Original Article
, Volume: 13( 1)Isolation and Identification of Iridoid Glucosides from Pedicularis flava Pall. Growing in Mongolia
- *Correspondence:
- Hans-Joachim Knolker, Department of Chemistry, Dresden University of Technology, Bergstr. 66, 01069 Dresden, Germany, Tel: (49)351/463-34659; Fax: (49)351/463-37030; E-mail: hans-joachim.knoelker@tu-dresden.de
Received: June 08, 2017; Accepted: June 21, 2017; Published: June 26, 2017
Citation: Tunsag J, Batsuren D, Ganpurev B, et al. Isolation and Identification of Iridoid Glucosides from Pedicularis flava Pall. Growing in Mongolia. Nat Prod Ind J. 2017;13(1):106.
Abstract
We have isolated and structurally elucidated by spectroscopic methods the monoterpenoid iridoid glucosides mussaenoside and euphroside from the aerial parts of the Mongolian medicinal plant Pedicularis flava Pall. Euphroside and mussaenoside have been isolated for the first time from this natural source.
Keywords
Pedicularis flava; Iridoid; Euphroside; Mussaenoside; Monoterpenoid glucoside
Introduction
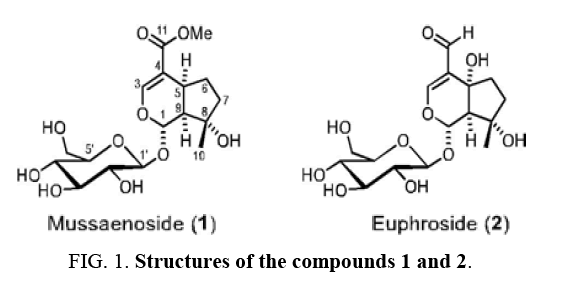
Mongolian medicinal plants still offer a great potential for the discovery of new drugs because most of them have not been studied in detail [1,2]. Only a few investigations focused on Pedicularis flava Pall. [3]. The flowers and leaves of this plant have been used for the treatment of joint inflammation and poisoning. Moreover, Pedicularis