Original Article
, Volume: 12( 2)Preparation of Series of Heteropoly ComplexâÂÂs Containing Ce4+ and Al3+ Cations with Isopoly Molybdate, Vanadate, Tungstate
- *Correspondence:
- Ranjeeta S Department of Chemistry, Ranchi University, Ranchi, Jharkhand, India, Tel: +91 651 220 4544; E-mail: contact@ranchiuniversitymba.org
Received Date: August 22, 2017 Accepted Date: August 29, 2017 Published Date: September 04, 2017
Citation: Ranjeeta S, Alok Kumar T, Nalini Kant R, et al. Preparation of Series of Heteropoly Complex’s Containing Ce4+ and Al3+ Cations with Isopoly Molybdate, Vanadate, Tungstate. Inorg Chem Ind J. 2017;12(2):115
Abstract
The series of heteropoly complexes were prepared by conventional reflux method in the water bath separately by taking Isopoly anions of Mo, V, W. All the products isolated were crystalline in nature appeared as dark yellow products. The chemical as well as physical methods were applied to characterize all the three isolated products the chemical analysis suggests the ionic nature of complexes since the complexes were dissolved in warm water. The inorganic wet analysis suggests ionic nature of sodium as its cation in association with complexes. The IR analysis applied for identification of group frequencies also suggests the ionic nature of sodium for the isolated complexes in solid state. The thermal analysis involving DTA and TGA support the stability of complexes in the atmosphere due to the presence of large no of water of hydration. The magnetic susceptibility analysis supports the paramagnetic nature of all the isolated series of products containing Al3+ and Ce4+ hetero cation. The structure of the heteropoly complexes synthesised may be suggested as Anderson’s structure. The study of elemental analysis and molecular weight determination as well as chemical and physical analysis of the products, the following formula based on the IUPAC system may be proposed as: 1. Na2 (Al2CeMo7O27) 150H2O (sodium-7-molybdo-1-cirate-2 aluminate hydrate) 2. Na2 (Al2CeV4O16) 72H2O (sodium-4 -vanado-1-cirate-2 aluminate hydrate) 3. Na4 (Al2CeW4O19) 177H2O (sodium-4-tungstato-1-citrate-2-aluminate hydrate)
Keywords
Preparation; Cryoscopy; TGA and DTA thermal studies; Magnetic susceptibility; I.R. spectral studies
Introduction
The triheteropoly complex compounds have important application in the field of absorption catalysis due to enhanced solid-state surface area. The contribution of presence of two different hetero cations further increase the surface area of the heteropoly complexes and thermal stability of the product which is evident from the thermal analysis of the entire product synthesised [1]. The chemistry of synthesis of heteropoly complex compound was started quite earlier by Tsigdimos and co-workers [2] in the moderate acidic medium formed with the help of addition of adequate quantity of CH3COOH. As per the literature survey of preparation of triheteropoly complex compounds the preparation of the product based on mixing of proper concentration of sodium tungstate, two different hetero cations aqueous solution and the mixture solution refluxed for about two to three hours at refluxing temperature. The mixture solution after refluxing is left for two to three days and then the lustre brown coloured crystalline residue in solid state was recovered. The condition of moderate acidic medium (~ pH 4.5) is necessary since the concentrated acidic medium or even concentrated alkaline medium may decompose the poly anion of tungsten, molybdenum, or/and vanadium in their respective mono anions. The mechanism of decomposition of poly anion may be shifted through the chemistry of dichromate anion formed from chromate anion in moderate acidic medium.
Moderate acidic medium
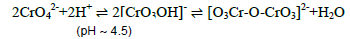
The triheteropoly anion formed contains oxygen metal bridges commonly known as oxometalate bridges possessing various structures namely Anderson, Anderson-Evans, Keggin, Lindqvist, Well-Dawson, Dexter-Silverton [3] depending upon formulas of the poly hetero complex anions. The most common among the above structure is the Keggin’s structure [4] having polyanion composition [M12O40]n-. The triheteropoly complex compound recovered as solid state lustre brown colour were subject to characterization for the evaluation of the components inserted including the presence of water molecules generally as water of hydration through IR studies [5-15] and the solubility of the triheteropoly complex were about examined through the TGA, DTA analysis [16-21] of the products.
