Original Article
, Volume: 15( 3)Synthesis and Biological Evaluation of New Sulfonamide Derivatives
- *Correspondence:
- Nief OA , Department of Chemistry, College of Science, Al-Mustansiriya University, Baghdad, Iraq, Tel: +964-770-5899622; E-mail: olfat_nife@yahoo.com
Received: May 03, 2017; Accepted: August 02, 2017; Published: August 07, 2017
Citation: Askar FW, Aldhalf YA, Jinzeel NA, et al. Synthesis and Biological Evaluation of New Sulfonamide Derivatives. Int J Chem Sci 2017;15(3):173
Abstract
New sulfonamide derivatives comprising azide, 1,2,3-triazole, azo, chalcone, and Schiff base moieties are proved. The structures of the compounds have been confirmed by FT-IR and ¹H-NMR spectra and element analysis. The synthesized derivatives have been screened for antimicrobial and in vitro antioxidant properties. The results of this investigation revealed that the newly synthesized compounds are potent antimicrobial and antioxidant agent.
Keywords
Sulfonamide; Chalcone; Anti-microbial activity; Anti-oxidant activity; Azo compounds
Introduction
Sulfonamide (sulfa drugs) has been the first drugs largely employed and systematically used as preventive and chemotherapeutic agents against meny diseases [1-4]. Triazoles are an essential category of heterocycles rings due to a wide range of pharmaceutical applications and synthetic medium [5,6]. Azo dye, aromatic rings associated together through azo (-N=N) chromophores, represent the largest category of dyes used in textile processing and other industries such as food colorant, printing, cosmetic, and pharmaceutical industries [7,8]. Chalcone derivatives contain α, β-unsaturated carbonyl moiety possesses broad spectrum of biological activity in both medicinal and pharmaceutical, such as antimicrobial [9], anti- inflammatory [10], Antitubercular [11], Antioxidant [12] and Anticancer [13].
Experimental Procedure
Materials and measurements
All of the reagents are commercially available in the (Aldrich Co.) was used without further purification. Melting points was recorder by electric thermal melting point apparatus and are uncorrected. FT-IR measurements have been recorded through the model Shimadzu FT-IR-8400S. Was obtained ¹H NMR spectra using Ultra Shield Bruker laboratory model in the 300 MHz uisg DMSO-D6 as a solvent and the use of TMS, according to internal standards. Element analyses were performed on EURO EA instrument in University of Al -Mustansiriyah.
Synthesis of N-(4-aminophenyl)-4-methylbenzenesulfonamide (1) [14]: A mixture of Toluene -4-Sulfonyl chloride (tosyl chloride) (0.01 moles) and 1,4- phenylene diamine (0.01 moles) with (0.01 moles) Triethyl amine in dry benzene (20 mL) has been refluxed for 6h. The excess of solvent has been evaporated and the product has been filtered off, recrystallized from chloroform. Yield: 88%, M.P: 182°C to 184°C, FT-IR (KBr, ν, cm-1): 3414, 3329 (NH2), 3246 (NH), 1317, 1155 (SO2). 1H NMR (300 MHz, δ, ppm, DMSO-D6): 7.55-6.28 (m, 8H, Ar-H), 6.15 (2H, NH2), 4.7 (s, 1H, NH), 2.24 (s, 3H, CH3); Anal. % calc./found for C13H14N2O2S (m.w.262): C,59.54/ 59.21; H,5.34/ 5.10; N,10.68/ 10.45; S;12.21/ 11.96.
Synthesis of N-(4-(chlorodiazenyl) phenyl)-4-methylbenzenesulfonamide (2): A solution of compound (1) (0.01 moles) in conc. HCL (3 mL) has been cooled to (0°C to 5°C). A chilled solution of sodium nitrite (0.01 moles, 1.5 g) in 10 mL of water has been added drop by drop through 15 min, and then stir the mixture reacted for 10 min.
