Original Article
, Volume: 11( 6)Mechanical Extraction and Fuel Properties Evaluation of Hura crepitans Seed Oil
- *Correspondence:
- Otoikhian SK , Department of Chemical Engineering Technology, Auchi Polytechnic, Auchi, Edo State, Nigeria, Tel: 2348029320114; E-mail: kev4za@yahoo.com
Received: September 12, 2016; Accepted: September 19, 2016; Published: September 25, 2016
Citation: Otoikhian SK, Aluyor EO, Audu TOK. Mechanical Extraction and Fuel Properties Evaluation of Hura crepitans Seed Oil. Chem Technol Ind J. 2016;11(6):104.
Abstract
Hura crepitans seeds (HCS) from H. crepitans tree were collected and extracted by mechanical screw press at a constant pressure of 25 N/m2. A study in terms of proximate, elemental and fatty acid contents of the oil was investigated using standard analytical techniques. The results were as follows acid value 8.49 mg·KOH/g, flash point 249°C, cloud point 11°C, pour point -6°C, density 919.38 kg/m3, kinematic viscosity 59.88 Cst, Sulphur 0.0174%, water and residue 2.686%, brownish colouration, free fatty acid 4.15%, iodine value 164.34 g/100, saponification value 236.12 mg·KOH/g, free glycerin 0.614%, total glycerin 8.253% and percentage oil yield 38.40%. The results of the fuel properties reveal its potential viability as biodiesel feedstock.
Keywords
Hura crepitans tree; Analytical techniques; Screw press; Fuel properties
Introduction
Most Nigerian oil crops are not yet economically exploited; many of them are regarded as wastes or underutilized [1]. Hura crepitans plant an evergreen tree of Euphobiaceae family commonly planted in cities and villages of Nigeria, despite its abundance is still been underutilized [1]. A number of seed oil has been characterized but vast majority has not been adequately evaluated [2], this is particularly valid for the Nigerian flora which has one of the most extensive floras in continental Africa [3]. H. crepitans falls into this group of underutilized plants in Nigeria [2,4]. They are among the largest trees of tropical America [5] and are interesting for their pumpkin-shaped seed capsules that explode with a loud report, scattering the seeds. They are sometimes grown as boulevard trees but have disadvantages in their poisonous leaves, bark, and seeds and the explosions of their capsules, which have force enough to injure persons or livestock. Capsule explodes to disperse seed up to 14 meters away [5]. It was introduced by humans to Africa as a shade plant [5]. Fruits are capsule, 3 cm to 5 cm in length with a diameter of 5 cm to 8 cm. Sixteen carpels are arranged radically around the central axis, seeds are flattened with 15 mm to 25 mm in diameter. Lowland tropical to subtropical moist forest habitat is what is associated with tree; its large seeds can germinate and grow in deep shade allowing the plant to invade undistributed forest. H. crepitans are essentially not among the popularly cultivated of oilseeds like groundnut, soybean, palm fruit and palm seed in Nigeria [6].
At best they can be allowed to waste away. It is often cultivated for shade in Nigeria. Fishermen are said to use the milky caustic sap to poison fish, and it is not a trees to be careless with. Of all the tropical trees none is better equipped both physically and perhaps chemically to repel careless handling. Its presumable poisonous latex and its highly explosive capsule are found to be most interesting and worthy of study [7,8]. Legitimate concerns are being raised about the possible effect of using vegetable oil for fuel on the prices of oil for food [9], this study is thus aimed at extracting raw oil from H. crepitans seed and examine its physico-chemical properties, with the specific objective of viewing its viability as a potential non-edible feedstock in the production of biodiesel.
