP may be attributed to the reduction of hydrocarbon content, which resulted from reaction of the starting material (raw powder) with the Fenton reagent and grafting with acrylic acid. On the other hand, the weakening of the C–O–C band for the grafted copolymer confirms that the cellulose become degraded during grafting [14].
Thermogravimetric (TGA) analysis
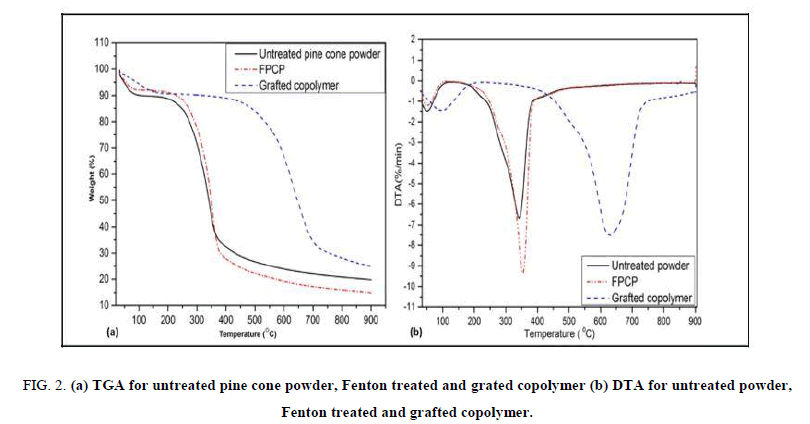
TGA was performed to determine mass change in the composite, which happens at a molecular level, as a function of time and temperature. Thermal degradation of the pine cone biomass occurred in three phases: (1) moisture evolution from 30°C-150°C, (2) hemicelluloses and cellulose decomposition from 200°C -350°C and (3) lignin decomposition from 160°C-700°C as shown in FIG. 2 (a) and (b) [15]. The mass losses are observed in the temperature range 200°C-375°C for untreated powder and grafted copolymer from 450°C-750°C.
Figure 2: (a) TGA for untreated pine cone powder, Fenton treated and grated copolymer (b) DTA for untreated powder, Fenton treated and grafted copolymer.
FIG. 2 (a) shows the first phase for initial mass loss at low temperatures below 150°C for the raw powder and FPCP, which can be explained by water molecules evaporating from the material due to the hygroscopic nature of the pine cone surface [4,16]. Grafted copolymer showed an initial weight loss at about 150°C, which is slightly different from that of raw powder and FPCP. Mass losses during this stage were about 10% for the raw pine, 7% for the FPCP and 8% for grafted copolymer. The difference in mass losses indicates a decrease in surface hydrophilic groups, which signifies an increase in the hydrophobic character for FPCP and grafted copolymer due to the Fe2+/H2O2 treatment and acrylic acid grafting. This information supports the results obtained from the FTIR in FIG. 1, which shows a loss of hydroxyl functions for both FPCP and grafted copolymer.
Second phase (FIG. 2 (a)) the decomposition of hemicelluloses at 100°C–200°C and cellulose 200°C–300°C for both raw and FPCP. Grafted copolymer was slightly different, hemicellulose decomposition was at 300°C–450°C, while the cellulose decomposed at about 450°C–700°C. The mass loss of about 55% and 50% for raw powder and FPCP, respectively. However, the mass loss for the grafted copolymer was about 60%. It was reported that hemicelluloses decomposes at lower temperatures due to their random and amorphous structure [17]. Cellulose consists of both amorphous and crystalline regions, with the amorphous regions being oxidized at a lower temperature, whereas the crystalline regions are oxidized at a higher temperature.
The third phase (FIG. 2 (a)) that is attributed to the decomposition of lignin at about 100°C–700°C for raw powder and FPCP, respectively while for the grafted copolymer at about 200°C–700°C was due to lignin degradation. Lignin is chemically different from hemicellulose and cellulose, because it is composed of three kinds of benzene–propane, units and is heavily cross-linked and has a relatively high molecular weight [17,18]. Additionally, the thermal stability of lignin is also relatively high. The shift that was observed for the grafted copolymer may be attributed to the grafting of acrylic acid onto FPCP that improved the thermal stability of grafted copolymer. Enhanced thermal and physico-chemical properties of cellulose pine needles grafted with butyl acrylate were observed [19].