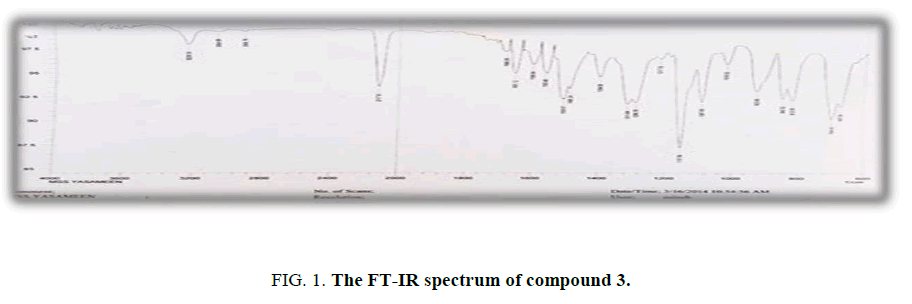
Synthesis of N-(4-azidophenyl)-4-methylbenzenesulfonamide (3): An aqueous solution of sodium azide (0.012 mole, 0.78 g) has been added drop wise to diazonium salt solution (2). The mixture has been stirred for 25 min to give dark brown solid compound (3). Yield: 84%, M.p.: 290°C to 292°C, FT-IR (KBr, ν, cm-1): 3228 (NH), 2117 (N3), 1311, 1151 (SO2). 1H-NMR (300 MHz, δ, ppm, DMSO-d6): 7.79-6.55 (m, 8H, Ar-H), 4.7 (s, 1H, NH), 2.24 (s, 3H, CH3); Anal. % calc./found for C13H14N2O2S (m.w. 288): C,54.16/53.92; H,4.16/4.25; N, 19.44/ 19.18: S,11.11/ 10.92.
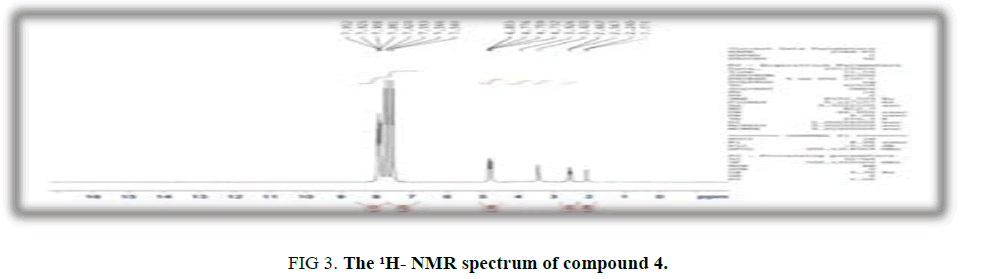
Synthesis of N-(4-(4-acetyl-5-methyl-1H-1,2,3-triazol-1-yl) phenyl)-4-methylbenzenesulfonamide (4): Azide compound (3) (0.01 moles) has been cautiously added to a cold solution of acetylacetone (0.01 mole, 1.3 g) and sodium ethoxide (7 mL), the mixture has been heated under reflux on a water bath for 3h. The resulting solid was separated and recrystallized from chloroform. Yield: 73%. M.p.: 198°C to 199°C. FT-IR (KBr, ν, cm-1): 3210 (N-H), 1690(C=O), 1352, 1161(SO2). 1H-NMR (300 MHz, δ, ppm, DMSO-d6): 7.87-6.35 (m, 8H, Ar-H), 4.66 (s, 1H, NH), 2.24 (s, 3H, CH3), 2.38 (s, 3H, CH3 triazole), 2.15 (s, 3H, CH3CO); Anal. % calc./found for C18H18N4O (m.w.370): C,58.37/ 58.21; H,4.86 /4.52; N,15.13/ 14.96; S,8.64/ 8.39.