Literature Review
Seed oil extraction
Separation of oil from oilseeds is a major process in the utilization of the oil. The process used has direct impact on the quality and quantity of the oil as well as the protein obtained from the oilseeds. There are basically two methods for the separation of oil from its oilseeds. They are solvent extraction and mechanical oil expression. In the solvent extraction method, the solvent is brought into close contact with the oilseed whereby the oil present in the seed is dissolved in the solvent and later recovered by evaporation. In the second method, which is employed in this research, the pre-treated oilseed is passed through a screw press where a combination of shear stress and heat or shear stress only are used to crush the oilseed to release the oil. Presently, the solvent extraction method is the most popular method for separation of oil from soybeans, mainly because of the method’s high extraction efficiency (over 99%) as well as its capability to handle large quantities. The main drawback of this method is that, unless highly sophisticated equipment, proper hygienic conditions, and control of process parameters are maintained, the quality of the soy meal produced will not be adequate for sustainable human consumption [10]. This is in view of the traces of residual chemical solvents remaining in meals beyond levels acceptable for edible grades. Solvent extraction is more efficient than mechanical expression in that over 99% oil recovery can be obtained compared to 70% to 80% with the latter. Alternative oilseparating methods, such as mechanical oil expression, appear to have potential for producing chemical free and edible-grade defatted soy flour suitable for human consumption [11]. However, mechanical oil-expression equipment and processes presently available are not considered adequate for this purpose as their oil extraction efficiency is quite low (<70% oil extraction). These need to be improved so that not more than 5% oil remains in the de-oiled meal to make this process economically viable [11].
Composition of seed oil
Triglycerides are the main constituents of vegetable oils and animal fats. Triglycerides have lower densities than water (they float on water), and at normal room temperatures may be solid or liquid. When solid, they are called "fats" or "butters" and when liquid they are called "oils". A triglyceride, also called triacylglycerol, is a chemical compound formed from one molecule of glycerol and three fatty acids [12]. The basis of fat is glycerol which has a chemical formula that can be illustrated by the shape shown below. Each of the 'arms' of this shape can combine with fatty acid, to build up a molecule shown below the glycerol symbol (Figure 1 and 2) .
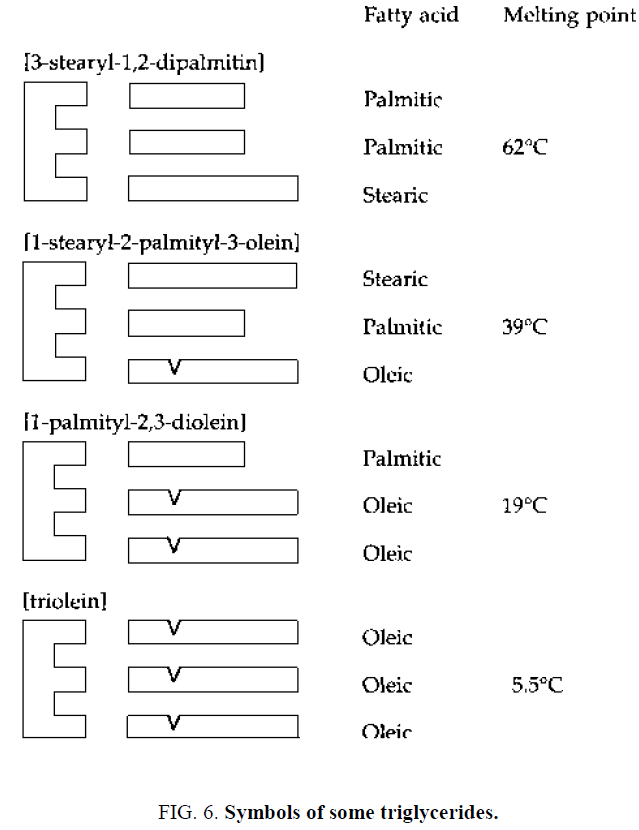
Because of the way in which its composition is built up, the chemical substance having this form is known as a triglyceride, and all oils and fats are made up of a mixture of these triglycerides (Figure 3) . A number of different fatty acids exist and, to a large extent, the character of a particular oil or fat depends on the actual fatty acids present in the individual triglyceride molecules [13]. Some of these components of fatty acids are longer than others and they can all combine with a glycerol 'arm'.
Figure 3: Symbolic representation of the combination of a glycerol with fatty acids of different lengths.
