Research
, Volume: 9( 1)Enhanced Direct Oxidation of Diclofenac (DCF) at a Carbon Paste Electrode (CPE) Modified with Cellulose and its Biodegradability by Scedosporium dehoogii
- *Correspondence:
- Maxime Pontie University of Angers, GEIHP EA 3142, Institute of Biology in Health PBH, IRIS, CHU, 4 Rue Larrey, 49933 Angers Cedex 01, France, Tel: 0244688361; E-mail: maxime.pontie@univ-angers.fr
Received: February 08,2018; Accepted: March 01,2018; Published: March 05, 2018
Citation: Pontie M, Bouchara JP, Mbokou SF, et al. Acetaminophen molecular imprinted p-phenylenediamine glassy carbon
electrode for pharmaceutical tablets control. Res Rev Electrochem. 2018;9(1):112.
Abstract
A novel carbon paste electrode modified with cellulose fibers and dedicated to diclofenac electroanalysis was prepared, optimized, and used for the determination of the kinetic parameters of DCF biodegradation by a filamentous fungus. The electrochemical response of the modified CPE was compared to that of the unmodified. This study conducted by cyclic voltammetry and linear sweep voltammetry allowed the optimization of the cellulose fibers modified CPE in terms of absence/presence of cellulose fibers, accumulation time (250 s), and initial potential (- 0.4 V/Ag/AgCl). Interestingly, in these conditions, the limit of detection observed through linear sweet voltammetry was found to be as low as 0.020 µmol L-1. This electrode was then used to follow the degradation of DCF. Our results demonstrated that among species belonging to the Scedosporium genus, S. dehoogii displayed the best assets in our process in terms of growth temperature and ability to metabolize DCF. More precisely, DCF biodegradation using S. dehoogii in the process revealed a kinetic of order of 1, a kinetic constant k of 0.012 day-1 and a half time of 57.8 days for an initial concentration of DCF of 1.65 ± 0.05 mg L-1 and at a temperature of 25°C. This study constitutes a solid proof of concept for future developments of fungal wastewater treatments for bioremediation of DCF which is refractory to standard bacterial-based bioprocesses.
Keywords
MIP-GCE; P-Phenylenediamine; Acetaminophen; Tablets; CV/SWV
Introduction
Acetaminophen (paracetamol, N-acetyl-4-aminophenol), a common analgesic and anti-inflammatory drug is used for humans and animals, it has been reported as the pharmaceutical compound present in largest concentration in French rivers [1] and it is known to be one of the most extensively employed drugs in the world. Considering the hazardous impacts of this molecule and its metabolites in the environment [1,2] the elaboration of sensitive and reliable methodologies for its control and removal from discharged effluents is required. The large scale therapeutic use of this drug generated the need for the development of fast, simple and accurate methods for its determination in wastewater treatment plants. A variety of techniques have been used for determining acetaminophen in various media, as recently reported [3-14] and also in pharmaceuticals preparations and human plasma [13,14]. Electrochemical analysis is an excellent technique for the sensitive determination of drugs and related compounds in pharmaceuticals samples and biological fluids. Nowadays, the popularity of electrochemical techniques in the field of drug analysis is due to their simplicity, high sensitivity, low cost and relatively short analysis time [14]. Electrochemical techniques have been widely explored for acetaminophen sensing due to its electroactive nature as reported elsewhere on GCE [3,12-16] where voltammetry mechanistic studies for the electrode processes of the N-acetyl-p-benzoquinone imine / acetaminophen redox system has been intensively presented.
Recently, molecular imprinting polymers (MIPs) as a recognition element for sensors, have attracted considerable attention [15,17-21]. Molecular imprinting is one of the most powerful tools for preparing materials that can bind analytes reversibly and selectively in the presence of their interferents [15,18,19]. MIPs are synthetic polymeric materials with specific recognition sites complementary in shape, size and functional groups to the template molecule involving an interaction mechanism based on molecular recognition. Molecular imprinting involves positioning functional monomers around the template molecules by non-covalent interaction or reversible covalent interaction followed by polymerization and the template removal. The major additional advantages of electro polymerized modification designing MIP sensors surfaces is its ability to coat very small or irregularly shaped surfaces [4, 21-24]. It’s a very simple modification, very easy to handle and with intrinsic robustness, low cost and long lifetime depending on media complexity. Today it remains a great challenge to develop a suitable technique following MIP methodology with monomers electro polymerization with an excellent specific recognition and simple detection, to rapidly detect paracetamol, as also recently reported for the following molecules: p-nitrophenol [25], oleanolic acid [26], dopamine [19], streptomycin [27] and very recently reported for an oral antidiabetic drug dedicated to the treatment of type 2 diabetes, the metformin [15].