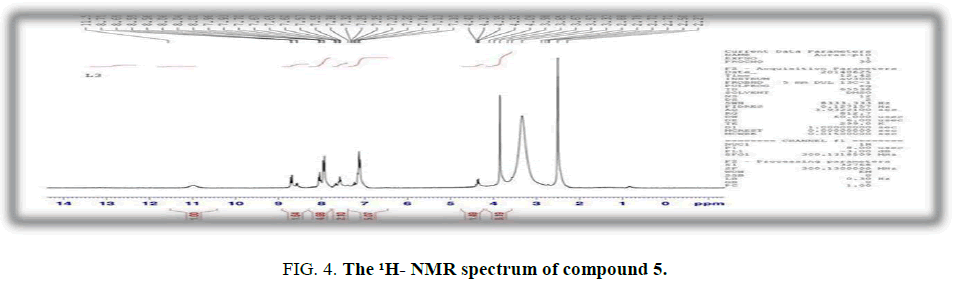
Synthesis of 5-methyl-1-(4-(4-methylphenylsulfonamido) phenyl)-1H-1,2,3-triazole-4-carboxylic acid (5): A mixture of ethyl acetoacetate (0.01 moles, 1.03 mL) and azide compound (3) (0.01 moles, 1.3 mL) in absolute ethanol (25 mL) has been chilled to 0?C. Sodium ethoxide (0.01 moles) in (25 mL) has been added progressively to the reaction mixture and heat under reflux for 6h. The XSZ product has been recrystallized from acetone. Yield: 80%; M.p: 250°C to 251°C; FT-IR (KBr, ν, cm-1): 3300-2900 (O-H), 3230 (N-H),1699 (C=O), 1355, 1160 (SO2); 1H-NMR (300 MHz, δ, ppm DMSO-d6): 11.32 (s,1H, O- H), 7.67-6.54 (m, 8H, Ar-H), 4.7 (s, 1H, NH), 2.23 (s, 3H, CH3),2.33 (s, 3H, triazole); Anal. % calc./found for C17H20N4O2S (m.w.372): C,58.83/ 54.70; H,5.37 / 5.11; N,15.05/ 14.85; S,8.60/ 8.35.
Synthesis of N-(4-((3-formyl-4-hydroxyphenyl) diazenyl) phenyl)-4-methylbenzenesulfonamide (6): To a cold solution of salisaldelyde (0.01 mole, 1.22 g) in %10 NaOH (12 mL) a solution of diazonium salt (2) was added gradually and very slowly. The solution wae lefe for 30 min in ice bath. The precipitate was filtered and washed with water. Yield: 65%; M.p.: 282°C; FT-IR (KBr, ν, cm-1): 3405-3100 (O-H), 2815, 2745 (C-H ald),1550(N=N), 1355-1160 (SO2). 1H-NMR (300 MHz, δ, ppm DMSO-d6): 9.96 (C-H ald), 8.33-7.40 (m, 8H, Ar-H),5.65 (s,1H, O-H), 4.65 (s, 1H, NH), 2.3 (s, 3H, CH3); Anal. % calc./found for C20H17N3O4S (m.w.395): C,60.75/ 60.57; H,4.30/4.12; N,10.63/ 10.44; S,8.10/ 7.91.
Synthesis of N-(4-((4-hydroxy-3-((pyrimidin-2-ylimino) methyl) phenyl) diazenyl) phenyl)-4- methylbenzenesulfonamide (7): A mixture of 2-amino pyrimidine (0.01 moles)) and compound 6 (0.01 mole, 9.5g) has been refluxed in ethanol absolute (30 mL) for 6 h. The mixture cools and the product recrystallized from acetone. Yield: 75% ; M.p.: 185°C to 187°C; FT-IR (KBr, ν, cm-1): 3430-3112 (O-H), 1635 (C=N), 1539, 1158 (SO2) ; 1H-NMR (300 MHz, δ, ppm, DMSO-D6):8.90-8.7 9(m, 3H, proton of pyrimidine) 8.5 (s, 1H, N=CH), 7.97-6.78 (m, 11H, Ar-H),5.60 (s,1H,O-H), 4.75 (s, 1H, NH), 2.32 (s, 3H, CH3); Anal. % calc./found for C24H20N6O3S (m.w.472): C,61.01/ 59.85; H,4.23 /4.02; N,17.79/17.58; S,6.77 /6.54.