Grafted copolymer showed improved thermal stability from both TGA and DTA curves. The initial decomposition temperature for grafted copolymer increased from 250 to 450°C. It was observed that Tmax shifted to 750°C for grated pine cone powder as seen in FIG. 2 (b), compared to a decomposition temperature of 350°C of untreated pine cone powder and FPCP. These observations clearly indicate that grafting improved the thermal stability of FPCP. Thermal stability was found to be dependent on degree of grafting onto pine [20].
X-ray diffraction (XRD) analysis
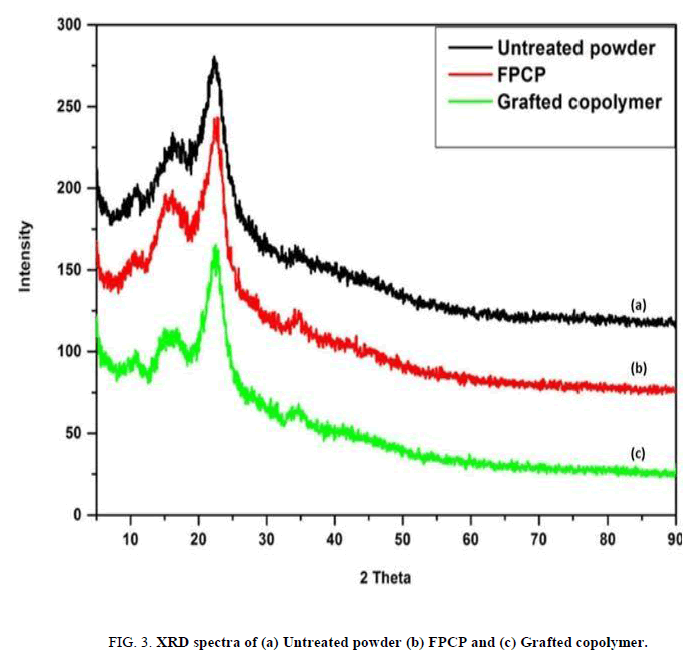
XRD was used to determine the crystallographic structure of the study materials. The corresponding spectra are represented in the FIG. 3. Compared to the spectrum of untreated powder, those of the FPCP and grafted copolymer show an increase in intensity and broadening of the peaks at 2θ=11°, 15.3°, 22.6° and 34.5°. The spectra reveal that pine cone is composed mainly of crystalline cellulose as indicated by the peak at 2θ=22.6° [17].
The peaks at 2θ=15° and 34.5° intensified because the crystalline nature of the FPCP and grafted copolymer increased. The further increase of peak 2θ=34.5° in FIG. 3 (c) may be because the amorphous phase of grafted copolymer was further enhanced by the initiator (CAN) creating more reaction sites on the cellulose backbone as a result of the incorporation of acrylic acid [21].
Scanning electron microscopy (SEM) analysis
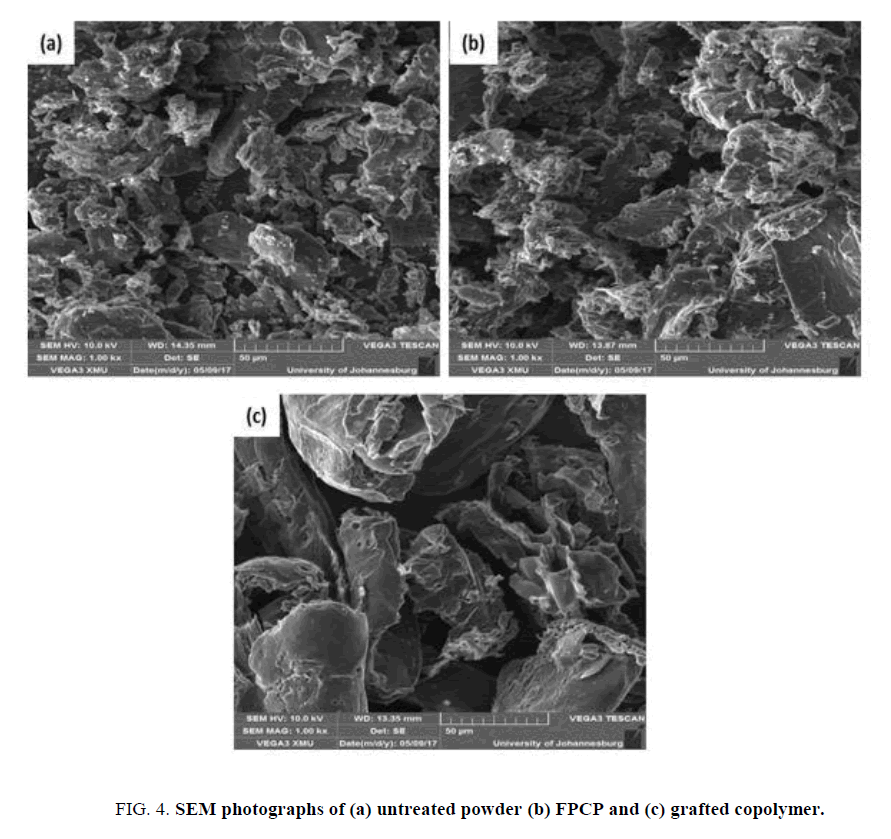
The surface features of the three study materials were examined by SEM, which are illustrated in FIG. 4. The surface of the raw powder was uneven, with small particles visible that may be due to the lignin and other extracts. The FPCP shows less surface roughness and the small particles are absent, which may be because of the removal of lignin and other extracts such as soluble organic substances [22]. The smooth surface of the grafted copolymer is an indication that acrylic acid was coated on the surface of the FPCP.