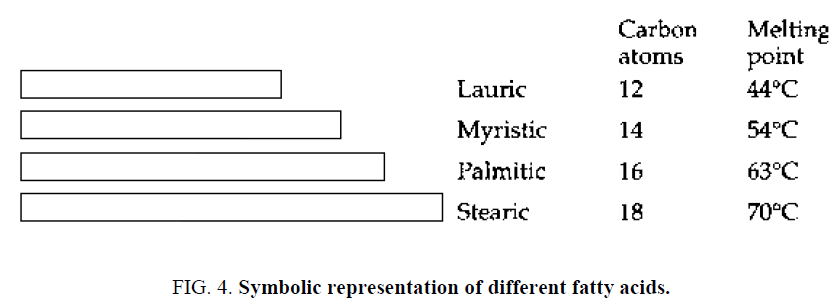
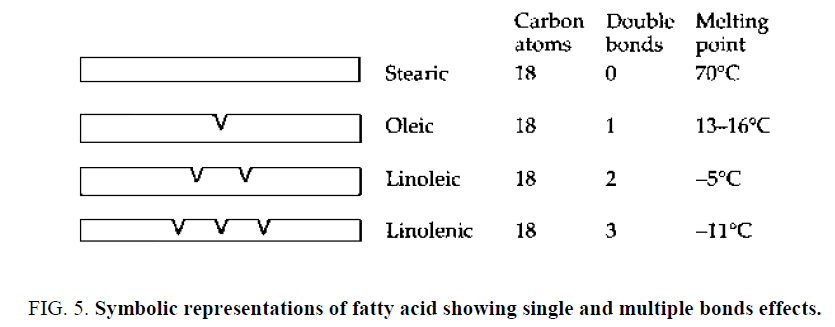
There are three different types of fatty acids: saturated, mono-unsaturated and polyunsaturated. Saturated fatty acids: In their simplest forms are fatty acids made up of linear chains of carbon atoms linked to a group that provides the acidic properties. Examples are lauric, myristic, palmitic, and stearic acids. They contained different numbers of carbon atoms (Figure 4) .
These fatty acids are solid at normal ambient temperatures and their presence in high proportions in a triglyceride mixture is likely to make it solid [13]. Monounsaturated fatty acids, shows sometimes carbon atom chains containing what chemists call a double bond. Fatty acids with just a double bond in their structures are said to be monounsaturated, an example is oleic acid having 18 carbon atoms with stearic acid [13]. Polyunsaturated fatty acids are made up of two or three double bonds and are said to be polyunsaturated. Linoleic and linolenic acids are common examples of them. They have the same number of carbon atoms as Stearic acid but linoleic acid has two double bonds and linolenic acid has three [13]. Relationships between fatty acids with 18 carbon atoms (C18 acids) can be represented as shown in Figure 5.
It can be seen that the presence of a double bond lowers the melting point [13]. Thus, a triglyceride mixture containing a high proportion of monounsaturated or polyunsaturated fatty acid is likely to be liquid at atmospheric temperature (Figure 6) . These triglycerides are some of the many that are found in palm oil [13].
The overall triglyceride composition is such that palm oil is liquid or semisolid at tropical temperatures but solid in temperate climates.
Deterioration of seed oil
Analytical chemists are able to measure the overall proportions of the different fatty acids present in oil or fat. The fatty acid composition of the oil tends to be characteristic of the oilseed from which it is extracted [13]. The following two types of deterioration can occur under poor storage conditions: oxidation, when the fat is attacked by the oxygen present in the air; and hydrolysis, when the fat is attacked by water [13]. Usually hydrolysis occurs in the presence of an enzyme which either exists naturally in the oilseed, or is present in moulds which grow on it. Hydrolysis can occur both in oilseeds and in extracted oil. Hydrolysis in oilseeds is accelerated by the presence of moulds. It is therefore very important to dry oilseeds to a moisture level which does not encourage mould growth [13]. Values of free fatty acid from good quality oilseed are found to be very low, thus, free fatty acid contents of oil are used as guide to the deterioration extent in the quality of oil. Coconut oil from high quality copra can have free fatty acid value of about 0.1% to 0.2%, but higher value of 5% can occur in oil from poor quality copra [13]. Even good quality oil will deteriorate quickly if it contains oilseed residues or is stored in wet drums. To avoid deterioration, particles of seed debris should be removed from the oil as soon as possible after extraction and certified oil should be stored dry clean containers [13].
Material and Methods
Material
Seeds of H. crepitans were collected from the premises of Auchi Polytechnics and Environs in the Northern Part of Edo State, Nigeria. All reagents used were of analytical grade obtained from SIGMA-ALDRICH Chemical and Co, USA.