flava Pall. has been applied to wound healing. We have isolated the two monoterpenoid iridoid glucosides 1 and 2 ( Figure 1) for the first time from Pedicularis flava Pall. and assigned their structures.
Isolation
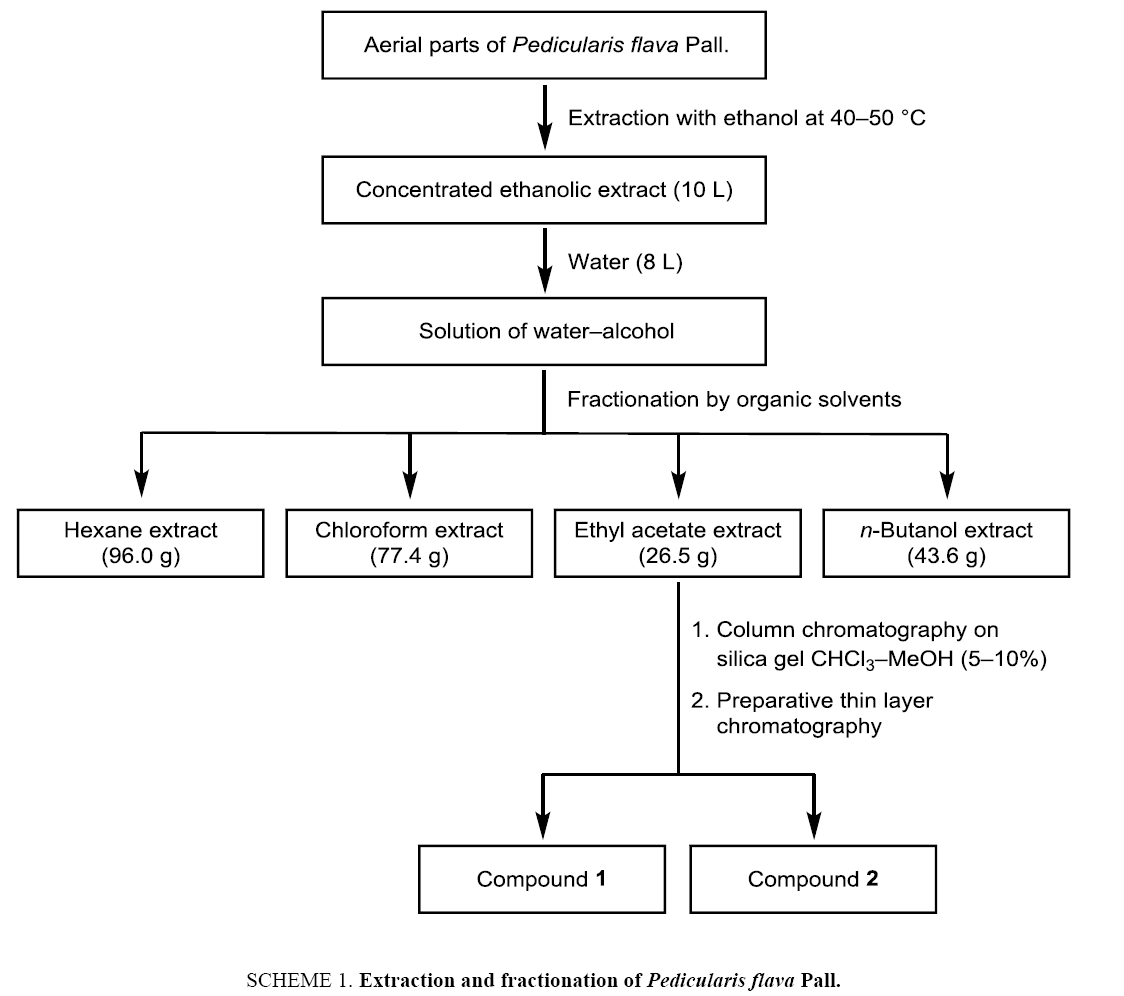
The aerial parts of Pedicularis flava Pall. (Orobanchaceae) have been collected in Sergelen soum Tuv aimag, Mongolia, during the plant flowering period (August). The botanical assignment was made by Dr. Ch. Sanchir (Institute of Botany, Mongolian Academy of Sciences). The dried and grinded aerial parts of Pedicularis flava (12 kg) were extracted with ethanol (72 L) at 40°C to 50°C for 2 h ( Scheme 1). The alcoholic extract was filtered and concentrated using an evaporator at reduced pressure to give 10 L of a concentrated extract. Distilled water (8 L) was poured to the alcoholic extract to give a water–ethanol solution which was fractionated by extraction with solvents of increasing polarity: hexane, chloroform, ethyl acetate, n-butanol. The extracts were dried using Na2SO4. Evaporation of the solvents provided the hexane (96.0 g), chloroform (77.4 g), ethyl acetate (26.5 g) and butanolic extracts (43.6 g). Purification of the ethyl acetate extract by column chromatography on silica gel using chloroform–methanol (5% to 10%) as eluent and subsequent preparative thin layer chromatography afforded the compounds 1 and 2.
