Original Article
, Volume: 13( 1)Phytoremediation Of Heavy Metal-polluted Mine Drainage By Eleocharis Acicularis
- *Correspondence:
- Nurfitri AG, Department of Earth Science, Graduate School of Science and Engineering, Ehime University, 2-5 Bunkyo-Cho, Matsuyama 7908577, Japan, Tel: +818062870755; E-mail: vivinurv3@gmail.com
Received: February 16, 2017; Accepted: February 27, 2017; Published: February 28, 2017
Citation: Nurfitri AG, Masayuki S, Koichiro S. Phytoremediation of heavy metal-polluted mine drainage by Eleocharis acicularis. Environ Sci Ind J. 2017;13(1):131.
Abstract
The aquatic macrophyte Eleocharis acicularis from Cyperaceae family have been reported to be a hyperaccumulator of heavy metals such as As, Fe, Ag, Cd, Cs, Cu, In, Pb, and Zn in laboratories and field experiments. This study aimed to determine heavy metal accumulation in E. acicularis at heavy metal-rich mine drainage and to reveal the plant ability to uptake heavy metals in waste drainage with addition of silicon fertilizer in E. acicularis for phytoremediation. The floating mats of E. acicularis were transplanted in the two of mining wastewater pond in a mining site of Southwest Japan. The result indicates that the bioconcentration factors of Fe, Cu, Zn, As, and Pb shows the E. acicularis is a hyperaccumulator of heavy metals in the water. However, there are no effects on the addition of silicon fertilizer in correlations between silicon concentration and heavy metal in E. acicularis.
Keywords
Eleocharis acicularis; Phytoremediation; Silicon fertilizer
Introduction
Soil and underground water contamination by heavy metals is a serious problem worldwide, and ongoing studies in several countries are addressing these environmental issues [1,2]. Heavy metals are defined as metallic elements that have a high density. They are released into the environment by natural or anthropogenic sources, such as mining, smelting, and other metal-based industrial operations [3-5]. One of the strategies used to remediate heavy metal pollution is phytoremediation, which utilizes plants to clean up metal-, metalloid-, and radionuclide-contaminated sites in both developing and transition nations [6].
This strategy is also known as ‘bioremediation’ and involves the soil microflora and fauna [7]. Phytoremediation is a technology based on the use of plants to remediate contaminated soil, water, and air, and involves various processes [8,9].
The aquatic macrophyte E. acicularis, which belongs to the family Cyperaceae, has the ability to accumulate copper (Cu), arsenic (As), antimony (Sb), zinc (Zn), and lead (Pb) [10]. The plants can grow in a wide range of climates, from Indonesia to Europe [11]. E. acicularis has the potential to adapt to different environments and remediate heavy metal contamination in water bodies, such as in rivers, paddy fields, lakes, and ponds, and to treat tailing waste worldwide [9,12].
E. acicularis is known as a hyperaccumulator plant because it has the capacity to accumulate high concentrations of heavy metals. The capacity of E. acicularis for the phytoremediation of water and soil contaminated by heavy metals and metalloids under natural conditions has been demonstrated [13-16].
As a higher plant, E. acicularis has the possibility to absorb silicon (Si) from the nature. The Si as nutrition has been proved to be beneficial for the plant growth and is associated with plant benefits to increasing resistance against biotic and abiotic stress [17-19]. The graminaceous plant such as rice, sugarcane and some of cyperaceous also known to absorb Si accumulation-plant [19,20]. The objectives of this study were to (1) measure heavy metal/metaloids (Fe, Cu, Zn, Pb, and As) accumulation, and (2) determine the ability of E. acicularis to take up Fe, Cu, Zn, Pb, and As after the addition of silicon fertilizer.
Materials and Methods
Study site and methods
The mine is located in the south west of Japan. The mining site produced arsenic trioxide (As2O3) as the main product, both of source rocks and wall rocks also contain several heavy metals such as cadmium (Cd), copper (Cu) and zinc (Zn) for 28 years between 1920 and 1962 which can contaminate the soils and underground water [21,22]. An analysis of the arsenic levels near the mine site revealed high arsenic contents in the dust from the ceiling boards of residences, the soil, and percolated water from slag, with concentrations of approximately 200 ppm to 800 ppm, 2.760 ppm, and 180 ppm, respectively [21]. The mining tunnel and mine drainage are located near a river and residential paddy fields, which are largely covered by plants. Their location is on a mountain side that is covered by snow in the winter season. This study focuses on the mine drainage system by using aaquatic macrophyte E. acicularis.