The first non-electropolymerized MIP dedicated to paracetamol was reported by Tan et al. [28] which was based on a bio-mimetic recognition material for a bulk acoustic wave sensor in the human serum and urine. The first electropolymerized MIP dedicated to paracetamol was a carbon fiber microelectrode modified by o-phenylenediamine (OPDA) + aniline electro-copolymerization, as reported by Gomes-Caballero et al. [29]. The detection limit was 212 μg/L (1.5 μM) determined with SWV. The second MIP paracetamol sensor was reported by Ozcan et al. [4] based on polypyrrole modified pencil graphite electrode, the authors observed a LOD for DPV at 119 μg/L (790 nmol/L) in a linear range of 5μmol/L to 4.5 mmol/L. Recently Wang et al. [20] reported a MIP based on photosensitive polymers micelles electrodeposited on GCE at +2 V after few complex steps of elaboration and dedicated to acetaminophen determination in commercial tablets. The LOD obtained was 150 μg/L with a wide linear range attained in DPV from 0.1 mmol/L to 8 mmol/L. Very recently Peng et al [21] reported an imprinted MIP based on a o-phenylenediamine film electropolymerized on a multi-walled carbon nanotube modified GCE by linear sweep voltammetry (LSV) with a linear concentration range response between 200 nmol./L to 40 μmol./L and a LOD of 50 nmol./L (30 μg/L), as resumed in the TABLE 1.
| Electrode | Modifier | Limit of detection (μM) | Sensitivity (μA mM-1 cm-2) |
Linear range (mM) |
Detection method |
Ref. |
|---|---|---|---|---|---|---|
| PGE | polypyrrole | 0.79 | - | 0.005 –0.50 1.25–4.5 |
DPV | [4] |
| GCE | photosensitive polymers micelles | 1.0 | - | 0.01 – 8.0 | DPV | [20] |
| GCE/MWCNT | o-phenylenediamine | 0.5 | - | 2×10-4 – 4×10-2 | LSV | [21] |
| CFME | o-phenylenediamine and aniline | 1.5 | 180 | 6.5×10−3 – 2 | SWV | [29] |
| GCE | p-phenylenediamine | 0.21 | - | 6.6×10−4 – 3.3 | CV + SWV | This work |
TABLE 1. Comparison of the efficiency of different electrochemical MIP sensors used in the analysis of acetaminophen.
The aim of the present paper was to elaborate a MIP based on electropolymerization of p-phenylenediamine (PPDA) in a very simple way, for the direct quantification of acetaminophen under low cost analysis, with a high selectivity to interferents and its validation to real samples.
In order to achieve our goals, this study was conducted by designing a very simple experimental procedure of MIP elaboration with electropolymerization of PPDA in a phosphate buffer solution (PBS) at a pH=7.4 and a ionic strength of 0.1 mol/L
i) engaging SWV as a very sensitive tool to prove acetaminophen presence in the MIP
ii) using CV in facilitating a total removal of acetaminophen to achieve MIP elaboration
iii) comparing NIP, MIP and unmodified GCE performances, their wide linear range of concentration and LOD proving MIP selectivity vs phenol interferents molecules
iv) determining acetaminophen in commercialized tablets
Experiments
Apparatus and glassy carbon electrodes (GCE) preparation
Cyclic voltammetry (CV) and square wave voltammetry (SWV) measurements were performed using an electrochemical analyzer PG580 (Uniscan Instruments, UK) connected to a personal computer. The electrochemical software used was UiEchem version 3.27 purchased from Uniscan Instruments (UK). A three-electrode configuration was employed, consisting of a glassy carbon electrode (GCE) (area=0.0706 cm2, Bioanalytical, UK) serving as a working electrode, a saturated calomel reference electrode (SCE) and a platinum wire counter electrode.
Electrochemical experiments were carried out in a 100 mL glass voltammetry cell at room temperature. The GCE surface was renewed by a homemade pre-treatment in three following steps: first step was a polishing operation during 15 min, with Al2O3 particles average size of 1μm following by abundant rinsing in ultrapure water and exposed to ultrawave (48 kHz) during 5 min. Second step was a polishing operation during 15 min, with Al2O3 particles average size 0.05 μm following by 5 min of immersion in ultrapure water under ultrawave (48 kHz). Third step is an electrochemical pre-treatment in phosphate buffer solution (pH 7.4, ionic strength 0.1 mol/L) during 10 cycles of CV at a potential scanning rate of 100 mV/s in a potential range between 0 2V/SCE and +1.2V/SCE.