Experiment
The following experimental procedures were selected for the preparation of the three products namely sodium-7-molybdo-1-cirate-2 aluminate hydrate, sodium-4 -vanado-1-cirate-2 aluminate hydrate, sodium-4-tungstato-1-citrate-2-aluminate hydrate, the water solution 70 ml having 0.36 (M) concentration of sodium molybdate was mixed with 40 ml of aqueous solution of 0.65 molar aluminium carbonate in acidic medium formed by the addition of the 10 ml glacial acetic acid. Now the step wise 60 ml cerium sulphate solution having 0.35 M concentration was added, during addition of cerium sulphate continuous stirring was also performed. Finally, the pH of the entire mixture solution was maintained nearly 4.5 whenever the pH goes above 4.5 the glacial acetic acid were added to maintain the pH of the mixture solution. This mixture solution was refluxed for about three hours. The bright lustre yellow colour solid product was appeared in the bottom of the beaker after three days. This solid product was treated with concentrated alcohol to remove impurities associated with the solid residue and then the product was left to dry. Similarly, other two products were also synthesised by taking sodium meta vanadate and sodium tungstate all the three products now kept for characterization to assign the chemical formulas.
Characterization
The characterization of all the three products involves the following experimental analysis such as Elemental analysis, Cryoscopic analysis, I.R. analysis, TGA and DTA analysis (Tables 1-3).
| Elements | Percentage found | Percentage calculated | |||
|---|---|---|---|---|---|
| Exp-1 | Exp-2 | Mean | |||
| Sodium | 1.15 | 1.13 | 1.14 | 1.14 | |
| Aluminium | 1.33 | 1.35 | 1.34 | 1.34 | |
| Cerium | 3.48 | 3.44 | 3.46 | 3.46 | |
| Molybdenum | 16.6 | 16.64 | 16.62 | 16.62 | |
| Hydrogen | 7.43 | 7.4 | 7.42 | 7.42 | |
| Oxygen | (By difference) | 70.03 | 70.03 | ||
Table 1. The elemental analysis of the products gives emphasis on the elemental constituents that is the elements involve in the formation of the triheteropoly complex compound. This elemental analysis process is based on the experiments for evaluation of percentage of each element as per the Vogel’s book.
| Elements | Percentage found | Percentage calculated | ||
|---|---|---|---|---|
| Exp-1 | Exp-2 | Mean | ||
| Sodium | 2.29 | 2.3 | 2.30 | 2.30 |
| Aluminium | 2.7 | 2.72 | 2.71 | 2.71 |
| Cerium | 7 | 7.02 | 7.01 | 7.01 |
| Vanadium | 10.21 | 10.23 | 10.22 | 10.22 |
| Hydrogen | 7.2 | 7.22 | 7.21 | 7.21 |
| Oxygen | (By difference) | 70.54 | 70.54 | |
Table 2. The elemental evaluation of the second product for determination of the constituent elements of the complex compound as follows.
| Elements | Percentage found | Percentage calculated | ||
|---|---|---|---|---|
| Exp-1 | Exp-2 | Mean | ||
| Sodium | 1.04 | 1.02 | 1.03 | 1.03 |
| Aluminium | 1.22 | 1.2 | 1.21 | 1.21 |
| Cerium | 3.13 | 3.14 | 3.14 | 3.14 |
| Tungsten | 16.5 | 16.53 | 16.52 | 16.515 |
| Hydrogen | 7.7 | 7.73 | 7.72 | 7.72 |
| Oxygen | (By difference) | 70.38 | 70.38 | |
Table 3. Finally, the third synthesised product elemental analysis indicates the following percentage composition of its constituent element.