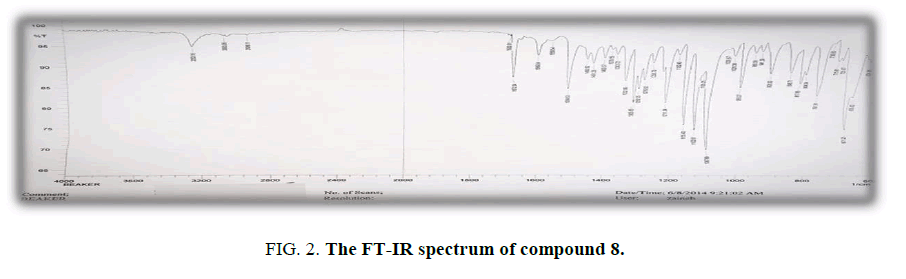
Synthesis of N-(4-((4-hydroxy-3-(3-oxo-3-phenylprop-1-en-1-yl) phenyl) diazenyl) phenyl)-4- methylbenzenesulfonamide (8): Compound (6) (0.01 moles, 3.95 g) in 30 mL absolute ethanol has been added to a solution of (0.01 mole, 1.20 g) of (acetonphenone) in (5 mL), 40% NaOH, after 6 h of stirring, let the mixture was left in the refrigerator for 24 h, then the precipitate was filtered and washed with solvent. Yield: 70%. M.p.: 194°C to 195°C; FT-IR (KBr, ν, cm-1): 3250 (N-H), 1672 (C=O), 1640, (C=C),1354,1162(SO2); 1H-NMR (300 MHz, δ, ppm, DMSO-d6,): 8.1 (s, 1H, CH=CH), 8.27-7.63 (m, 16H, Ar-H and CH-CO), 5.6(s, 1H,O-H), 4.7 (s, 1H, NH), 2.3 (s, 3H,CH3); Anal. % calc./found for C28H23N3O4S (m.w. 497): C,67.60/ 67.41; H,4.62/4.43; N,8.45/ 8.30; S,6.43/6.25 [15].
Study of Biological Activities
Anti-microbial activity
The Sulfonamide deravitived (4-8) were used against Escherichia coli, K. pneumonia, Staphylococcus aureus, Streptococcus pyogenes and two fungal as Aspergillus niger, and Candida albicans by using diffusion method [16,17]. As a control DMSO has been test at 10 mg/mL concentration by using DMSO as solvent. The bacteria and fungi have been sub-commission cultured in agar and potato dextrose agar medium and these plates have been incubated for 24 hours for bacteria and 48 hours for fungi at 37°C. The zone inhibition observed around the cups after respective incubation has been measured in mm (Table 1).
| Variables | Antibacterial Activity | Antifungal | |||||
|---|---|---|---|---|---|---|---|
| Zone of inhibition (mm) | |||||||
| Gram positive | Gram negative | Fungi | |||||
| Compound | E. coli | K. pneumonia | S. aureus | S. pyogenes | A. niger | C. albicans | |
| 4 | 4 | 8 | 2 | 4 | 8 | 9 | |
| 5 | 6 | 10 | 2 | 6 | 7 | 10 | |
| 6 | 12 | 12 | 2 | 15 | 12 | 12 | |
| 7 | 18 | 15 | 8 | 18 | 15 | 14 | |
| 8 | 18 | 15 | 15 | 20 | 14 | 16 | |
| Ampicillin | 24 | 25 | 22 | 26 | - | - | |
| Fluconazole | - | - | - | - | 24 | 25 | |
Table 1: Anti-microbial evaluation compound [4-8].
Anti-oxidant activity
The free radical scavenging activity of the derivatives to the radical 1,1?diphenyl?2?picryl hydrazyl has been measured as shown by reference [18]. The use of methanol as the solvent and ascorbic acid as the standard. Sulfonamide stock solution (1 mg/mL) has been diluted to final concentration 20?100 μg/mL. Methanolic DPPH solution (1 mL, 0.3 mmol) has been added to sample solution in DMSO (3 mL) at different concentrations. Shaken strongly mix and allow to stand at room temperature for 30 min to measure in 517 nm (As), using the "Shimadzu 175 laboratory" (Table 2) and has been used. The Methanol solution of DPPH as a sample control of the Ac. Clear the capacity calculated using the following equation:
| Compound | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 |
| 4 | 10 | 22 | 30 | 40 | 51 | 60 | 71 | 81 | 90 |
| 5 | 12 | 23 | 32 | 43 | 52 | 63 | 72 | 82 | 91 |
| 6 | 13 | 24 | 33 | 44 | 54 | 64 | 73 | 84 | 92 |
| 7 | 13 | 26 | 34 | 45 | 55 | 66 | 75 | 85 | 93 |
| 8 | 14 | 27 | 36 | 47 | 57 | 67 | 76 | 86 | 94 |
| Ascorbic Acid | 9 | 15 | 25 | 35 | 43 | 55 | 62 | 75 | 82 |
Table 2 : Antiradical activity of compounds 4-8 (expressed as % inhibition).