Reagents
Acetaminophen was purchased from Aldrich as a powder with analytical grade. A 0.1 mol/L PBS (pH 7.4) was used as the supporting electrolyte (salts used for PBS elaboration are Na2HPO4 and NaH2PO4 purchased by Labosi (OSI, France) with analytical grade and used as received). Acetaminophen solutions were prepared in a concentration range from 0.0001 mg/L to 500 mg/L from a concentrated solution of 5 g/L in PBS. Deionized water was obtained from a Elga Labwater ultrapure-water system (Purelab-UV-UF, Elga, France) (pH 6.5, conductivity < 1 μS/cm and TOC < 0.1mg/L). Paraphenylenediamine (PPDA) monomer (FIG. 1) was purchased from Sigma (batch n°115K1318) with analytical grade and used as received. Dopamine (3-hydroxytyramine), dopac (3,4-dihydroxyphenylacetic acid) and PAP (4-aminophenol) were used as purchased from Aldrich as powders of analytical grades. Doliprane®500 tablets were purchased by Sanofi Aventis (France) delivered under the batch n°5768 and elaborated in February 2014.
Procedures
The GCE was immersed into PBS containing the desired concentration of acetaminophen in a 100 ml electrochemical cell. SWV parameters were optimized (results not shown) for acetaminophen determination, in a similar approach, as previously described [30]. We obtained the following optimized parameters: pulse height 50 mV, frequency 50 Hz and scan increment 10 mV.
The pre-treated GCE was coated by a film of poly-PPDA electrodeposited. The electrochemical deposition of poly-PPDA was achieved in 2 mmol/L PPDA deaerated PBS solution by 10 repetitive potential scans between 0.0 and +0.8 V/SCE (potential scan rate: 100 mV/s) and return to 0.0 V/SCE, a well-established deposition process, as previously reported [22-24,31,32]. The same procedure was followed for MIP elaboration in presence of 2 mmol/L PPDA + 2 mmol/L of acetaminophen. But during MIP/NIP elaboration we have voluntary limited the upper potential to +0.8 V/SCE in order to limit the risk of dimers and/or polyphenols formation on GCE, as reported recently [33,34] and also to limit formation of very reactive species like OH° or intermediates appearing at a higher oxidation potential. For template extraction, CV was applied by scanning 300 times between 0 V/SCE to +0.8 V/SCE and return to 0.0 V/SCE, under a potential scanning rate of 100 mV/s in PBS. The complete template removal from electrode was ensured till no SWV response was noticed. The LOD was calculated as three times the standard deviation from the blank measurement (in the absence of acetaminophen) divided by the slope of calibration plot between acetaminophen concentration and SWV current.
The purchased commercial tablets were examined for estimation of paracetamol. The tablets were finely powdered and dissolved in PBS with 1 tablet for 1 liter of PBS. This solution was directly analysed inside the electrochemical cell with MP GCE. Similar analyzed were realized for 3 tablets and the RSD calculated. The samples were then spiked with known appropriate amount of paracetamol for experiments All SWV runs for each concentration of test analyte were quantified using the method of standard addition.
Field Emission Gun Scanning Electron Microscopy (FEGSEM) observations
Unmodified pre-treated and modified NIP/MIP with template and MIP GCEs were observed by FEGSEM analysis for a morphological study of their surface. The apparatus used was a JEOL type JSM-6301F (SCIAM, Angers University, France). Images obtained were from secondary electrons under 3 keV-5 keV with magnifications situated between 25 to 20,000. GCEs were rinsed with ultrapure water after electrochemical use, dried in a dessicator chamber for one night and introduced in the FEGSEM chamber for analysis without further treatment.