The apparent molecular weight of the product-I is 4038 as per cryoscopic method of evaluation. This 4038 is the apparent (approx) molecular weight of the complex compound. However, the formula molecular weight of the product comes to 4044 which is slightly higher than the apparent molecular weight. This higher formula weight is suggested for the synthesised product since the apparent molecular weight calculation is based on partial ionization of the complex compound.
The molecular weight determination involving cryoscopic method suggests 1991 while formula weight of the complex comes 1996. Again, the slight higher formula weight indicates the incomplete ionization of the complex compound.
The apparent molecular weight based on cryoscopic method evaluation was determined as 4451which is again slightly lower than the formula weight 4456 of the product.
Results and Discussion
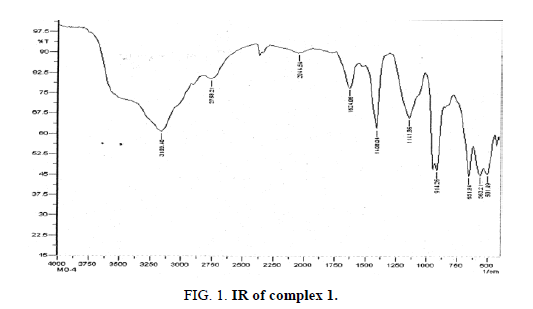
The final results for the characterization of the three products involve IR, DTA and TGA analysis. The IR graph for complex-I indicates broader peaks at 3159 cm-1, 2758.21 cm-1, 2011.51 cm-1, 1624.06 cm-1 and 1408.01 cm-1 indicating the group frequencies due to the presence of water of hydration. It may be possible that some of the peaks among them may be due to the presence of water of crystallization. However, it is difficult to differentiate between the above peaks appeared due to water of hydration or/and water of crystallization. The peak appeared at 1141.36 cm-1 may be visible due to the presence of Mo=O group frequencies again further the peaks at 914.26 cm-1 indicated in the graph assigned to sodium cation of the complex. Another peak appeared at 651.24 cm-1 assigned to Al-O, the 563.21 cm-1 peak indicate the presence of Ce=O and finally the peak at 501.49 cm-1 indicate the group frequency due to existence of Ce-Mo bond.
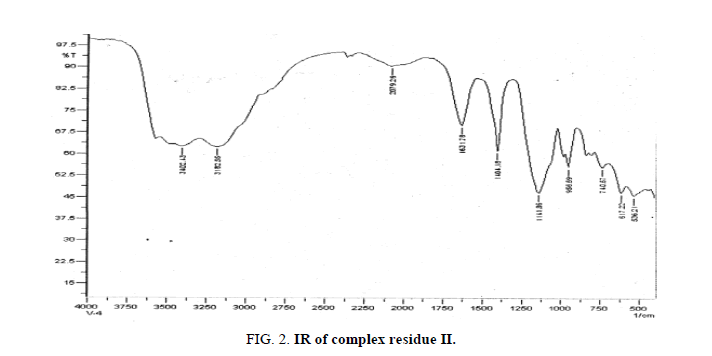
The IR peaks 3443 cm-1, 3282.55 cm-1, 2079.26 cm-1, 1631.78 cm-1 and 1141.36 cm-1 in the IR spectral graph appeared due to the presence of water of hydration as well as water of conjugation of the dried and purified product. Again the IR peak at 956.69 cm-1 assigned to the Na cation of the product. The shifting in sodium cation peak may be considered due to the presence of different constituent elements compared to the first product. The presence of V=O confirmed on the basis of peak appeared at 740.67 cm-1 in the IR spectral graph. The group frequency appeared at 617.27 cm-1 indicates the presence of Al-O bond. Finally, the 536.21 cm-1 IR spectral peak assigned to the presence of Ce-O.