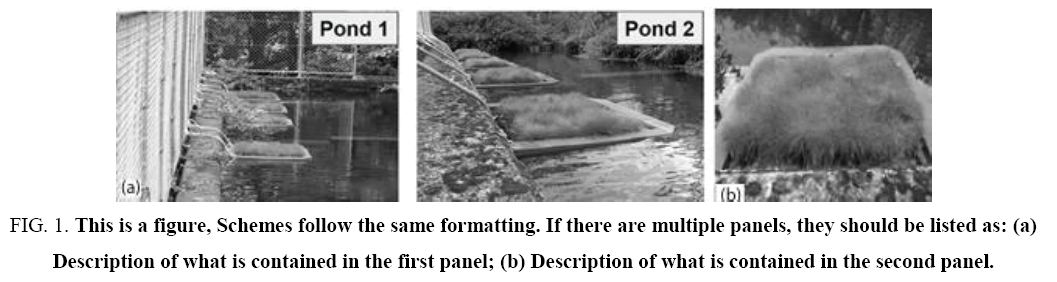
The aquatic macrophyte E. acicularis was transplanted for up to 7 months during summer to winter season from August into February to a heavy metal-contaminated mine drainage effluent from the abandoned mine. The main drainage system has two ponds into which water flows from the mining tunnel. Each pond measures 3 m in width, 20 m in length, and 3 m in depth. E. acicularis was cultivated in a two ponds using a floating cultivation method ( Figure 1). In pond 1, there are four floating cultivation of E. acicularis were used with a granulated silicon-based fertilizer, and ± 500 g was applied to the bottom of the E. acicularis mat. This method was used to determine the plant’s ability to take up heavy metals with the addition of silicon fertilizer. No silicon fertilizer was used in pond 2 that there are five floating of E. acicularis. The plants were analyzed before transplanting and again at 44, 71, 99, 137, and 177 days. Water samples were collected in ponds 1 and 2 at the time of transplantation.
Figure 1: This is a figure, Schemes follow the same formatting. If there are multiple panels, they should be listed as: (a) Description of what is contained in the first panel; (b) Description of what is contained in the second panel.
Analytical methods
A particle-induced X-ray emission (PIXE) analysis was used to determine the heavy metal concentrations in the water and E. acicularis at the Cyclotron Center of Iwate Medical University, Iwate, Japan. The plant samples were separated into roots and shoots, rinsed with Milli-Q water, and dried in a ventilated oven at 80°C for 48 h. The dried samples were ground into a fine powder with an agate mortar. The powdered plant samples were digested with 1 mL of nitric acid (HNO3) and heated in a microwave at 150 W for the PIXE analysis.
Statistical analysis
The statistical analyses of experimental data obtained using Independent-Sample T-test performed with SPSS 16.0 for Windows.
Results and Discussion
Growth of Eleocharis acicularis in mine drainage
E. acicularis adapted well to the mine drainage effluent during the experiment, showing a good growth rate in the floating cultivation system used in each pond. In this study, the main drainage in the study area was contaminated by As, Pb, and Cu with the concentration exceeding WHO standards for drinking water (>0.01 mg/L) [23]. The highest concentrations of As, Pb and Cu from mine drainage water were 59.4 ± 4.6, 4.36 ± 1.37, and 80.3 ± 5.03. The plants in pond 2 grew to a higher density than those in pond 1 and maintained a green color throughout the cultivation period until day 137. A proportion of the E. acicularis population in pond 1 became brown and finally decomposed on day 177, whereas the other plants only became brown. Because the plants were cultivated for 177 days during winter, the E. acicularis plants gradually turned brown, as most common plants do during this season. This condition affected the heavy metal absorption in the plant shoot. The pH of the water did not differ substantially between the two ponds, with pH values of 7.19-7.95 and 7.13-8.25 in ponds 1 and 2, respectively. These values are close to the neutral alkaline phase of pH based on the classification proposed by the United States Geological Survey [24].