Methods
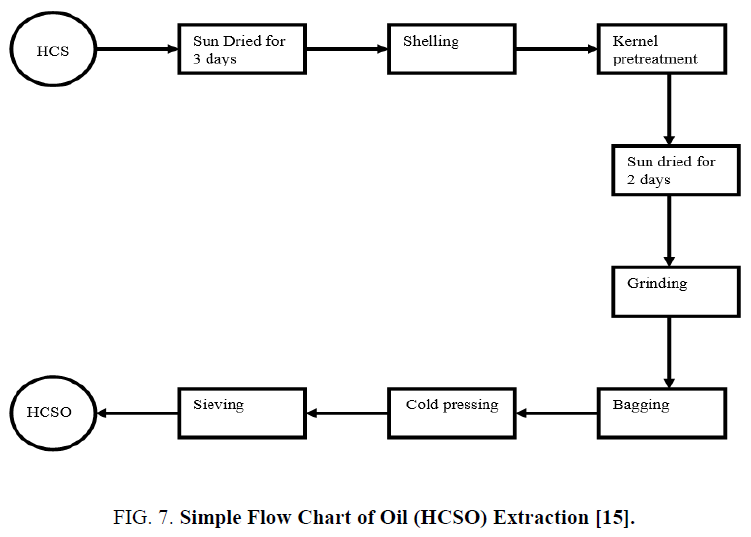
Extraction of H. crepitans seed oil (HCSO): Hydraulic press method was used in this study to extract the oil of H. crepitans from its seeds [14]. The extraction process is represented in a simple flowchart shown below (Figure 7) :
Seeds of H. crepitans were collected locally from Owan-East local government area of Edo State. Mature seed pods were sun dried for three days and stored at ambient temperature in sacks before use. During drying period most of the pods explode to bring out the seed nuts through explosive mechanism. The few ones that could not explode themselves were later bagged and hammered with sick to decorticate the seed nuts from the pods. The seed nuts consisting of the nut shell and the kernel are collectively picked together and pretreated by sun drying for two (2) days to enable easy removal of the shell to recover the kernel. After drying for two days the seed nuts are locally shelled with mallets and stones to get the white kernel which is again sun dried for 3 days so as to eliminate much of the water moisture present in the kernel before grinding. This drying operation was conducted to ease grinding and remove moisture. The dried kernel was then crushed; this was effectively done to reduce the particle size so as to allow the oil content expelled easily. After grinding, the crushed kernel was then bagged in a perforated sack made from baft. The sack was initially weighed before loading the crushed kernel [14]. The loaded sack was then placed inside the pan of a 50 Tons hydraulic press machine. The screw handle was adjusted to exert pressure of 25 N/m2 on the sack by moving the moveable plate closer to the fixed plate carrying the pan [10,11,13]. The oil in the crushed kernel is expelled through the perforation chamber on the pan into a reservoir. After much pressure has been exerted and dripping of the oil into the reservoir has finally stopped, the weight of the cake left and that of the oil collected in the reservoir were recorded according to Wiemer et al. [14] report. The final weight of the oil recovered was then used in calculating the percentage yield as follow.
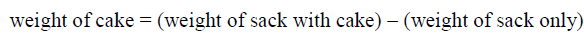 (1)
(1)
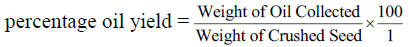 (2)
(2)
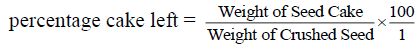 (3)
(3)
Physiochemical analysis of extracted H. crepitans seed oil (HCSO)
Fatty acid methyl ester analysis: The crude oil extract was made to be free of water by filtering through anhydrous sodium sulphate salt. The extracting solvent was removed from the oil/solvent mixture by using rotary evaporator. Fatty acid profile saturated, mono and polyunsaturated analysis were carried out by following the modified AOAC 965.49 and AOAC 996.06 official methods.
A 50 mg of the oil sample was saponified (esterifies) for 5 min at 95°C with 3.4 ml of the 0.5 M KOH in dry methanol. The mixture was neutralized by using 0.7 M HCl, 3 ml of the 14% boron triflouride in methanol was added. The mixture was heated for 5 min at 95°C to achieve complete methylation process. The fatty acid methyl esters were thrice extracted from the mixture with redistilled n-hexane. The content was concentrated to 1 ml for gas chromatography analysis and 1 μl was injected to the injection port of GC. The chromatography conditions of analysis are as attached in the analysis print out [16].