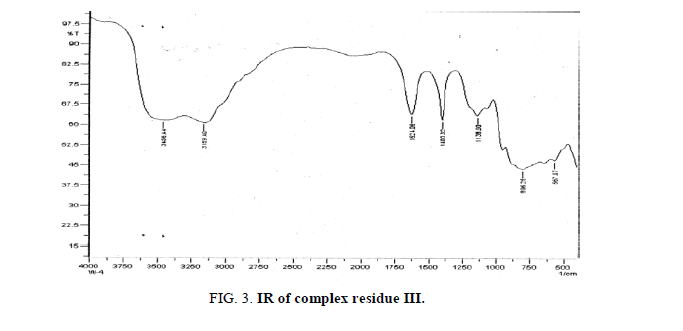
The strong broad peaks 3456.44 cm-1, 3159.40 cm-1 indicates the association of water molecules also supported by presence of two sharp peaks at 1624.06 cm-1 and 1400.32 cm-1 also. The appearance of 1138.00 cm-1 may be attributed due to the presence of W=O. The peak at 806.25 cm-1 may be due to the association of Al-O and finally the 567.07 cm-1 small peak may be assigned for the Ce-O group frequency.
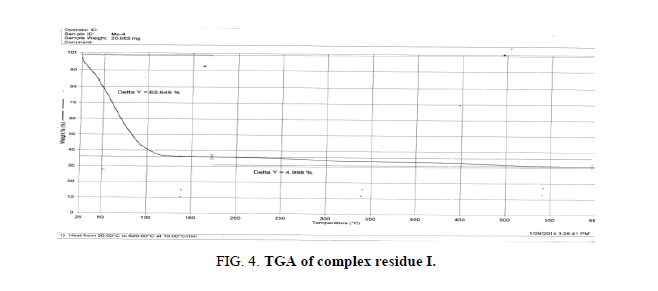
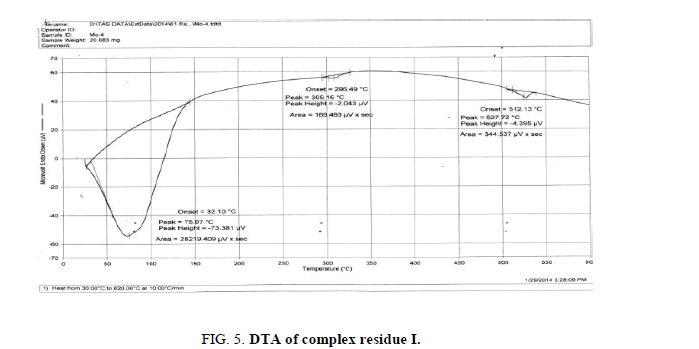
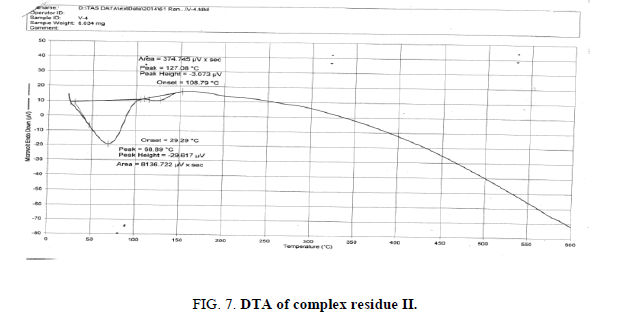
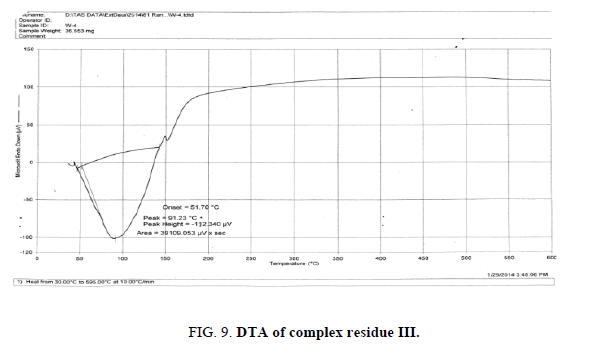
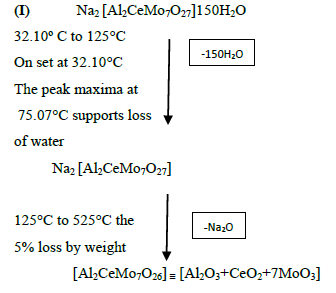
The DTA graph of the product suggest exothermal decomposition reaction for loss of water molecules and also the other minor extreme graph at 309°C and 527.72°C peak indicates the internal rearrangement of the product. The DTA peak on set at 29.29°C and peak at 68.89°C temperature range 30°C to 140°C support the loss of water of association of the product, apprpox 60% by weight. Another peak at 127.08°C, onset at 108.79°C supports loss of Na2O with further 1.5% loss by weight. The DTA graph supports the loss of entire water molecule associated with product by the strong exothermic peak appeared at 91.23°C which onset at 51.70°C. Loss of Na2O is supported by unnoticed small endothermic peak at about 150°C (Figures 1-9).