Heavy metal accumulation in E. acicularis
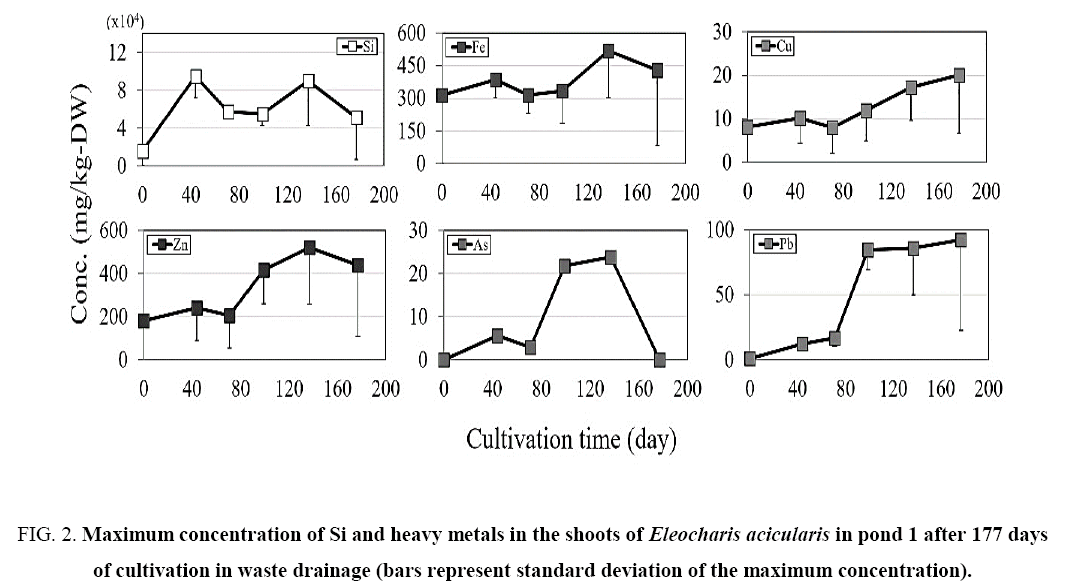
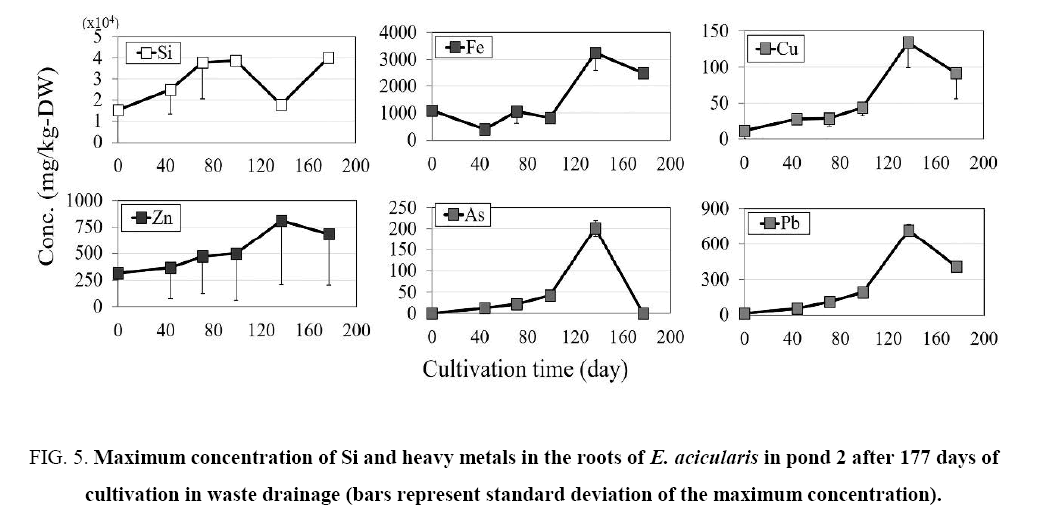
The heavy metal accumulation in E. acicularis was measured in the shoots, roots, and water samples from the mine drainage system. Figure 1 and 2 show the silicon (Si) and heavy metal concentrations in the mine drainage effluent and E. acicularis, respectively. The concentrations are shown by maximum concentration to consider the maximum capacity of E. acicularis in this field.
Figure 2: Maximum concentration of Si and heavy metals in the shoots of Eleocharis acicularis in pond 1 after 177 days of cultivation in waste drainage (bars represent standard deviation of the maximum concentration).
Silicon (Si)
In this experiment, roots of aquatic macrophyte E. acicularis are attached and accumulate and directly with the granular silicon fertilizer. Maximum concentration of Si in E. acicularis between two ponds shows that the highest accumulation was observed in pond 1 with the main route of Si uptake was through the roots.