Flash point analysis: The sample was dried in the laboratory on the bench to remove the traces of the water moisture in the sample. The dried sample was poured into the cup of the tester to the mark and it was followed by the replacement of the cup and the cup cover with the left hand pointing towards the left front corner of the test unit. Stirrer driver was fitted into the tester properly and it was followed by the connection of the resistance thermometer probe. Flame and the pilot light were carried out by lighting and the draught screen was closed [17]. The tester was put on and the heater temperature was regulated and switched for the stirrer was on simultaneously with the tester for homogeneity. The flash occurs when a large flame was observed on the cup and the temperature at which this occurs was recorded as the flash point for the oil sample [17]. The fire point was observed temperature at which the oil combustion was sustained after the flash point of the sample was recorded. The temperature was recorded for the fire point for the sample.
Pour point analysis: The sample was homogenized and poured into the test jar to the mark level. The jar was closed tightly with the cork carrying the high pour thermometer that was placed 3 mm below the surface of the oil. The disc was placed in the bottom of the jacket and the ring gasket was placed around the jar at the 25 mm from the bottom. The test jar was then placed in the jacket. The oil was allowed to cool without disturbance to avoid fictitious results. The test jar from the jacket was removed carefully and tilted to ascertain whether there is a movement of the oil. The procedure continued in this manner until a point was reached at which the oil in the test jar showed no movement when the test jar was held in a horizontal position for 5 seconds [17].
Kinematic viscosity analysis: The bath was maintained at the operating temperature by regulating and thermosetting the bath temperature. The wide capillary viscometer tube was selected for the analysis. The sample was filtered through the N200 sieve to remove the associated solid particles. The charged viscometer was allowed to stay in the water bath long enough to reach the test temperature. Suction was used to adjust the head level of the test sample to a position in the capillary arm of the instrument to about 5 mm ahead of the first timing mark. With the sample flowing freely, measurement was done to 0.2 s, the time required for the meniscus to pass from the first time mark to the second [17].
Acid value analysis: Oil sample of 0.40 g was dissolved in the 5.0 ml in 1:1 mixture of ethanol diethyl ether in 100 ml volumetric capacity flask. The mixture was homogenized and allowed to stay on the bench until the indicator was added. Swirled and adding of phenolphthalein indicator was followed [17].
Density/specific gravity analysis: The sample was homogenized and 40 ml was poured into the clean 500 ml measuring cylinder, air bubbles were avoided during pouring. The temperature was controlled to avoid being lean on the wall of the cylinder. When the hydrometer has come to rest, the reading was taken [17].
Colour analysis: Two reference columns were filled up with distilled water to the marks. The sample column was filled up to the mark and was placed in between the two reference columns in the equipment compartment. While closing one eye and adjusting of the two knobs on the equipment, the equilibrium reading was observed to know when the system will give no difference in colouration. The value was then recorded for colour.
Cloud point analysis: A method of determining cloud point using a high precision cloud point meter comprising a waveguide sensor of a total-reflection type, the waveguide sensor including a waveguide having an incidence channel, an emergence channel and a detection surface all formed on a substrate, the incidence channel and emergence channel intersecting along the detection surface, an incidence optical fiber connected to the emergence channel; and a cooling/heating means in contact with the waveguide sensor for cooling/heating the waveguide sensor within a desired temperature range, the method comprising the steps of [17]:
• Placing a sample on the detection surface;
• Introducing light into the incidence optical fiber, thereby introducing light into the incidence channel;
• Detecting light in the emergence optical fiber, the light being received from the emergence channel;
• Cooling/heating the waveguide sensor using the cooling/heating means, thereby cooling/heating the sample; and
• Determining the temperature wherein a total reflection of light occurs as detected in the emergence optical fiber, the temperature being the cloud point of the sample.
Glycerin analysis: The method was based on the modified ASTM D7637 by cold oxidation of the glycerol content of the oil sample by sodium meta periodate in strong acidic medium. The formaldehyde and formic acid produced in the reaction were used to measure the glycerol content by titration with standard sodium hydroxide solution at pH 8.1-10. The quantitative determination of the glycerin level by the titrimetric method expressed as percentage.
Water/residue analysis: The sample was shaking thoroughly in the container before filling the two centrifuge tubes to the 50 ml mark. A 50 ml of the water saturated xylene was added to each of tube and then followed with 0.2 ml of the emulsifier solution. The tubes were stopped and inverted severally for mixing which was later placed in the water bath at 60°C for minutes. The content was centrifuged for 10 min at the rate of 1500 rpm. The combination volume of the water and residue at the bottom of the cup was read and recorded. The cup with the content without agitation was returned to the centrifuge and centrifuged for another 10 min at the same rate. The process was repeated until the combined volume of water and residue remained constant for two consecutive reading.