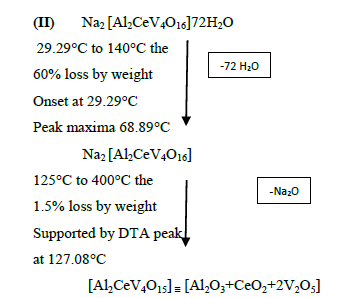
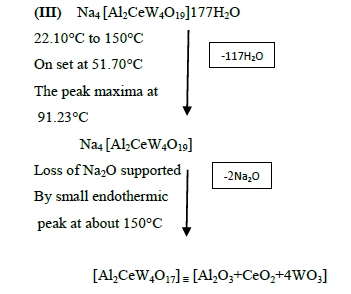
Conclusion
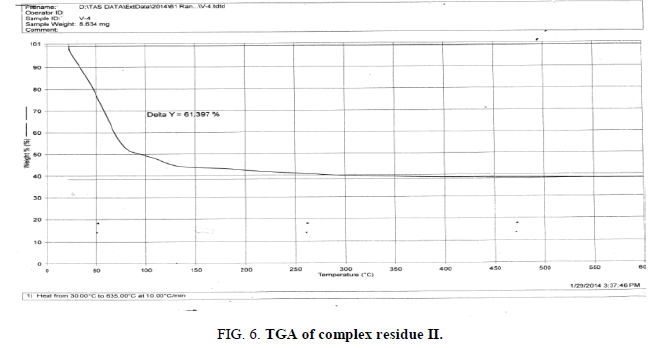
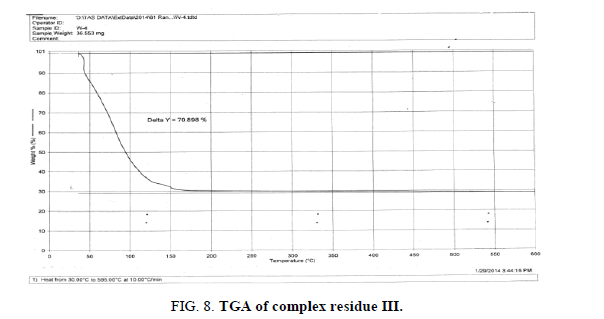
The above analysis, results and discussion concluded on the fact that the three different triheteropoly complexes are stable in the atmospheric condition up to almost 60°C temperature. These complex compounds were recovered in the solid state in the room temperature condition. The solubility analysis of all the three different product observed as they were about insoluble in water at room temperature but completely soluble in boiling water. The association of the ionic sodium with all the three different triheteropoly complexes revel the fact that these complex compounds are ionic in nature the association of sodium cation was confirmed on the basis of IR spectral peaks as well as also from the sodium flame photometric experiment suggesting the presence of sodium element as during the experiment he golden yellow flame is produced in the flame photometer. The magnetic moment of the three complex compounds i.e., triheteropoly complex compound by the Gouy balance method at room temperature revel that the three different heteropoly complexes are paramagnetic in nature. The triheteropoly complexes show the curves indicating the association of water of molecules but it is not possible to evaluate the water of molecules as water of hydration or/and water of constitution however the thermal analysis curves indicate first the loss of water of crystallization and then the loss of water of constitution as the TGA graph shows the loss of water about 30°C temperature up to 150°C temperature, Finally on the basis of entire analysis involved for the characterization concluded the might be the presence of water of constitution is the basis of the building of triheteropoly compound structure.