Results and Discussion
Synthesis
The new sulfonamide compounds have been synthesis following the reaction sequences describe in Scheme 1. Reaction of toluene-4-Sulfonyl chloride with 1,4-phenylene di amine and triethyl amine in dry benzene afforded of N-(4-aminophenyl)-4- methylbenzenesulfonamide (1). The structure of all compounds was conformed based on melting point (m.p), thin layer chromatography (TLC) and spectral data. FT-IR spectrum of compound (1) shows the characteristic bands at 3414,3329,3246 and 1317, 1155 cm-1 which due to NH2, NH, and (SO2), While the 1H-NMR spectrum indicated singlet signal at 2.24 ppm belonged for proton (CH3) group and singlet at 6.15 ppm connected to protons amino group, while a signal as multiplet at 7.55-6.28 ppm due to eight phenyl protons.
Treatment of sulfonamide (1) with sodium nitrite in hydrochloric acid at 0°C to 5°C afforded the diazonium salt (2). Reaction of diazonium salt (2) with sodium azide gave N-(4-azidophenyl)-4-methylbenzenesulfonamide (3) The IR spectrum of derivative (3) shows new absorption bandat 2117 cm-1 due to stretching vibration of N3 and band at 3228 cm-1 belonged for stretching vibration of N-H. The 1H-NMR showed singlet signals 2.24ppm assigned to three protons of methyl group and 4.7 ppm was attributed to N-H proton. The aromatic protons were appeared at 7.79-6.55 ppm.
Cyclization of azide derivatives (3) with acetylacetone in the presence of sodium ethoxide afforded compound (4). FTIR absorption bands of triazole compound exhibited the disappearancs of absorption bands due to N3 stretching of compound (3) together with the presence of stretching band at 1690 cm-1 due to carbonyl group. 1H-NMR spectrum exhibited four singlet signals 2.15 ppm was assigned to three protons of acetyl group, 2.24 ppm was attributed to protons of p-substituted methyl group, 2.38 ppm belong to protons of methyl triazole and 4.66 ppm due to N-H. The aromatic protons were appeared at 7.87- 6.35 ppm.
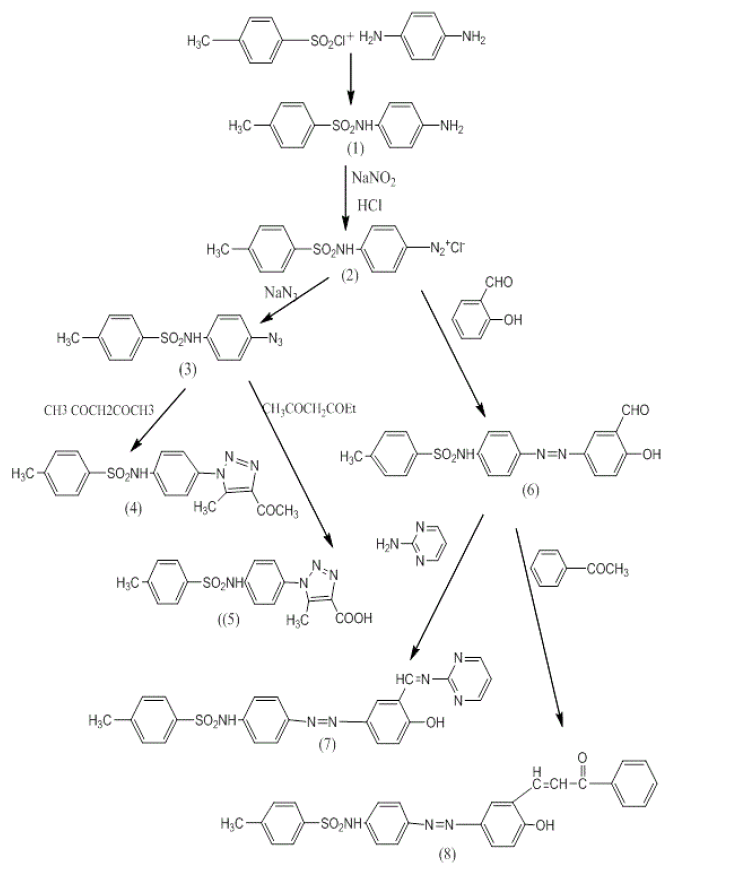
Moreover, cyclization of azide compound with ethyl acetoacetate afforded triazole derivative (5). The FTIR spectrum of derivative (5) shows sharp absorption band at 1699 cm-1 which was attributed to carbonyl group of the carboxylic acid and the abroad band at 3300-2900 cm-1 due to O-H group. 1H-NMR spectrum of compound (5) singlet signals 2.23 ppm was assigned to CH3 protons, 2.33 ppm belong to CH3 of triazole, 4.7 ppm was attributed to N-H proton and 11.32 ppm due to proton of hydroxyl group. The aromatic protons were appeared at 7.67-6.54 ppm.