Energy dispersive spectroscopy (EDS) analysis
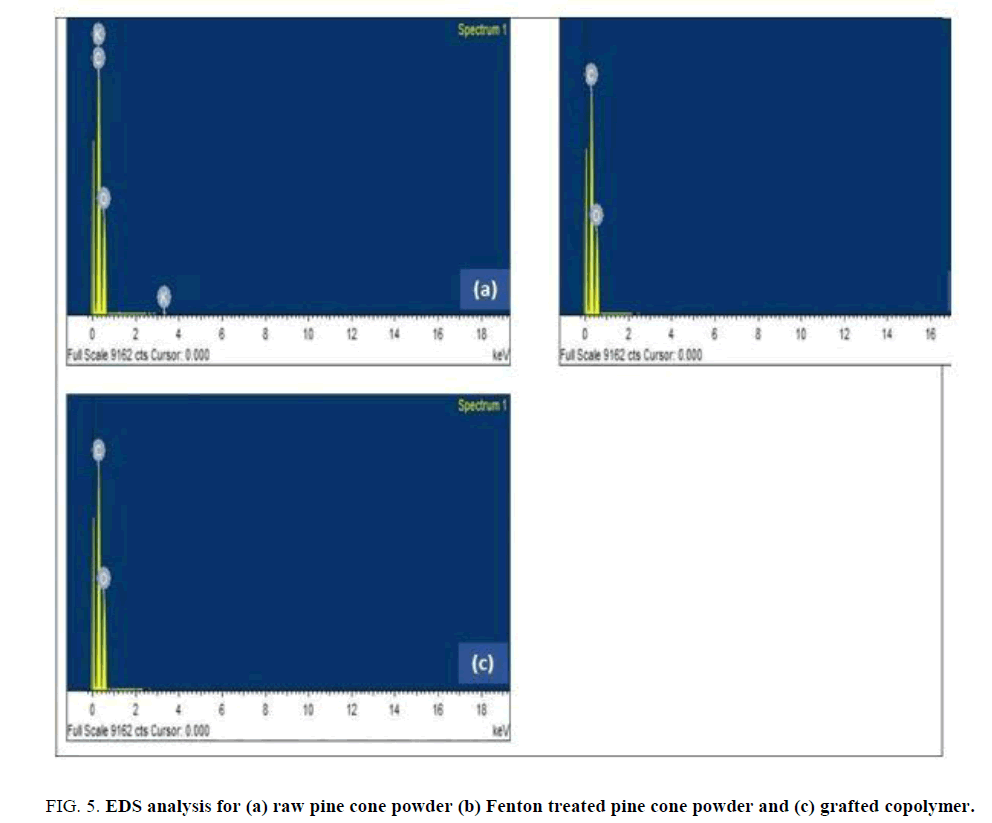
FIG. 5 shows the EDS analysis that was performed on raw pine cone powder, FPCP and grafted copolymer. The EDS spectrum (FIG. 5 (a)) represents the raw pine cone powder peaks corresponding to the energy levels for which the potassium, carbon and oxygen. The comparison of potassium, carbon and oxygen percentage, both potassium and carbon percentage are higher than the oxygen. The potassium can be due to the impurity or the extracts.