Sulphur analysis: The digested sample of 10 ml aliquot was measured into the 25 ml volumetric flask with the addition of the deionized water to 20 ml. Gelatin-barium chloride reagent (1.0 ml) was added and later made up to the volume with deionized water. The content was mixed thoroughly and allowed to stand for 30 min. The reading was made at 420 nm within 30 min on UV-visible spectrophotometer [17].
Free fatty acid analysis: The procedure involves the measurement of the level of concentration of oleic acid in the oil sample. The preparation of the 95% alcohol was carried out by measuring the alcohol into the beaker. Phenolphthalein (1.0%) was prepared into a separate beaker and kept on the laboratory bench. Also prepared was the 0.1 N standard solution of the sodium hydroxide in a conical flask. A 10.0 g of the homogenized oil sample was weighed into the 300 ml capacity of the Erlenmeyer flask. This was followed by the addition of the hot neutralized alcohol into the content of the beaker. The mixture was heated up to the almost boiling and the titration was performed by the addition of the sodium hydroxide solution to get to the end point. The mixture was allowed to settle in the flask and the pink colouration was observed above the sample after few seconds [17].
Result and Discussion
Table 1 shows the results of the physico-chemical properties of H. crepitans seed oil, the acid value was found to be 8.49 mg·KOH/g, this value obtained from H. crepitans seed oil extracted through mechanical techniques in this study was observed to be higher when compared with the value obtained for Jatropha seed oil (0.95 mg·KOH/g), Luffa cylindrical seed oil (3.72 mg·KOH/g), Chrysophyllum albidum seed oil (2.87 mg·KOH/g), green algae seed oil (0.374 mg·KOH/g) and castor seed oil (8.49 mg·KOH/g) [18], but lower than 27.09 mg·KOH/g reported from H. crepitans seed oil extracted through solvent extraction [19].
| Parameter | Value |
|---|---|
| Acid Value (mg·KOH/g) | 8.49 |
| Flash Point (°C) | 249 |
| Cloud Point (°C) | 11 |
| Pour Point (°C) | -6 |
| Density (kg/m3) | 919.38 |
| Kinematic Viscosity (Cst) at 40°C | 59.88 |
| Sulphur (%) | 0.0174 |
| Water and Residue (%) | 2.686 |
| Colour | Brownish |
| Free Fatty Acid (% oleic acid) | 4.15 |
| Iodine Value (g/100) | 164.34 |
| Saponification Value (mg·KOH/g) | 236.12 |
| Free Glycerin (%) | 0.614 |
| Total Glycerin (%) | 8.253 |
| Percentage Oil Yield (%) | 38.40 |
Table 1: Physico-chemical properties of H. crepitans seed oil.
The flash point of the seed oil was found to be 249°C. This value is lower than the reported for fresh Jatropha seed oil (204°C) [18]. The cloud point and pour point of the seed oil were found to be 11°C and -6°C respectively, the cloud point and pour point are lower than 15°C and 0°C respectively reported for Jatropha seed oil [18]. The density of H. crepitans seed oil used in this research was found to be 0.91938 g/cm3 which is lower than 0.94 g/cm3 [19] and 0.8711 g/cm3 [2] reported for H. crepitans seed oil extracted in their experiment, and also lower than 0.9186 g/cm3, 0.9169 g/cm3 and 0.9090 g/cm3 [6] reported from seed oils extracted from Cucumeropsis edulis, Hevea brasiliensis and Citrus sinensis respectively. The kinematic viscosity was found to be 59.88 Cst at 40°C and significantly higher than 36.02 Cst at 40°C reported for Jatropha seed oil [18]. The sulphur content was found to be 0.0174%, this value is lower than 0.0310% [20] reported for Cucumeropsis mannii vegetable oil. The oil yield of H. crepitans seed oil was found to be 38.40%. This value is lower than 51% [21] and 53.61% [19] reported for H. crepitans seed oil samples extracted through solvent extraction, the method (mechanical extraction) employed in this study in extracting crude sample of H. crepitans seed oil is devoid of impurities from solvents used in the solvent extraction method that could mal transesterification process [10,11], but the yield value was found to be higher than 21.57% [18,22] reported for Chrysophyllum albidum seed oil. The proximate composition; water and residue value was found to be 2.686%, this value is lower than 8.02% [1] reported for H. crepitans seed oil extracted as sample in their experiment. The iodine and saponification values were found to be 164.34 g/100 and 236.12 mg·KOH/g respectively, the saponification value is lower than 294.04 mg·KOH/g [2] reported for H. crepitans seed oil while the iodine value is significantly lower than 82.56 g/100 [18] reported for Luffa cylindrical seed oil and 119.76 g/100 [18] reported for green algae.