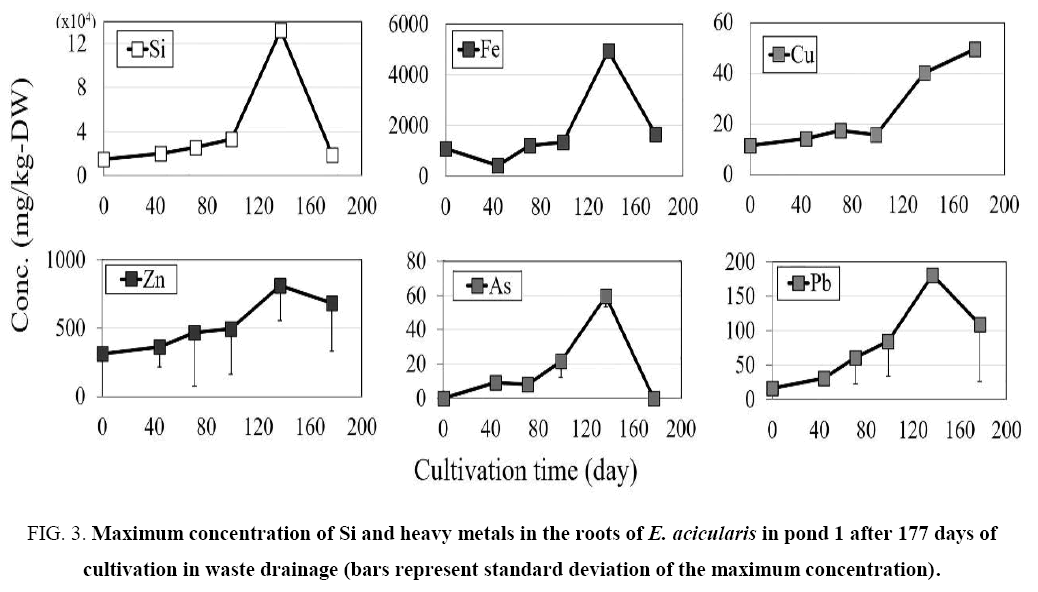
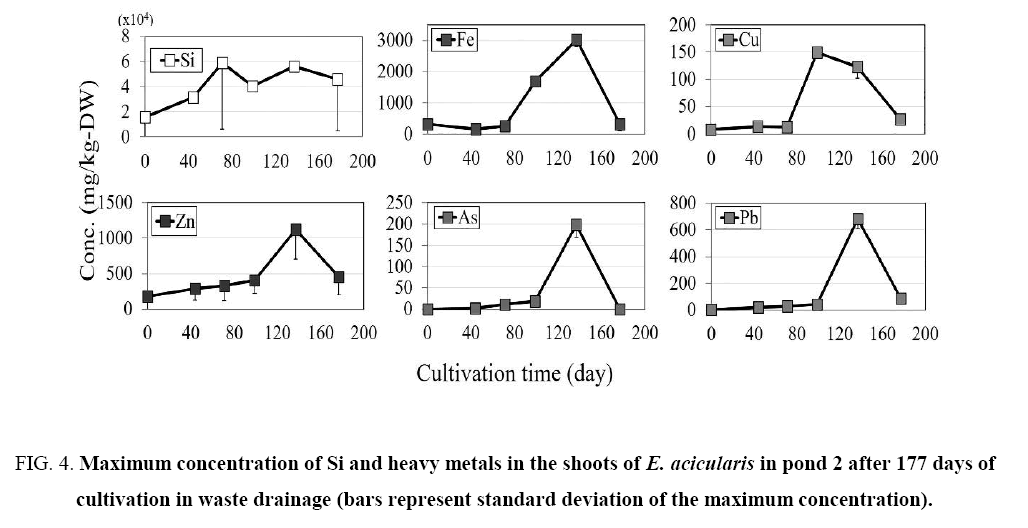
In pond 1, the Si concentration showed a similar pattern in the shoots and roots of E. acicularis ( Figure 2) during the cultivation period. Variations in the Si concentration in the shoots were observed on days 0, 44, 71, 99, 137, and 177. The Si concentration increased from summer to autumn (0 to 137 days) and then decreased during winter, between days 137 and 177. In pond 2, E. acicularis was transplanted without Si fertilizer. The maximum initial Si concentrations in the shoots and roots of were 15600 and 15100 mg/kg dry weight (DW), respectively. The Si concentration increased from summer to autumn, from day 0 to day 137, then decreased in the winter season between days 137 and 177.
Iron (Fe)
The maximum Fe concentration was lower in the shoots than that in roots ( Figure 2 and 3). The shoot concentration of Fe in pond 1 increased slightly between days 44 and 177, with values of 387, 316, 335, 518, and 430 mg/kg DW on days 44, 71, 99, 137, and 177, respectively. Also Fe in pond 2 increased between days 44 and 177, with values of 151, 259, 1700, 3030, and 303 mg/kg DW on days 44, 71, 99, 137, and 177, respectively. In both ponds, the Fe concentration showed a similar trend in the shoots and roots, with a slight increase from day 0 to 137 and a slight reduction at 177 days of cultivation.
Figure 3: Maximum concentration of Si and heavy metals in the roots of E. acicularis in pond 1 after 177 days of cultivation in waste drainage (bars represent standard deviation of the maximum concentration).