Results
NIP/MIP elaboration
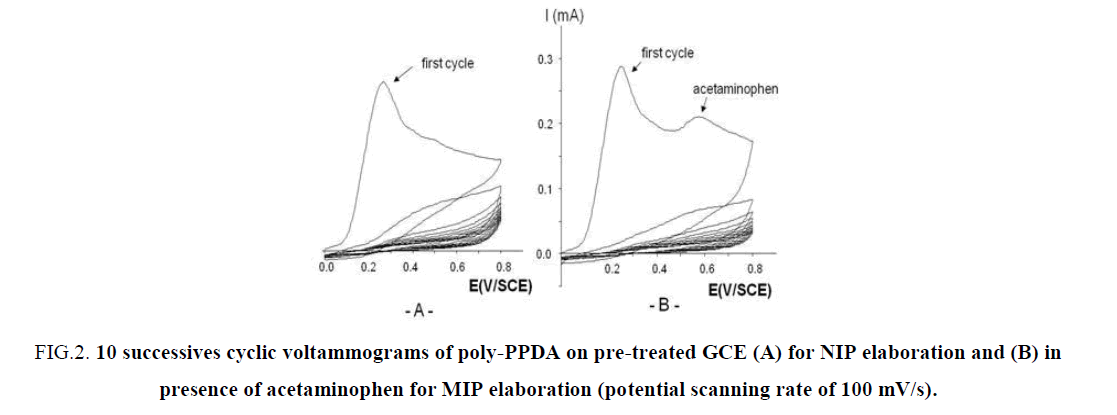
A typical example of the cyclic voltammograms deposition of a poly-PPDA coating is shown in FIG. 2. During 10 cycles of electropolymerization, respectively without template (FIG. 2A) and with template (FIG. 2B). FIG. 2 show a rapid decrease in the current intensity at 0.3 V/SCE corresponding to an ultrathin film growth of poly-PPDA. As detailed by Lakard et al. [32] the first step mechanism of deposition is the adsorption of the monomer PPDA on the electrode surface. Then this monomer is oxidized with the loss of an electron and the formation of a cation radical. This step is followed by the cleavage of the C-N bond with formation of a primary carbocation, which attacks another molecule of PPDA. After the expulsion of the proton from the protonated amine, an additional loss of an electron and C-N bond cleavage take place. Therefore poly-PPDA, (C6H4-NH)n, grows progressively on the electrode surface. The question stay during the electropolymerisation in presence of the template if the structure of the template is intact imprinted in the MIP membrane. The way to return to 0.0V/SCE at the end of the electropolymerisation help us to return to the initial redox state of the template due to the fact that the oxidation of acetaminophen in NAPQI is a reversible process, as reported recently [14].
FIG 2: 10 successives cyclic voltammograms of poly-PPDA on pre-treated GCE (A) for NIP elaboration and (B) in presence of acetaminophen for MIP elaboration (potential scanning rate of 100 mV/s).
As reported elsewhere for the poly-o-phenylenediamine) (poly-OPDA) coating [20], the mass variations measured by a quartz crystal microbalance (QCM), obtained during 500 s at a fixed potential of +0.9 V/SCE have shown a regular increase of the mass uptake during the first 120 s, followed by a slight stabilization. Those experiments allowed to estimate a surface coverage of 9 μg/cm2. More recently Cot et al [24] reported for 10 scans at a potential scan rate of 100 mV/s a thickness deposited on silicate substrate of 5.6 μm. These two observations may be explained by the fact that the insulating poly-PPDA or poly-OPDA films are generally known to be uniform, very compact and adherent to the electrode surface and growth thick enough until the surface of the electrode is completely covered. Then the current decreased to a minimum, because the monomers cannot penetrate the film anymore. But in presence of acetaminophen the N-H groups of the poly-PPDA interact with the –OH and –NH groups of the acetaminophen for the imprinted sites with which the template molecules are expected to bind with high affinity and selectivity through hydrogen bonding and π-π interactions as recently described for paranitrophenol (PNP) molecule with chitosan/phenyltrimethoxysilane material MIP coating [25].