The azo compound was synthesized by coupling between diazoniuim salt of amino sulfonamide derivative with salicylaldehyde. FT-IR absorption bands of compound (6) exhibited the disappearances of two absorption bands due to NH2 stretching of compound (1) together with the appearance of stretching band at 1550 cm-1 due to N=N group, which it also shows stretching abroad band 3405-3100 cm-1 due to O-H group.
1H-NMR spectrum of azo compound exhibited singlet signals 2.3 ppm was assigned to three protons of methyl group, 5.65 ppm was attributed to O-H proton, singlet at 4.65 ppm related to NH, doublet of doublet at 7.40-7.45 and 7.61-7.69 ppm belong to (8H, 2ph), which is interference with the proton of salicylaldehyde ring, singlet at 9.96 ppm due to proton of aldehyde.
Condensation of compound (6) with 2-amino pyrimidine in ethanol afforded Schiff base (7). The formulation of Schiff base was showed by the disappearance of NH2 stretching band of amine and carbonyl group of compounds (6) combined with the presence of azomethine (CH=N) stretching band at 1635 cm-1.
The ¹H-NMR spectrum of compound (7) exhibited singlet signals at 2.32 ppm was assigned to CH3 protons,4.75 ppm was attributed to N-H proton and 5.60 ppm due to proton of O-H (Figure. 1-4). The aromatic protons were appeared at 7.97-6.78 ppm and a multiplet signals at 8.90-8.79 ppm due to protons of pyrimidine ring. On the other hand, the reaction of compound (6) with acetophenone afforded chalcones derivative (8). FT-IR spectrum of (8) shows a band at 1672, 1640 cm-1 due to (C=O and (C=C) respectively. 1H-NMR spectrum of chalcones (8) exhibited singlet signal: at 2.3 ppm which was assigned to CH3 Protons, 4.7 ppm was attributed to N-H proton, 5.6 ppm due to O-H proton. A multiplet signals at 8.27-7.63 ppm due to 16H aromatic protons and (CH-CO), singlet peak at 8.1 ppm belong to (C=CH).
Figure 3: The 1H- NMR spectrum of compound 4.
Figure 4: The 1H- NMR spectrum of compound 5.
Anti-microbial activity
The synthesized sulfonamide carrying azo,1,2,3-triazole, Schiff base, chalcone moieties which is accountable for antimicrobial activity. It seems that the compounds 7, 8 are very significant for activity against both bacterial for antimicrobial activity. All the compounds were found to exhibit moderate to good antifungal. Standard antibacterial medication (Ampicillin) and antifungal medication (Fluconazole) were utilized for comparison. The examinations have been performed in triplicate keeping in mind minimize blunders (Figure.5).