Copper (Cu)
The maximum concentration of Cu in the shoots and roots in ponds 1 and 2 increased slightly until day 137 of cultivation The Cu concentration showed a similar trend in the shoots and roots, with a slight increase until day 137 and a slight reduction at 177 days of transplantation.
Zinc (Zn)
The concentration of Zn in the shoots and roots increased slightly in pond 1. The maximum concentrations of Zn in the shoots and roots in pond 2 showed similar trends ( Figure 2- 5). The concentration of Zn increased slightly until day 137 and decreased slightly at 177 days of cultivation, showing similar patterns in ponds 1 and 2.
Figure 4: Maximum concentration of Si and heavy metals in the shoots of E. acicularis in pond 2 after 177 days of cultivation in waste drainage (bars represent standard deviation of the maximum concentration).
Figure 5: Maximum concentration of Si and heavy metals in the roots of E. acicularis in pond 2 after 177 days of cultivation in waste drainage (bars represent standard deviation of the maximum concentration).
Arsenic (As)
The maximum concentration of As in the plants is shown in Figure 2- 5. The concentration of As was higher in the roots than in the shoots. The concentration in shoots and roots showed similar patterns in the two ponds, with a slight increase until day 137 and a slight reduction from day 137 to 177 of transplantation.
Lead (Pb)
The maximum concentration of Pb was higher in the roots than in the shoots, as shown in Figure 2- 5. The Pb concentration in the shoots and roots in pond 1 increased slightly until day 177 of cultivation, whereas the Pb concentration in shoots and roots in pond 2 increased slightly until day 137 and decreased slightly from day 137 to day 177 of cultivation.
Phytoremediation using E. acicularis was previously carried out in field experiment [13,15,16,25]. The highest concentration of Fe, Cu, Zn, As, and Pb in E. acicularis were about 109800, 20200, 13700, 1790 and, 1120 mg/kg-DW, respectively.
Previous studies reported concentrations of Fe, Cu, Zn, As, and Pb in water of 101, 14.6, 1220, 13.7, and 566 μg/L, respectively [13,25]. By contrast, the highest concentrations of Fe, Cu, Zn, As, and Pb in the water in the present study were 144, 4.36, 106, 59.4, and 80.3 μg/L, respectively. The concentrations of heavy metals in the waste water were not stable, but there was little accumulation in the pond because the waste water flowed continuously to the river. It is possible that the heavy metals flowed to the river as well.
The accumulation of Fe, Cu, Zn, As, and Pb in wetland plant species has been reported in previous studies. De Lacerda et al. and Weis et al. reported high concentrations of Zn in Spartina alterniflora. Other wetland plant species, such as Eichornia crassipes, Avicennia marina, and S. maritima, have also been shown to accumulate Zn, Cu, and Pb in plant tissues.
In the present study, the roots of E. acicularis accumulated more Fe, Cu, Zn, As, and Pb than the shoot. The highest Fe, Cu, Zn, As, and Pb concentrations in the shoot were 3030, 123, 1120, 198, and 682 mg/kg DW, respectively. This indicates that the shoots of E. acicularis are tolerant of heavy metals. The Fe, Cu, Zn, As, and Pb concentrations in the roots were 4930, 91.7, 1260, 201, and 719 mg/kg DW, respectively, indicating that the roots of E. acicularis are tolerant of heavy metals.