References
- Moffat JB. Metal-oxygen clusters: The surface and catalytic properties of heteropoly oxometalates. Springer Science & Business Media. 2006;Apr 11.
- Tsigdimos GA. Heteropoly verbindungen (Method Chimicum). In: Niedenzu K, Zimmer H, Stutgart G, editors. Thiems, Veriog. 1974;8:32.
- Dawson B. The structure of the 9 (18)-heteropoly anion in potassium 9 (18)-tungstophosphate, K6 (P2W18O62). 14H2O. Acta Cryst. 1953;6:113-26.
- Keggin JF. The structure and formula of 12-phosphotungstic acid. In: Proceedings of the Royal Society of London A: Mathematical, Physical and Engineering Sciences. The Royal Society. 1934;144:75-100.
- West SF, RiethAud LF. Differential thermal analysis of some heteropoly acids of molybdenum and tungsten. J Phys Chem. 1955;59:1069-72.
- Matijevi? E, Kerker M. Influence of electrolytes on the light scattering of inorganic compounds. Light scattering of phosphotungstic acids1. J Am Chemsoc. 1959;81:1307-10.
- Hegedus A, Dvorsky M, Magr Tud, et al. OsztKozlemen. 1959;11:327.
- Zakhryapin B, Neorg AAA. Khim. 1959;1,445,2403.
- Eriks K, Yannoni NF, Agarwala UC, et al. Crystal structures of 2 heteropoly salts-potassium 12-tungstocobaltiate, k5 [cow12o40]. 20H2O and sodium 6-tungstonickelate, Na4 [niw6o24h6]. 16H2o. ACTA CRYST. 1960;13:1139.
- Dexter DD, Silverton JV. A new structural type for heteropoly anions. The crystal structure of (NH4) 2H6 (CeMo12O42).12H2O. J Am Chemsoc. 1968; 90:3589.
- Han HHK. Ph.D. Thesis Boston University. 1970.
- Wells AF. Structural Inorganic Chem. 3rd ed. Oxford. 1962; p:451.
- Perloff A. Crystal structure of sodium hexamolybdochromate (III) octahydrate, Na3 (CrMo6O24H6). 8H2O. Inorg Chem. 1970;9:2228-39.
- Mishra HC, Roy SK, Ojha AN. Solid state studies on polymerization and depolymerization of 12-heteropoly molybdochromate. J Indian Chem Soc. 1978;55:307-8.
- Langinestraand A, Cerric R, Gazz R. Chim ltal. 1965;95:26.
- Burkholder E, Golub V, O'Connor CJ, et al. Solid state coordination chemistry: One-, two-, and three-dimensional materials constructed from molybdophosphonate subunits linked through binuclear copper tetra-2-pyridylpyrazine groups. Inorg Chem. 2003;42:6729-40.
- Ferraro JR. Pleniumpress, New York. Chapter V. 1971.
- Okuhara T, Mizuno N, Misono M. Catalytic chemistry of heteropoly compounds. Advances in Catalysis. 1996;41:113-252.
- Morizzi J, Hobday M, Rix C. Some bidentate amine adducts of indium (III) organophosphonates. J Mater Chem. 2001;11:794-8.
- Firouzabadi H, Jafari AA. Heteropoly acids, their salts and polyoxometalates as heterogenous, efficient and eco-friendly catalysts in organic reactions: Some recent advances. JICS. 2005;2:85-114.
- Mendham J, Denney RC, Barnes JD, et al. Vogel's Quantitative Chemical Analysis. Prentice Hall, New York. 2000;71:65-70.