In vitro antioxidant screening
The antioxidant screening of sulfonamide derivatives was identified on the basis of their scavenging of the stable (DPPH) free radical. The results of antioxidant screening were depicted in (Table 2). DPPH radical scavenging is considered a good in vitro model and is widely reduced by an antioxidant compounds or a radical species to become a stable diamagnetic molecule. The potential is similar with antioxidant activity of ascorbic. The highest scavenger activity was observed in compounds 7,8 are probably due to the presence of azomethine and α, β-unsaturated group (Figure. 6).
Conclusion
Novel sulfonamide derivatives are prepared and characterized on the basis of analytical and spectral data. Screening of these compounds against pathogenic microorganism reveals that these sulfonamide derivatives showed moderate to noticeable antimicrobial and antioxidant activities.
References
- Oana MD, Florentina L, Cornelia V, et al. Synthesis and Biological Evaluation of New 2-Azetidinones with Sulfonamide Structures.Molecules. 2013;18:4140-57.
- Lal J, Sushil K, Gupta D, et al. Biological activity, design, synthesis and structure activity relationship of some novel derivatives of curcumin containing sulfonamides. Eur J Med Chem. 2013;64:579-88.
- Lili O, Shuang H, Ding W, et al. Parallel synthesis of novel antitumor agents: 1,2,3-triazles bearing biologically active sulfonamide moiety and their 3D-QSAR. Mol Divers. 2011;15:927-46.
- Ashish B, Ravi K, Rajesh KS. Synthesis of some bioactive sulfonamide and amide derivatives of piperazine incorporating imidazo[1,2-B ]pyridazine moiety. Med Chem. 2016;6:257-63.
- Katritzky AR, Zhang Y, Singh SK. 1,2,3-Triazole formation under mild conditions via 1,3-dipolar cycloaddition of acetylenes with azides. Heterocycles. 2003;60:1225-39.
- Antonino L, Riccardo D, Francesco M, et al. 1,2,3-Triazole in heterocyclic compounds, endowed with biological activity, through 1,3-dipolar cycloadditions. Eur J Org Chem 2014;16:33289-306.
- Kiran V, Mehta M. Studies on some isoxazoline-azo compounds and their colourant performance and fastness evaluation on synthetic fabrics. Int J Chem Tech Res. 2012;4:409-14.
- Mohammed MN.Preparation, characterization and biological activity studies of new azo compounds. J Chem Chem Eng. 2012;6:885-8.
- Amita SR, Simon L, Srinivasan KK, et al. Synthesis and in vitro antimicrobial evaluation of 5'-acetamido-2'-hydroxy chalcone derivatives. Res J Chem Sci. 2014;4:56-9.
- Yadav HL, Gupta P, Pawar PS, et al. Synthesis and biological evaluation of anti-inflammatory activity of 1,3-diphenyl propenone derivatives. Med Chem Res. 2010;19:1-8.
- Monica K, Rakesh P, Yadavendra Y. Der Pharma Chemica. 2014;6:352-9.
- Tan Nhut D, Dao Thanh T. Synthesis, antioxidant and antimicrobial activities of a novel series of chalcones, pyrazolic chalcones, and allylic chalcones. Pharmacol Pharm. 2011;2:282-8.
- Hery-Jumina S, Mustofa A, Novi K, et al. Chalcones: Synthesis, structure diversity and pharmacological aspects. J Chem Pharm Res. 2014;6:1076-88.
- Wright SW, Hallstrom KN. A convenient preparation of heteroaryl sulfonamides and sulfonyl fluorides from heteroaryl thiols. J Org Chem. 2006;71:1080.
- Stolz A. Basic and applied aspects in the microbial degradation of azo dyes Appl. Microbiol Biotechnol. 2001;17:56-69.
- Mcmurry J. Organic Chemistry Thomson Learning Academic Resource Center, 6th ed. 2004
- Greenwood D. Snack R, Peurtherer A. A guide to microbial infections: Pathogensis, immunity, laboratory diagnosis and control. J Med Microbiol. 1997;690.
- Mina S, Fereshteh G, Mohammad M, et al. Synthesis and biological investigation of some novel sulfonamide and amide derivatives containing coumarin moieties. Iran J Pharm Res. 2014;13:881-92.