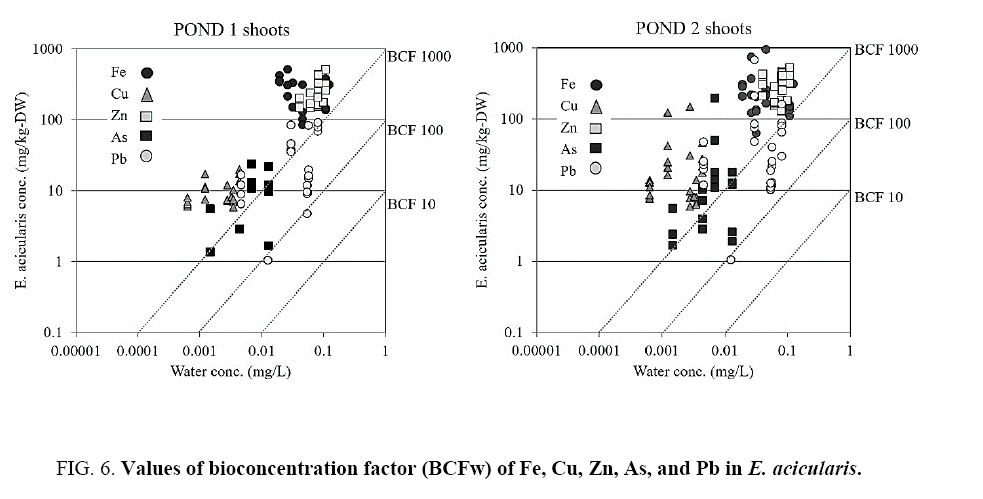
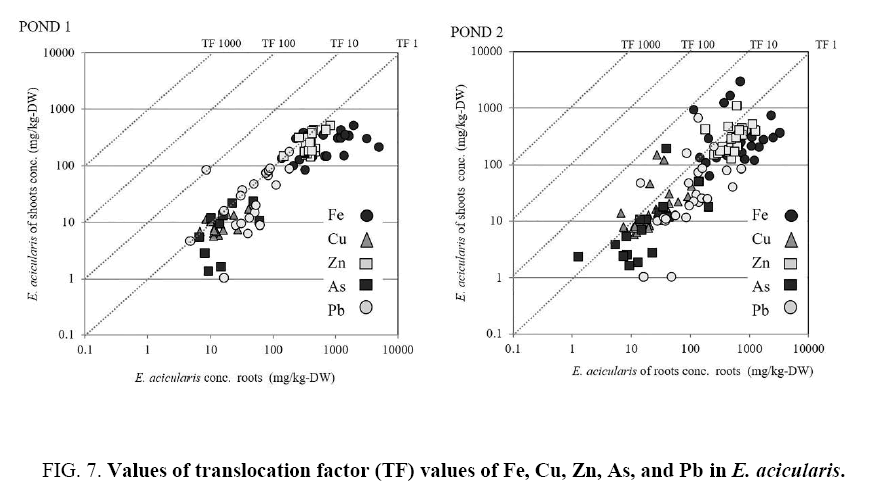
A plant is classified as a hyperaccumulator for heavy metals if it meets four criteria: (a) TF (the level of heavy metals in the shoot divided by the level of heavy metals in the root) >1; (b) BCFw (the level of heavy metals in the shoot divided by the total level of heavy metals in water) >100; (c) heavy metal levels are 10-500 times higher than the levels in normal (uncontaminated) plants [26]; and (d) it contains more than 1000 mg/g of copper, lead, nickel, and chromium, or more than 100 mg/g of cadmium or more than 10000 mg/g of zinc. A plant with high levels of heavy metals in the roots and a shoot/root ratio<1 is classified as a heavy metal excluder. In addition another research have reported that a plant should not be classified as a hyperaccumulator based solely on its TF and BCF values, recommending that levels of accumulation should be considered as well as concentrations [27].
The BCF and TF of E. acicularis are shown in Figure 6 and 7. A BCF value >100 indicates the potential utility of a plant species for phytoremediation in water. The BCF values for Fe, Cu, Zn, As, and Pb were >100 in this plant. This is consistent with the results of a previous study of E. acicularis that showed the ability of the plant to hyperaccumulate those elements in field and laboratory experiments. The TF of this plant was opposite to the BCF. The TF value indicates a plant’s efficacy as a metal transporter. The TF value of <1 in this study is consistent with that reported by Ha et al. [16]. The TF values for Fe, Cu, Zn, As, and Pb indicated that this plant is tolerant to heavy metals.
Regarding the criteria for hyperaccumulator plants, in the present study, the E. acicularis in both ponds showed BCF value >100, whereas the TF values and the concentrations of heavy metal were below the predetermined values that define a hyperaccumulator. In contrast to previous studies, E. acicularis showed the capacity to accumulate high concentrations of heavy metals. This prevented the plants from absorbing heavy metals. However, high Si concentrations facilitate the decomposition of plants, and may also cause plant disease and inhibit their absorption of heavy metals.
Previous studies have shown that E. acicularis is a strong candidate for phytoremediation because of its capacity to accumulate high concentrations of multiple heavy metals at values that define a hyperaccumulator. The BCF is an index of the efficiency of metal accumulation in plants, and is defined as the ratio of the metal concentration in the plant to that in water. Hyperaccumulator plants are defined by different BCF values in different growth media. In the soil, BCF values >1 are used to evaluate the potential utility of a plant as a hyperaccumulator in phytoremediation [28] whereas in water, the BCF values for metal accumulation are generally >100 [15]. The present study demonstrated the potential utility of E. acicularis as a hyperaccumulator plant for phytoremediation and showed that it can be classified as a tolerant plant, capable of heavy metal accumulation in its tissues ( Figure 6 and 7).