Results and Discussion
Compound 1 (Mussaenoside)
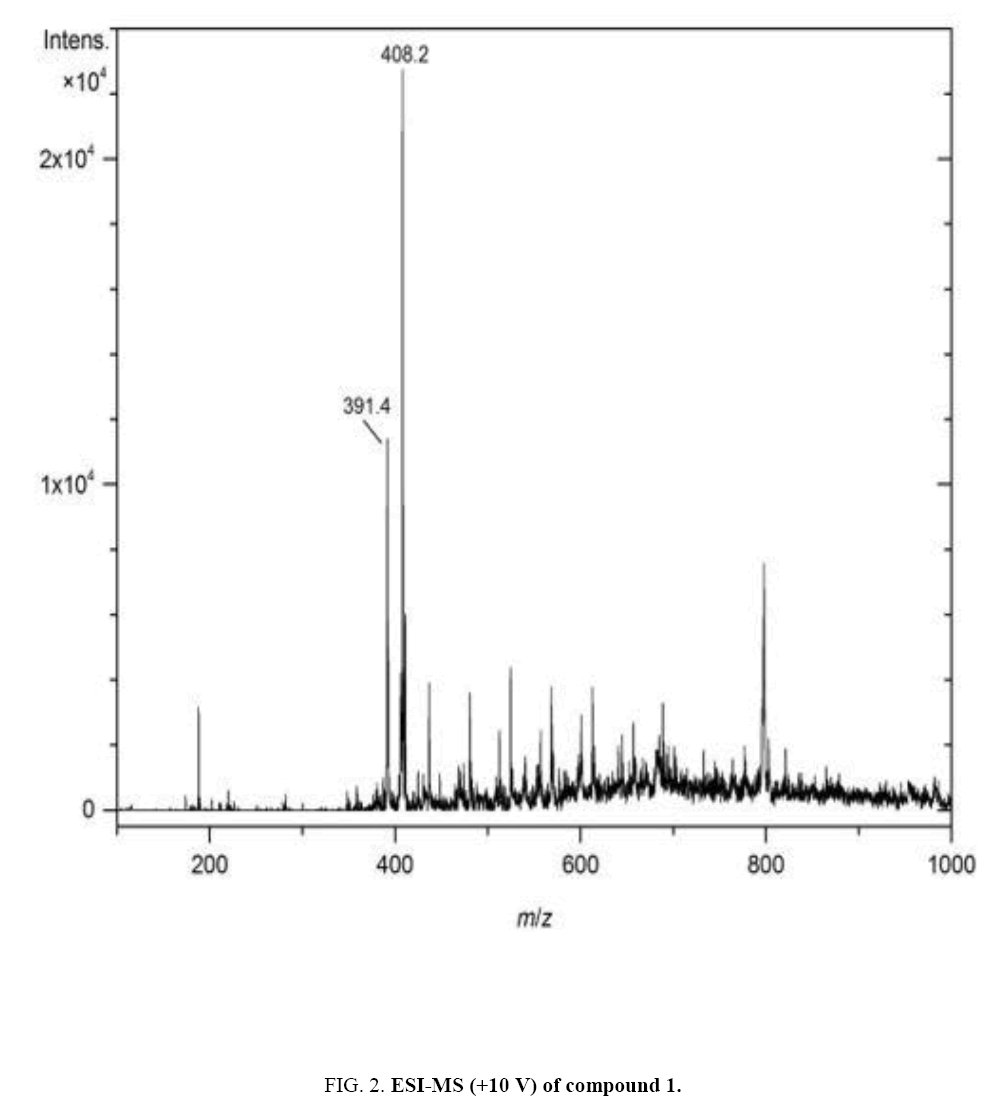
Compound 1 (mussaenoside) was obtained as an amorphous white powder. For the NMR spectra (500 MHz-1H, 125 MHz-13C and DEPT, and HSQC), CD3OD was used as solvent. On the basis of ESI-MS (m/z = 391.4 [M+H]+ and 408.2 [M+NH4]+) ( Figure 2) and 13C NMR spectra, compound 1 was assigned the molecular formula C17H26O10. The 1H and 13C NMR spectra confirmed that compound 1 is an iridoid monoterpenoid glucoside which by comparison with literature data [4–12] was found to be identical with mussaenoside (Table 1).