Table 2 shows fatty acid profile of H. crepitans seed oil extracted by mechanical method in this study. The result (%) of both oleic and linoleic acids from the results were found to be 20.225% and 65.997% respectively, these two fatty acids were seen to be in abundant in the oil seed samples with a total composition of 86.222%. The percentage composition obtained for oleic acid and linoleic acid are observed to be higher than 6.138% and 8.265% respectively earlier reported from H. crepitans seed oil sample by Oyeleke et al. [1]. The oleic value was found to be lower than 53.4% and 26.9% [23] reported for Thevetia peruviana (milk bush) oil and H. crepitans oil respectively, while the linoleic acid was higher than 19.03% and 36.6% [23] respectively reported for T. peruviana (milk bush) oil and H. crepitans oil. Palmitic, stearic and linolenic acids were found in low but measurable amounts in the sample. The palmitic acid content of the H. crepitans seed oil was 8.184%, which is lower than 14.398% reported for Jatropha curcas seed oil [23] but higher than 0.389% [1] reported for their H. crepitans seed oil sample and 19.10% [23] reported for T. peruviana (milk bush) oil. Moderate amount of stearic acid content (3.299%) was found which was found to be lower than 7.3% reported for T. peruviana oil [23], while linolenic acid was found very low but measurable quantity of 2.295% which was found to be lower than 0.3% and 0.8% reported for T. peruviana oil and H. crepitans oil respectively [23]. Result on fatty acid composition of H. crepitans seed oil, revealed that total saturated fatty acid composition of 11.48% is in line with 8.994, 35.2 and 18.92% total saturated fatty acid composition [19,23,24] reported for H. crepitans seed oil, but the total unsaturated fatty acid value of 88.52% was found to be higher than 66.483%, 64.9% and 52.46% total unsaturated fatty acid [19,23,24] reported for H. crepitans seed oil. 26.9% and 73.1% [23] total saturated and total unsaturated fatty acids were both reported for T. peruviana oil respectively. The differences may have possibly arisen from the different GC spectrometry employed in attaining these results.
| Fatty Acids | Class | % Composition |
|---|---|---|
| Palmitic | C16:0 | 8.183871 |
| Stearic | C18:0 | 3.299359 |
| Oleic | C18:1 | 20.22528 |
| Linoleic | C18:2 | 65.99681 |
| Linolenic | C18:3 | 2.294679 |
| Total diunsaturated | C18:2 | 65.99681 |
| Total Triunsaturated | C18:3 | 2.294679 |
| Total polyunsaturated | C18:2 | 68.291493 |
| Total monounsaturated | C18:1 | 20.225276 |
| Total unsaturated | C18:1, C18:2, C18:3 | 88.516769 |
| Total saturated | C16:0, C18:0 | 11.483230 |
| Ground total | C16:0 to C18:3 | 100.00000 |
| Number of fatty acids | C16:0, C18:0, C18:1, C18:2 and C18:3 | 5 |
Table 2: Fatty acid composition (wt %) of H. crepitans seed oil.
Conclusion
The study provided sufficient data and useful information on the characterization of H. crepitans seed oil, which is a low-valued and non-edible agricultural product grown in Nigeria. Hence, there is no competition with the food chain. The result from the physico-chemical properties and its fatty acid composition reveals the potential viability of H. crepitans seed oil as biodiesel non-edible feedstock.
References
- Oyeleke GO, Olayiwola OA, Latonia DF. Chemical Examination of Sandbox (Hura crepitans) Seed: Proximate, Elemental and Fatty Acid Profile. IOSR J Appl Chem. 2012;1(2):10-3.
- Oderinde RA, Ajayi IA, Dewuyi AA. Characterization of seed and seed oil of Hura crepitans and the kinetics of degradation of the oil during heating. Electronic J Env Agri Food Chem. 2009; 8(3):201-8.