Effect of Si Concentration to the Heavy Metal Accumulation by E. acicularis
The heavy metal accumulation by E. acicularis was higher in pond 2, which had a lower concentration of Si, than in pond 1. The variations in the Si and heavy metal concentrations are shown in a box plot, which demonstrates the association between the high Si concentrations and low heavy metal concentrations in pond 1 (to which Si fertilizer was added) in the plant shoots and roots. The lower concentration of Si in pond 2 was associated with higher heavy metal concentrations than were observed in pond 1.
Silicon fertilizers affect plants in two ways: with an indirect effect on soil fertility or a direct effect on the plant. Most investigations of the effects of monosilicic acid on soil properties have addressed its interactions with soil phosphates. The effects of silicon fertilizers on the soil are mediated by two processes. An initial increase in the concentration of monosilicic acid results in the transformation of slightly soluble phosphates into plant-available phosphates. The Si fertilizer then absorbs phosphate, reducing the leaching of phosphate by 40% to 90% [29,30].
The concentration of Si correlated inversely with that of As (n=107). Si also substantially reduced the retention of Cd in the roots and reduced its translocation to the shoots. Previous studies have reported similar relationships to those identified in the present study, showing that increased Si concentrations in rice leaves and stems correlated negatively with the concentrations of Cd, Zn, Cu, and Pb.
In addition to its role in metal toxicity, the effect of Si on metal tolerance has been studied. The Zn coexists with Si in the cytoplasm and that Zn-silicate is a transient storage compound for Zn, slowly degrading to SiO2 [31]. Zn translocates into vacuoles and accumulates in an unknown form. It has been suggested that the formation of Zn-silicate is part of the mechanism of heavy metal tolerance and may be responsible for the alleviation of Zn toxicity in Cardaminopsis. A similar mechanism has been reported in maize, which suggests that Si plays a role in the detoxification of excessive Cd in the plant.
Consistent with previous research, the Si in the shoots of E. acicularis in pond 1 played a role in the detoxification of heavy metals in the plants. However, the accumulation of heavy metals did not correlate with the concentration of Si in the plants. A weak correlation (R2=0.0-0.2) was observed because E. acicularis itself was not treated with the Si fertilizer. This could be one reason why the plants in pond 2 accumulated slightly higher concentrations of heavy metals than the E. acicularis in pond 1.
However, as Phytolits in plants, Si helps to uptake more heavy metals. It may play an important law in phytoremediation in the environment [16]. In this study, the role of Si in plants must be clarified and analyzed further. Which form of Si in plant plays a role to uptake more heavy metals and which form of Si in plant plays a role as detoxification of heavy metals.
Conclusions
In this study, field experiments showed that E. acicularis can grow on highly toxic mine drainage and accumulate heavy metals in the winter. The application of silicon fertilizer to E. acicularis mats had no effect on the heavy metal accumulation by the plants. The absorption of the different heavy metals by E. acicularis was not competitive, indicating that absorption of a specific metals by E. acicularis did not affect its absorption of other heavy metal cations. The accumulation of heavy metals by E. acicularis in a highly toxic mine drainage effluent showed that E. acicularis is a hyperaccumulator of Fe, Cu, Zn, As, and Pb, based on the BCF for the main drainage effluent. The efficiency of E. acicularis in phytoremediation was not only evident in its BCF values, but also in its ability to accumulate high concentrations of heavy metals. A possible explanation is the high concentration of Si present and the role of Si fertilizer in preventing the accumulation of heavy metals in the plant shoots. Further research is necessary to determine which form of Si plays a role in the uptake of heavy metals and which form of Si plays a role in the detoxification of heavy metals in E. acicularis.
Acknowledgments
The author Nurfitri Abdul Gafur wishes to thank the Japanese Government for providing a Monbukagakusho Scholarship for Graduate study at Ehime University.
Author Contributions
Nurfitri Abdul Gafur contribute to study design and data analysis, also drafted the manuscript which was undertaken in association with his PhD program at Ehime University. Masayuki Sakakibara, as PhD. Supervisor also assisted in data analysis and manuscript revisions. Koichiro Sera provided the PIXE measurement of w, which is also a substantial contribution to the manuscript
Conflicts of Interest
The authors declare no conflict of interest.
References
- Miretzky P, Munoz C, Carrillo-Chavez A. Cd (II) removal from aqueous solution by Eleocharis acicularis biomass, equilibrium and kinetic studies. Bioresour Technol. 2010;101(8):2637-42.
- Ogundiran MB and Osibanjo O. Heavy metal concentrations in soils and accumulation in plants growing in a deserted slag dumpsite in Nigeria.Afr J Biotechnol.2008;7(17):3053-60.