| Compound 1 (500 MHz) |
Mussaenoside (400 MHz) |
Compound 1 (125 MHz) |
Mussaenoside (100 MHz) |
|||
|---|---|---|---|---|---|---|
| H | δ (ppm), J (Hz) | C | δ (ppm) | |||
| Aglucone | Aglucone | |||||
| 1 | 5.53 (1H, d, J = 4.2) | 5.45 (1H, d, J = 4.2) | 1 | 95.36 CH | 95.4 CH | |
| 3 | 7.47 (1H, d, J = 1.0) | 7.39 (1H, d, J = 1.0) | 3 | 152.2 CH | 152.1 CH | |
| 4 | - | - | 4 | 113.40 C | 113.4 C | |
| 5 | 2.36 (1H, m ) | 2.28 (1H, m ) | 5 | 32.03 CH |
32.1 CH | |
| 6 | 1.5 (2H, m) | 1.43 (2H, m) | 6 | 30.74 CH2 |
30.8 CH2 |
|
| 7 | 1.79 (2H, m) | 1.7 (2H, m) | 7 | 40.7 CH2 |
40.8 CH2 |
|
| 8 | - | - | 8 | 80.52 C |
80.5 C |
|
| 9 | 2.29(1H, dd, J = 4.2, 9.2) | 2.22(1H, dd, J = 4.2, 9.2) | 9 | 52.31 CH |
52.4 CH |
|
| 10 | 1.38(3H, s) | 1.32 (3H, s) |
10 | 24.63 CH3 |
24.7 CH3 |
|
| 11 | - | - | 11 | 169.40 C=O | 169.4 C=O | |
| OMe | 3.76(3H, s) | 3.69(3H, s) | OMe | 51.65 CH3 | 51.7 CH3 | |
| Glucosyl | Glucosyl | |||||
| 1′ | 4.74 (1H, d, J = 7.9) | 4.67 (1H, d, J = 7.9) | 1′ | 99.81 CH | 99.9 CH | |
| 2′ | 3.27–3.45 (1H, m) | 3.18(1H, dd, J = 7.9, 9.2) | 2′ | 74.74 CH | 74.8 CH | |
| 3′ | 3.27–3.45 (1H, m) | 3.24(1H, dd, J = 8.9, 9.2) | 3′ | 78.42 CH | 78.4 CH | |
| 4′ | 3.27–3.45 (1H, m) | 3.36(1H, t, J = 9.2) | 4′ | 71.72 CH | 71.8 CH | |
| 5′ | 3.27–3.45 (1H, m) | 3.18(1H, m) | 5′ | 78.01 CH | 78.1 CH | |
| 6′ | 3.71(1H, dd, J = 6.4, 12.0);3.97(1H, dd, J = 2.0, 12.0) | 3.64 (1H, dd, J = 6.1,11.9); 3.90 (1H, dd, J =2.2, 11.9) | 6′ | 62.93 CH2 | 63.0 CH2 | |
Table 1: 1H and 13C NMR data (CD3OD) of compound 1 and comparison with mussaenoside.
Compound 2 (Euphroside)
For the NMR spectra (500 MHz-1H, 125 MHz-13C NMR and DEPT, COSY, and HSQC), CD3OD was used as solvent. A comparison of the 1H and 13C NMR data of compound 2 with those reported for euphroside in the literature [9–15] confirmed that both compounds are identical (Table 2).
| Compound 2 (500 MHz) | Euphroside (500 MHz) | Compound 2 (125 MHz) | Euphroside (125 MHz) | ||
|---|---|---|---|---|---|
| H | δ (ppm), J (Hz) | C | δ (ppm) | ||
| Aglucone | Aglucone | ||||
| 1 | 5.97 (1H, s) | 5.85 (1H, br s) | 1 | 95.34 | 95.3 CH |
| 3 | 7.41 (1H, s) | 7.35 (1H, s) | 3 | 163.09 CH | 163.1 CH |
| 4 | - | - | 4 | 126.52 C | 126.4 C |
| 5 | - | - | 5 | 71.24 C | 71.3 C |
| 6 | 2.3 (1H, m); 2.15 (1H, m) | 2.25 (1H, m); 1.90 (1H, m) | 6 | 37.8 CH2 | 37.6 CH2 |
| 7 | 2 (1H, m); 1.57 (1H, m) | 2.1 (1H, m); 1.50 (1H, m) | 7 | 40.33 CH2 | 40.3 CH2 |
| 8 | - | - | 8 | 78.85 C | 78.9 C |
| 9 | 2.5 (1H, s) | 2.45 (br s) | 9 | 61.46 CH | 61.3 CH |
| 10 | 1.2 (3H, s) | 1.2 (3H, s) | 10 | 23.64 CH3 | 23.6 CH3 |
| 11 | 9.3 (1H, s) | 9.25 (1H, s) | 11 | 192.53 CHO | 192.6 C?O |
| Glucosyl | Glucosyl | ||||
| 1′ | 4.69 (1H, d, J = 7.9) | 4.62 (1H, d, J = 7.8) | 1′ | 99.83 CH | 99.8 CH |
| 2′ | 3.26 (1H, dd, J = 8.0, 9.3) | 3.18 (1H, dd, J = 7.8, 9.0) | 2′ | 74.36 CH | 74.2 CH |
| 3′ | 3.43 (1H, m) | 3.38(1H, t, J = 8.5) | 3′ | 78.29 CH | 78.3 CH |
| 4′ | 3.34 (1H, m) | 3.34(1H, t, J = 8.5) | 4′ | 71.65 CH | 71.6 CH |
| 5′ | 3.31 (1?, m) | 3.32(1H, m) | 5′ | 77.43 CH | 77.3 CH |
| 6′ | 3.96 (1H, m); | 3.85(1H, dd, J = 2.0, 11.7); | 6′ | 62.81 CH2 | 62.7 CH2 |
| 3.73 (1H, m) | 3.65(1H, dd, J = 6.1, 11.7) | ||||
Table 2: 1H and 13C NMR data (CD3OD) of compound 2 and comparison with euphroside.