- Oderinde RA, Tairu AO, Dawodu FA, et al. Chemical Investigation of the Cyperaceae family. Preliminary report on Cyperous tuberous. Riv Italy Delle Sostanze Grasse. 1990;67:301-3.
- Burkill HM. Entry for Adenodolichos paniculatus (Hua) Hutch. & Dalz. [family LEGUMINOSAE-PAPILIONOIDEAE]. In: The useful plants of west tropical Africa. Vol 3, London: Royal Botanic Gardens, UK; 1985.
- Swaine MD, Beer T. Explosive seed dispersal in Hura crepitans L. (Euphorbiaceae). New Phytologist. 1977;78(3):695-708.
- Oyekunle JAO, Omonde AA, Akinnifesi JO. Physical properties of oils extracted from some nigeria non-conventional oilseeds. J Appl Sci. 2007;7(6):835-40.
- Francisco OE. Ornamental trees and other tropical plants. Caracas: Ediciones Armitano, Venezuela; 1969.
- Gordon WF, Herbert GB, Paul AO. Comparative phonological studies of trees in tropical wet and dry forest in the lowlands of Costa Rica. J Ecol. 1974;62:881-919.
- Trestle R. Global Agricultural Supply and Demand: Factors Contributing to the Increase in Food Commodity Prices. US Department of Agriculture, Economic Research Service Report. WRS-0801; 2008.
- Bargale PC, Ford RJ, Sosulski FW, et al. Mechanical oil expression from extruded soybean samples. J Am Oil Chem Soc. 1999;76(2):223-9.
- Nelson AI, Wijeratne WB, Yeh SW, et al. Dry Extrusion as an aid to mechanical expelling of oil from soybeans. J Am Oil Chem Soc. 1987;64:1341-7.
- Bookock DGB. Fast Formation of High-Purity Methyl Esters from Vegetable Oils. J Am Oil Chem Soc. 1998;75(9):1167-72.
- Bodger D. An Investigation of the Extraction, Refining and Composition of Oil from Winged Bean (Psophocarpus Tetragonolobus). J Am Oil Chem Soc. 1982;59(12):523-30.
- Wiemer HJ, Altes FWK. Small Scale Processing of Oil Fruits and Oilseeds. Eschborn, Germany; 1989.
- Otoikhian SK, Aluyor EO, Audu TOK. Synthesis of alkyl ester (biodiesel) from Jatropha curcas seed oil. J Sci Technol Res. 2009;8(1)232-8.
- Association of Official Analytical Chemists (AOAC). Official Method of Analysis. 16th ed. Arlington; 1995. p. 1-45.
- Gerpen Van J, Shanks B, Pruszko R, et al. Biodiesel Analytical Methods. National Renewable Energy Lab. 1617 Cole Boulevard, Golden, Colorado; 2002-2004. p. 1-95.
- Audu TOK. Development of a viable technology for the production of biodiesel from oils of non-edible seeds. PTDF Professional Chair Lecture Series University of Benin Press, Ekehuan Campus, Benin-City, Nigeria; 2012.
- Okolie PN, Uaboi-Egbenni PO, Ajekwene AE. Extraction and Quality Evaluation of Sandbox Tree Seed (Hura crepitans) Oil. World J Agri Sci. 2012;8(4):359-65.
- Igbum OG, Leke L, Ande S, et al. Effects of Transesterification Variables on the Characteristics of the Methyl Esters Obtained from Four Virgin Tropical Seed Oils in Nigeria. Res J Pure Appl Chem. 2012;2(4):230-46.
- Fowomola MA, Akindahunsi AA. Nutritional quality of sandbox tree (Hura crepitans Linn.). J Med Food. 2007;10:159-64.
- Egualeona S. Catalytic Production of Biodiesel from African Star apple (Chrysophyllum albidum) Seeds. Energy Fuels. 2011;14(6):2293-5.
- Alabi KA, Lajide L, Owolabi BJ. Analysis of Fatty Acid Composition of Thevetia Peruviana and Hura crepitans Seed Oil using GC-FID. Fountain J Nat Appl Sci. 2013;2(2):32-7.
- Muhammed NA, Isiaka AA, Adeniyi OA. Chemical composition of Hura crepitans seeds and antimicrobial activities of its oil. Int J Sci Res. 2014;2(3):440-5.