Finalization of MIP film elaboration on GCE
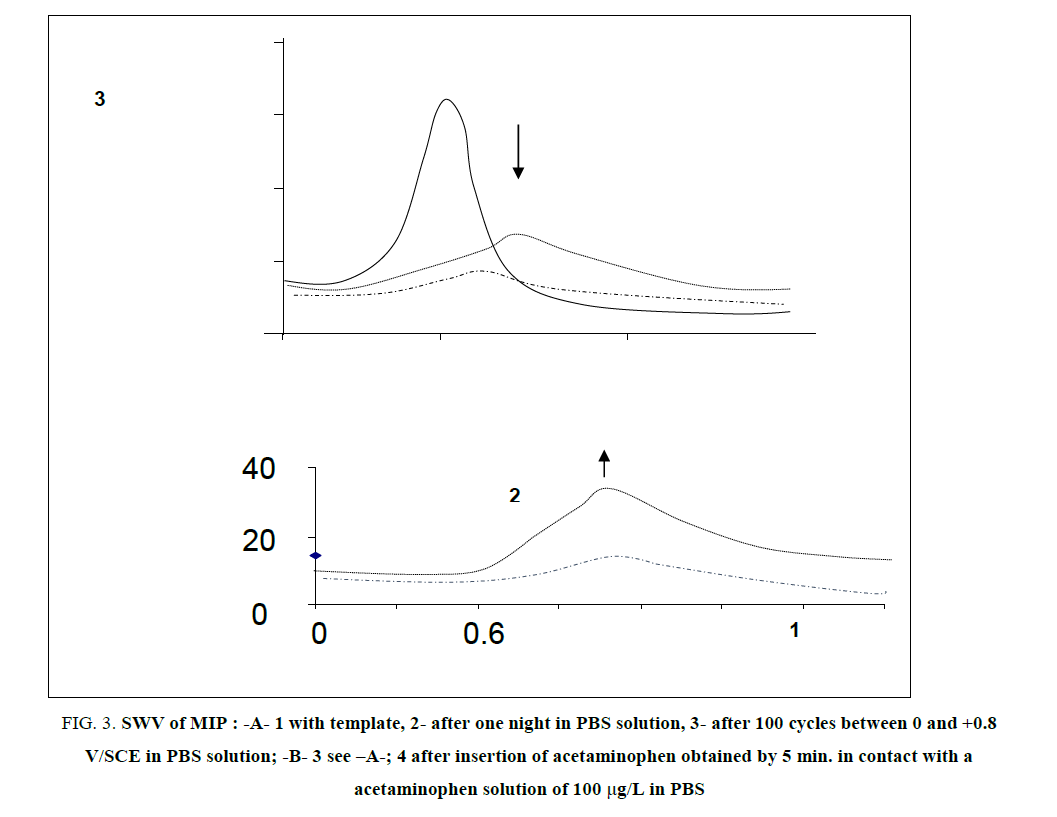
FIG. 3 resumed the experimental procedure elaborated for the complete removal of acetaminophen in MIP coating deposited on GCE. We tested 2 different ways, as illustrated on FIG. 3-A: (i) one night immersion in PBS and (ii) 100 cycles between 0 V/SCE to 0.8 V/SCE in PBS solution with a potential scan rate of 100 mV/s. As illustrated also on the FIG. 3-A, the best way was the second one because CV cycles permitted to attain more rapidly a lower peak intensity than pure diffusion during one night. Other numbers of cycles were tested, 50, 200 and 300 (results not shown) from where we decided to adopt 300 cycles because the oxidation peak of acetaminophen in the film had completely disappeared as lightened by SWV. The number of 300 cycles between 0 V/SCE to +0.8V/SCE at a potential scan rate of 100 mV/s is the procedure adopted for acetaminophen removal in MIP, for the rest of the experiments.
FIG 3: SWV of MIP : -A- 1 with template, 2- after one night in PBS solution, 3- after 100 cycles between 0 and +0.8 V/SCE in PBS solution; -B- 3 see –A-; 4 after insertion of acetaminophen obtained by 5 min. in contact with a acetaminophen solution of 100 μg/L in PBS
Furthermore, we tested the possibility of acetaminophen insertion again inside MIP and we succeeded after only 5 min (FIG. 3-B, curve 4) in contact to a acetaminophen PBS at a concentration of 100μg/L. Indeed, we observed that the gain in current was around 150%. Then, electrochemical removal and sorption, successively applied, was a good procedure to help the poly-PPDA coating to acquire the practice of acetaminophen exchange between MIP and the solution to be analysed.
FEGSEM 2D-Images of the Unmodified and Modified GCE
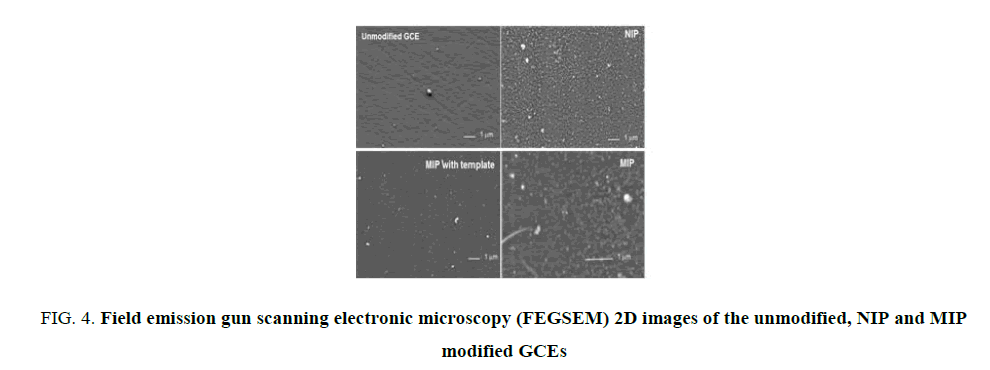
As observed on FIG. 4 the morphology of the GCE after polymers deposition is well distinct to the polished unmodified GCE. The presence of an homogeneous film of poly-PDDA show a surface covered by agregates (whites holes, see MIP image). Also the initial striations due to the polishing process on the bare unmodified GCE have completely disappeared. Furthermore, MIP with template surface is different to NIP. NIP surface appeared more dense. Furthermore, the final MIP appeared quite different from NIP and MIP with template. The elimination of the template changed the morphology of the modified GCE surface with the apparition of a favorable environment verry well designed to acetaminophen molecule, as described very clearly elsewhere for the p-nitrophenol [25].