Experimental Section, General
NMR spectra were recorded on a Bruker DRX 500 spectrometer operating at 500 MHz for 1H NMR and 125 MHz for 13C NMR. The chemical shifts δ are given in ppm; the solvent signals were used as reference (1H: δH = 3.35 ppm for residual CD2HOD, 13C: δc = 49.00 ppm). The coupling constants J are given in Hertz. ESI-MS were recorded on a Bruker-Esquire mass spectrometer with an ion trap detector. Positive and negative ions were detected.
Acknowledgements
This work was supported by the DAAD in the frame of a bilateral academic exchange program.
References
- Medicinal Plants in Mongolia, WHO Regional Office for the Western Pacific. 2013;1–235.
- Kletter C, Glasl S, Thalhammer T, et al. Traditional Mongolian Medicine – A potential for drug discovery. Sci Pharm. 2008;79:49-63.
- Gonchig E, Erdenebat S, Togtoo O,et al. Antimicrobial activity of mongolian medicinal plants. Nat Prod Sci. 2008;14 :1-5.
- Takeda Y, Nishimura H, Inouye H. Two new iridoidglucosides from MussaendaparvifloraandMussaendashikokiana. Phytochemistry. 1977;16:1401–04.
- Afifi-Yazar F, Sticher O, Uesato S, et al. Ladroside (=6'-Caffeoyl-mussaenoside), a New IridoidGlucoside from Veronica officinalis L. (Scrophulariaceae) and the elucidation of the absolute configuration at C(8) of Mussaenoside. HelvChimActa. 1981;64:16-24.
- Damtoft S, Hansen SB, Jacobsen B, et al.Iridoidglucosides from Melampyrum. Phytochemistry. 1984;23:2387–89.
- Otsuka H, Watanabe E, Yuasa K, et al. A Verbascosideiridoidglucoside fromPremnaCorymbosa Var. Obtusifolia. Phytochemistry. 1993;32:983–6.
- Jianmin Y, Shaonong C, Shengping Y, et al. Chemical components from Craniotomefurcata. Acta Bot Sin. 2001;43:1199–1201.
- Berg T, Damtoft S, Jensen SR, et al. IridoidGlucosides from Pedicularis. Phytochemistry. 1985;24:491-3.
- Akdemir Z, Çalis I, Junior P. Iridoids and phenylpropanoid Glycosides from Pedicularisnordmannia. Planta Med. 1991;57:584-5.
- Yuan CS, Zhang Q, Xie WD, et al. Iridoids from Pediculariskansuensisformaalbiflora. Pharmazie. 2003;58:428-30.
- Chu HN, Tan NH, Zhang YM. Chemical constituents from Pedicularisrex C. B. Clarke. Z. Naturforsch. 2007;62:1465–70.
- Sticher O, Salama O. Euphroside, A new iridoidglucosidefrom EuphrasiasalisburgensisHoppe. Helv. Chim. Acta 1981;64:78-81.
- Damtoft S, Rosendal S, Nielsen BJ. 13C and 1H NMR Spectroscopy as a tool in the configurational analysis of iridoidglucosides. Phytochemistry. 1981;20:2717-32.
- Ersöz T, Berkman MZ, Tasdemir D, et al. Iridoid and phenylethanoidglycosides from Euphrasiapectinata. Turk J Chem. 2002;26:179-88.