Figure 5: EDS analysis for (a) raw pine cone powder (b) Fenton treated pine cone powder and (c) grafted copolymer.
The Fenton treated pine cone and grafted pine cone powder (FIG. 5 (b) and (c)) possess the skeleton of only carbon, oxygen and hydrogen. The chemicals associated with Fenton treatment were not detected in the final grafted copolymer, which has been confirmed by EDS analysis [23].
Grafting optimization
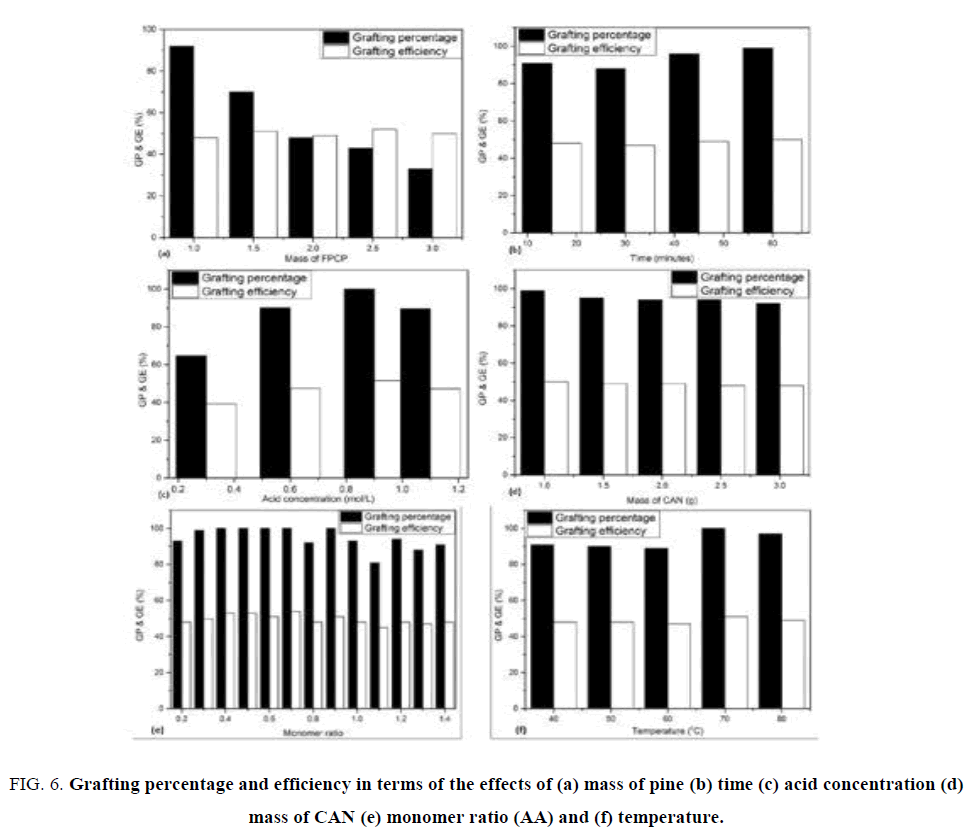
The optimum conditions for copolymerization were investigated in terms of FPCP mass, time, HNO3 concentration, mass of CAN, temperature and monomer ratio individually, keeping all other reaction conditions constant (e.g. time 30 minutes, HNO3 concentration 0.9 M, mass of initiator 1.0 g, temperature of 50°C and monomer ratio 2:10). The corresponding results in respect to grafting percentage and efficiency are shown in FIG. 6.
Figure 6: Grafting percentage and efficiency in terms of the effects of (a) mass of pine (b) time (c) acid concentration (d) mass of CAN (e) monomer ratio (AA) and (f) temperature.
Effects of FPCP mass: The effects of the mass of FPCP were studied when the copolymerization was carried out with various masses ranging from 1.0 g to 3.0 g (FIG.6 (a)). FIG. 1 (a) reveals that when the mass of FPCP was 1.0 g, the highest grafting percentage and efficiency were 92% and 50% respectively. A further increase in the amount of FPCP showed a decrease in both parameters. This may be because at 1.0 g of FPCP free radicals are generated for grafting the monomer. A further increase in the quantity of FPCP was therefore pointless because the amounts of acid, initiator and monomer were reduced and grafting had stopped since there were no more active sites available.