FIG 4: Field emission gun scanning electronic microscopy (FEGSEM) 2D images of the unmodified, NIP and MIP modified GCEs
SWV for Template Insertion Illustration in the MIP
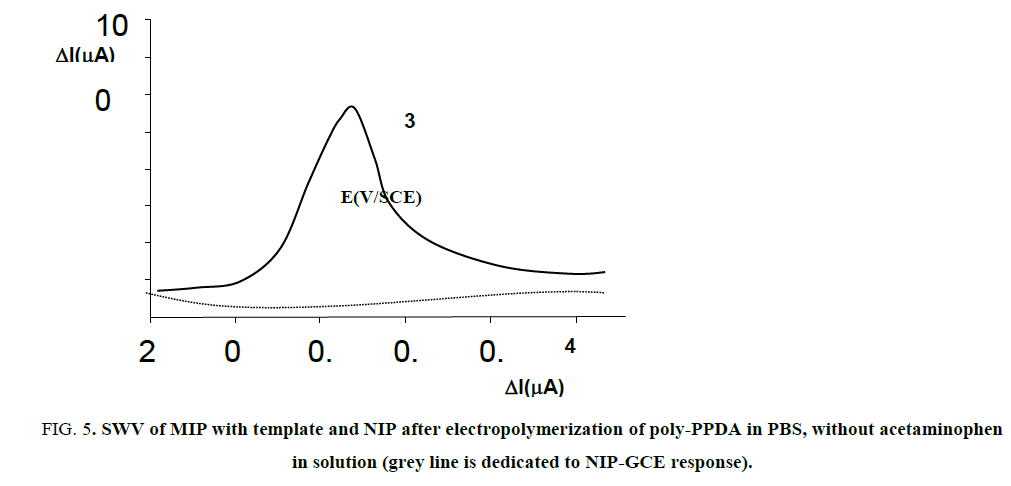
We tried few times to exhibit the presence of acetaminophen in the poly-PPDA film using CV technic, but very poor signal was observed, under the noise, very limited by the low sensitivity of this electrochemical method. Using a more sensitive technique like SWV was helpful to obtain a very well peak at a potential of +0.32 V/SCE, as illustrated on FIG. 5 and similarly reported by very recent works using DPV with PNP reduction [25] and metformin reduction [15]. On the other side, as attempted, no signal was observed for the NIP coating, as also illustrated on FIG. 5.
FIG 5: SWV of MIP with template and NIP after electropolymerization of poly-PPDA in PBS, without acetaminophen in solution (grey line is dedicated to NIP-GCE response).
We determined the calibration curves for acetaminophen in PBS for modified and non-modified GCEs, in a concentration range of 0.0001 mg/L to 500 mg/L.
For the MIP we obtained a wide linear range with a slope (see Eq. (1)) and a LOD of 30 μg/L:
Ipeak (+0.52V/SCE) (μA) = 0.06. [acetaminophen] (μg/L) + 2.7 with R2 = 0.97 (1)
For the unmodified GCE we obtained a higher slope and also a lower LOD (10 μg/L) (see Eq. (2):
Ipeak (+0.42V/SCE) (μA) = 0.20. [acetaminophen] (μg/L) + 0.8 with R2 = 0.98 (2)
Furthermore, as attempted, no signals were observed for the NIP in all range of acetaminophen concentration tested.
Our MIP presents a lower LOD in comparison to Gomez-Caballero et al. [29] with an electro-copolymerized film of OPDA/aniline modified ultramicroelectrodes with a LOD of 220 μg/L. Our hypothesis to explain such observations is based on hydrophilicity/hydrophobicity considerations of the coatings deposited, as reported elsewhere [33-35]. Indeed the degree of hydrophilicity of the outer coating help us to attain a lower LOD for the acetaminophen MIP as it was not the case for Gomez-Cabellero et al. in their work because aniline used increase the hydrophobicity of the polymer and then decrease its affinity to acetaminophen, a very hydrophilic molecule. Peng et al. [21] reported very recently an imprinted acetaminophen MIP based on a OPDA film electropolymerized on a multi-walled carbon nanotube modified GCE analyzed by linear sweep voltammetry (LSV) and they observed a very low LOD at 30 μg/L, very similar to the LOD reported in the present work, due to both hydrophilicity of OPDA and PPDA films which is favourable to a better sensitivity of the sensors developed.