- Nagajyoti PC, Lee KD, Sreekanth TVM. Heavy metals, occurrence and toxicity for plants: A review. Environ Chem Lett. 2010;8(3):199-216.
- Demirak A, Yilmaz F, Levent TA,et al. Heavy metals in water, sediment and tissues of Leuciscus cephalus from a stream in southwestern Turkey. Chemosphere.2006;63(9):1451-8.
- Liao S and Chang W. Heavy metal phytoremediation by water hyacinth at constructed wetlands in Taiwan.J Aquat Plant Manage.2004;42:60-8.
- Wuana RA and Okieimen FE. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. 2011:1-20.
- Souza RB de, Maziviero TG, Fontanetti CS et al. Soil contamination with heavy metals and petroleum derivates: Impact on edaphic fauna and remediation strategies. In: Maria CHS, editor.Soil processes and current trends in quality assessment.Croatia: Intech; 2013. 175-203 p.
- Khan FI, Husain T, Hejazi R. An overview and analysis of site remediation technologies. J Environ Manage. 2004;71(2):95-122.
- Mulligan CN, Yong RN, Gibbs BF. Surfactant-enhanced remediation of contaminated soil: A review.Eng Geol. 2001;60(1-4):371-80.
- Verma R and Dwivedi P. Heavy metal water pollution: A case study. Recent Res Sci Technol. 2013;5(5):98-9.
- Yeh TY, Chou CC, Pan CT. Heavy metal removal within pilot-scale constructed wetlands receiving river water contaminated by confined swine operations. Desalination.2009;249(1):368-73.
- Mulligan CN, Yong RN, Gibbs BF. Remediation technologies for metal-contaminated soils and groundwater: An evaluation. Eng Geol.2001;60(1-4):193-207.
- Ha NTH, Sakakibara M, Sano S. Phytoremediation of Sb, As, Cu, and Zn from contaminated water by the aquatic macrophyte Eleocharis acicularis. Clean-Soil, Air, Water.2009;37(9):720-5.
- Basu N, Nam DH, Kwansaa AE, et al. Multiple metals exposure in a small-scale artisanal gold mining community Environ Res. 2011;111:463-7.
- Sakakibara M, Sugawara M, Sano S, et al. Phytoremediation of heavy metal-contaminated river water by aquatic macrophyte Eleocharis acicularis in a mine site, southwestern Japan. 2013;20:226-33.
- Ha NTH, Sakakibara M, Sano S, et al.The potential of Eleocharis acicularis for phytoremediation: Case study at an abandoned mine site. Clean-Soil, Air, Water.2009;37(3):203-8.
- Romero A. Silicon and plant diseases. A review. Agron Colomb. 2011;29(3):473-80.
- Epstein E. The anomaly of silicon in plant biology. Proc Natl Acad Sci USA.1994;91(1):11-7.
- Liang Y, Sun W, Zhu YG,et al. Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: A review.Environ Pollut. 2007;147(2):422-8.
- Ma JF, Tamai K, Yamaji N, et al. A silicon transporter in rice. Nature.2006;440(7084):688-91.
- Almost I S 1990 An Epidemiological 26 53-62.
- Hiroki M. Effect of arsenic pollution on soil microbial population. Soil Sci Plant Nutr.1993;39:227-35.
- Anon WHO’s drinking water standards.
- Reston U and Organic VE. Standard Operating Procedure. Qual Assur. 1993;1-11.
- Ha NTH, Sakakibara M, Sano S. Accumulation of Indium and other heavy metals by Eleocharis acicularis: An option for phytoremediation and phytomining.Bioresour Technol. 2011;102(3)2228-34.
- Mganga N, Manoko MLK, Rulangaranga ZK. Classification of plants according to their heavy metal content around north mara gold mine, Tanzania: Implication for phytoremediation. Tanz J Sci. 2011;37:109-19.
- Prasetia H, Sakakibara M, Sueoka Y,et al.Pteris cretica as a potential biomarker and hyperaccumulator in an abandoned mine site, Southwest Japan. Environments.2016;3(3):15.
- Ashraf MA, Maah MJ,Yusoff I.Heavy metals accumulation in plants growing in ex tin mining catchment. Int J Environ Sci Technol. 2011;8(2):401-16.
- Matichenkov V and Calvert D. Silicon as a beneficial element for sugarcane. J Am Soc Sugarcane.2002;22:21-30.
- Ma JF. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses.Soil Sci Plant Nutr. 2004;50(1):11-8.
- Neumann D and Nieden U. Silicon and heavy metal tolerance of higher plants. Phytochemistry.2001;56(7):685-92.