Effect of time: There was a slight increase in grafting percentage and efficiency with increase in time (FIG. 6 (b)), the optimum values achieved being 99% and 50%, respectively, at 60 minutes. Increased grafting percentage with time could be explained by the increased interactions between initiator, monomer and FPCP, which resulted in further generation of free radical sites [21].
Effect of nitric acid concentration: The optimum grafting percentage and efficiency of 100% and 51%, respectively, were achieved when concentration of nitric acid was 0.9 M (FIG. 6 (c)). Further increase in acid concentration showed slight decrease in grafting percentage but grafting efficiency remained at 51%.
Effect of initiator (CAN): Grafting percentage (99%) and efficiency (50%) were highest when 1.0 g of initiator was used (FIG.6 (d)). Further increase from 1.5 to 3 g in the mass of initiator led to a slight decrease. This might be attributed to the facts that only initiator was increased while the concentration of nitric acid, mass of FPCP and monomer remained constant. Nitric acid in the grafting medium allows initiator to create free radicals on FPCP by oxidizing Ce4+ ions. Therefore, a further increase in initiator while the concentration of nitric acid is constant resulted to an excess because the monomer was finished can no longer be attached to free radicals. The initial increase in grafting percentage and efficiency was attributed to the availability of more free radicals generated for grafting of monomer. Maximum yields were observed when initiator concentration was 1.0 g and 10 ml of HNO3 [24].
Effect of monomer: Grafting parameters started to fluctuate when the concentration of nitric acid was 0.8 M. The highest optimum grafting percentage of 100% and efficiency 54% were observed when monomer ratio was 7:10 (FIG. 6 (e)). This may be explained by assuming that initially active sites of FPCP were available for the monomer, resulting with an increase in grafting percentage and efficiency. However, a further increase in monomer concentration caused more homopolymer to be formed, leading to a decrease in grafting parameters because there was excess monomer and limited availability of FPCP.
Effect of temperature: The maximum grafting percentage of 100% was obtained at 70°C while efficiency was 51%. Grafting parameters started to decrease with a further rise in temperature. These results indicate that grafting parameters depend on temperature. It was reported that at 70°C, higher dissociation rate, diffusion of initiator and mobility of monomer to the active sites of cellulose produced a considerable improvement in grafting parameters [25]. A further increase in temperature beyond 70°C resulted in radical termination, which led to the decrease of grafting percentage and efficiency. It was observed that at temperature above 70°C more homopolymer is formed and grafting decreased [26]. They further reported that optimum temperature for the grafting reaction was dependent on the initiator used. Grafting of acrylic acid onto cellulose in the heterogeneous medium by CAN-HNO3 initiator resulted in higher grafting percentages at temperatures between 30°C and 70°C but lower at 90°C [9].
Conclusion
The grafting of acrylic acid onto Fenton treated pine cone powder was successfully conducted by using the free-radical grafting procedure. The optimum conditions for the graft polymerization were 70°C, monomer ratio 7:10, initiator 1.0 g, Fenton pine cone powder 1.0 g, 0.9 mol/L HNO3 and time was 60 minutes. The results obtained from the FTIR, TGA, DSC, XRD and SEM confirmed the presence of acrylic acid on the grafted product. Morphological and structural changes showed that grafting was definitely achieved because the crystallinity of the grafted copolymer increased in comparison with the untreated pine cone powder and FPCP.
References
- Jain P, Vigneshwaran N. Effect of Fenton?s pre-treatment on cotton cellulosic substrates to enhance its enzymatic hydrolysis response. Bioresour Technol. 2012;10:219-26.
- Ofomaja AE, Naidoo EB, Modise SJ. Surface modification of pine cone powder and its application for removal of Cu (II) from wastewater. Desalin Water Treat. 2010;19:275-85.
- Teli M D, Sheikh J. Grafting of bamboo rayon with acrylic acid and its effect on cationic dyeing. Cellulose Chem Technol. 2012;46:53-59.
- Ofomaja AE, Naidoo EB, Modise SJ. Biosorption of copper (II) and lead (II) onto potassium hydroxide treated pine cone powder. J Environ Manage. 2010;91:1674-85.
- Madhavi M, Ahmad MB, Haron MJ, et al. Optimmized conditions for graft copolymerization of poly (acrylamide) onto rubberwood fibre. Bioresources. 2011;6:5110-20.
- Ofomaja AE, Ngema SL, Naidoo EB. The grafting of acrylic acid onto biosorbents: Effect of plant components and initiator concentration. Carbohydr Polym. 2012;90:201-9.