The LOD observed for the unmodified GCE was lower than the MIP-GCE elaborated, we attributed this observation to the fact that the poly-PDDA film present also the properties of a membrane, with nanopores and the mass transfer is based on a sieving exclusion as on the pre-treated GCE no sieving exclusion occurred. MIP coating is a way to increase electrodes selectivity/specificity as the unmodified electrodes are not selective.
MIP selectivity to interferents
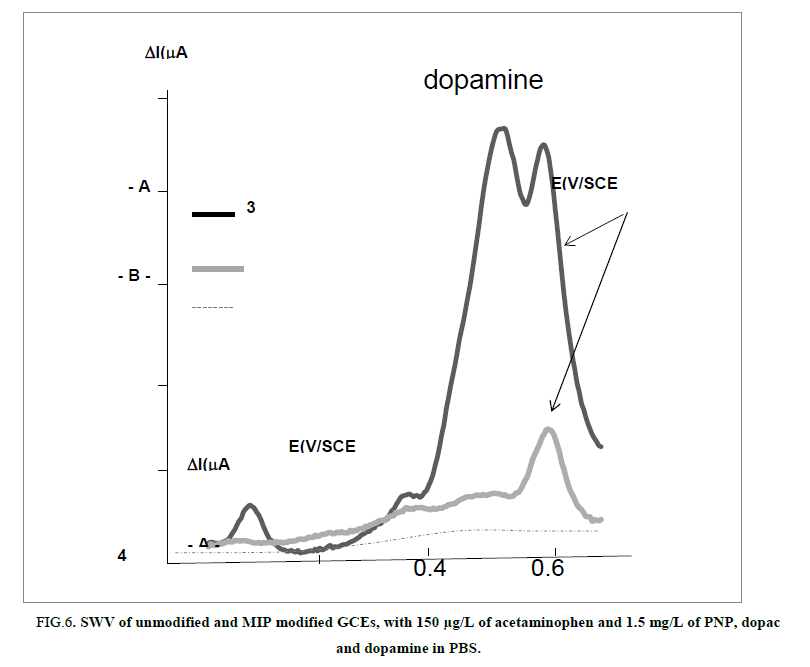
A series of different organic phenolic compounds were tested from their molecular weight near acetaminophen in order to examine the selectivity of the designed MIP-GCE. The response of paracetamol imprinted poly-PPDA film was researched using a solution containing the analyte and the interferents’ molecules in a concentration ratio of 1:10 respectively as reported on the FIG. 6.
FIG 6: SWV of unmodified and MIP modified GCEs, with 150 μg/L of acetaminophen and 1.5 mg/L of PNP, dopac and dopamine in PBS.
PNP, dopac and dopamine phenolic interferents’ molecules are well observed on the unmodified GCE respectively at -0.8 V/SCE, +0.1 V/SCE and +0.35 V/SCE as acetaminophen is visible at +0.52 V/SCE.
On the MIP-GCE we observed a higher peak intensity for the acetaminophen (with a 4 times decrease in its current intensity as a consequence of the sieving barrier elaborated) as all the others interferents signals decreased dramatically and are not very well observed on the modified MIP-GCE. Furthermore, no peak is observed for NIP (results not shown) as attempted.
In FIG. 6 selectivity between acetaminophen and the interferents is well illustrated in comparison to the unmodified GCE electrode. When comparing the signals of dopamine relatively to the acetaminophen there is an equal peak intensity on the unmodified GCE, but dopamine disappeared drastically on the MIP. We have attributed this observation to two mechanisms of exclusion- a sieving exclusion of dopamine which present the higher MW (189 g/Mol) and chemical exclusion due to the designing of cages formed for acetaminophen and not chemically/physically designed for the interferents. As a consequence MIP has increased acetaminophen partition coefficient between poly-PPDA and PBS solution and not for the interferents.
We can conclude that the recognition sites formed in the polymerized film have the capability to distinguish target molecules through their size and functional group distribution.
Determination of acetaminophen in tablets
To investigate the real world application of the MIP sensor elaborated, it was applied to determine acetaminophen in a pharmaceutical drug, very commonly used by patient and entitled Doliprane®500 (TABLE 2).
| Doliprane®500 | RSD (%) | ||
| Mass tablet weighted after powdered usual acetaminophen mass in a Tablet | 582 ± 2 | 1.5 (n=3) | |
| Acetaminophen mass calculated in the weighted tablet (mg) | 483 ± 1 | ||
| Acetaminophen mass determined with MIP-GCE in the weighted tablet (mg) | 480 ± 10 | 2.5 (n=3) | |
| Acetaminophen calculated in the initial mass tablet and determined with MIP-GCE (mg) | 497 ± 10* | ||
| Recovery range (%) | 97.4-101.4 |
*(3% error admitted by the European drug regulation for a mass value range admitted equal to 500 mg ± 15 mg)
TABLE 2. Real applicability of the proposed MIP for commercial tablets.