- Pholosi A, Ofomaja AE, Naidoo EB. Effect of chemical extractants on the biosorptive properties of pine cone powder: Influence on lead (II) removal mechanism. J Saudi Chem Soc. 2013;17:77-86.
- Abu-Ilaiwi FA, Ahmad MB, Ibrahim NA, et al. Optimized conditions for the grafting reaction of poly (methyl acrylate) onto rubberwood fiber. Polym Int. 2004;53:386-91.
- Gurdag G, Sarmad S. Cellulose graft copolymers: Synthesis, properties and applications. Kalia S, Sabaa MW, editors. In: Polysaccharide Based Graft Copolymers. © Springer-Verlag, Berlin Heidelberg, (Chapter 2), 2013;15-57.
- Neira A, Tarraga M, Catalan R. Degradation of acrylic acid-grafted cellulose in aqueous medium with radical initiators. J Chil Chem Soc. 2008;53.
- Garside P, Wyeth P. Identification of cellulosic fibres by FTIR spectroscopy: Thread and single fibre analysis by attenuated total reflectance. Stud Conserv. 2003;48:269-75.
- Yang H, Yan R, Chen H, et al. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Science Direct. Fuel. 2007;86:1781-88.
- He Y, Pang Y, Liu Y, et al. Physicochemical characteristics of rice straw pretreated with sodium hydroxide in the solid state for enhancing biogas production. Energy Fuels. 2008;22:2775-81.
- Nada AM, Alkady MY, Fekry HM. Synthesis and characterization of grafted cellulose for water and metal ions sorption. Bioresources. 2008;31:46-59.
- Gao N, Li A, Quana C, et al. TG-FTIR and Py-GC/MS analysis on pyrolysis and combustion of pine sawdust. J Anal Appl Pyrolysis. 2013;100:26-32.
- Carrier M, Loppinet-Serani A, Denux D, et al. Thermogravimetric analysis as a new method to determine the lignocellulosic composition of biomass. Biomass Bioenergy. 2013;35:298-307.
- Poletto M, Ornaghi HL Junior, Zattera AJ. Native cellulose: Structure, characterization and thermal properties. Materials. 2014;7:6105-119.
- Idris SS, Rahman NA, Ismail K, et al. Investigation on thermochemical behaviour of low rank Malaysian coal, oil palm biomass and their blends during pyrolysis via thermogravimetric analysis (TGA). Bioresource Technology. 2010;101:4584-492.
- Thakur VK. Cellulose-based graft copolymers. In: Structure and Chemistry. Thakur VK, editor. Taylor & Francis Group. Boca Raton, 2015;165.
- Gupta KC, Khandekar K. Graft copolymerization of acrylamide-methylacrylate comonomers onto cellulose using ceric ammonium nitrate. J Appl Polym Sci. 2002;86:2631-42.
- Wan Z, Xiong Z, Ren H, et al. Graft copolymerization of methyl methacrylate onto bamboo cellulose under microwave irradiation. Carbohydr Polym. 2011;83:264-69.
- Micales JA, Han JS, Davis JL, et al. Chemical composition and fungitoxic activities of pine cone extractives. In: Llewellyn GC, Dashek WV, O?Rear CE, editors. Biodeterioration Research 4: Mycotoxins, Wood Decay, Plant Stress, Biocorrosion and General Biodeterioration: Proceedings of 4th meeting of the Pan American Biodeterioration Society, 1991 August 20-25; as an Electronic Symposium. New York: Plenum Press. 1994;317-332.
- Krishnan VN, Ramesh A. Synthesis and characterization of cellulose nanofibers from coconut coir fibers. IOSR-JAC. 2013;6:18-23.
- Shanmugapriya A, Ramammurthy R, Venkatachalam M, et al. Optimization of ceric ammonium nitrile initiated graft copolymerization of acrylonitrile onto chitosan. J Water Resource Prot. 2011;3:380-86.
- Khullar R, Varshney VK, Nathani S, et al. Grafting of acrylonitrile onto cellulosic material derived from bamboo (Dendroccalamus strictus). Express Polymer Letters. 2008;2:12-8.
- Witono JR, Noordegraf IW, Heeres HJ, et al. Grafting co-polymerisation of acrylic acid to cassava starch: Evaluation on the influences of process parameters by an experimental design method. Carbohydr Polym. 2012;90:1522-9.