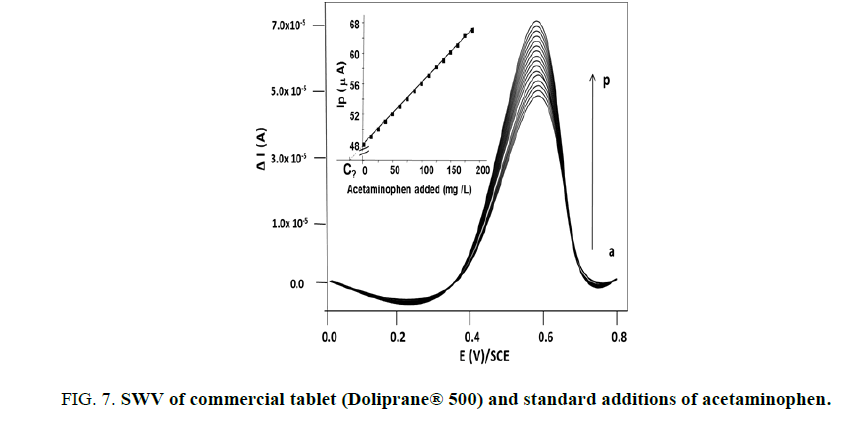
A quality control must be engaged everywhere in the world and our work present a very simple way to validate quality control to commercialized acetaminophen tablets. The common analytical method of internal standards was applied, as illustrated on FIG. 7.
We obtained the following Equation (3) between peak intensity observed at +0.6 V/SCE and the acetaminophen concentrations added:
Ip(μA) = 0.1 [acetaminophen] (in mg/L) + 48 with R2=0.999 (3)
The results obtained were in the range of the commercial limit of quality admitted by the European regulation (3%) (see Table 1) and we obtained recoveries between 97.4% and 101.4%, indicating the accuracy and repeatability of the proposed method in real sample.
Furthermore, comparison between a standard solution of acetaminophen with tablet solution following SWV signal was found in the studied potential window near the oxidation potential reported for pure solution of acetaminophen indicating that there are no significant interferences from excipient species (inorganic cations, anions and some organic molecules) found in commercialized pharmaceutical tablets.
Conclusion
The present study succeeded in designing a rapid and simple experimental procedure for the fabrication of a molecular imprinting polymer based on electrochemical deposition of PPDA via a one-step electrodeposition of constitutive components, triggered by applying an optimal electrode potential range between 0.0 V/SCE to +0.8V/SCE.
SWV was used with efficacy in three ways: (i) to follow acetaminophen direct oxidation located at +0.52V/SCE, (ii) to prove the existence of acetaminophen in the poly-PDDA electrodeposited film and (iii) to check acetaminophen total removal attained after 300 cycling occurred in CV.
Furthermore, a linear evolution between the response currents at Ep=+0.52V/SCE and acetaminophen concentration ranging from 0.0001 to 500 mg/L was determined using SWV for MIP poly-PPDA sensor with a limit of detection of 30 μg/L (or 200 nmol/L) at a S/N ratio of 3. We obtained also an excellent selectivity of the MIP for the paracetamol vs dopamine, 4-aminophenol and dopac interferents molecules. Finally recovery values obtained and situated between 97.4 to 101.4% indicated the accuracy and repeatability of the proposed method in real sample of commercial tablets.
Further experiments have to be conducted to study the morphology change after the test particularly focalizing on the accumulation of electrolysis acetaminophen oxidation by-products in the MIP.
To conclude the present methodology is extendable to others analytes of interest for developing electrochemical sensors, i.e ornithine, with specific determination of various electroactive species, under very low cost analysis. Work is in progress to extend its exploration and evaluate its merit.
Conflict of Interest
No conflict of interest.
Acknowledgements
Lots of thanks to Romain MALLET, Microscopy Dept. of Angers University (SCIAM, Angers, France) for FEGSEM images and his patience and inventiveness to make a success the insertion of our GCE into SEM chamber. The authors also thank ARIANES 2014 research program from the university of Angers for a help to Mbokou in its short internship research visit in France (laboratories GEPEA UMR CNRS 6144 and GEIHP EA 3142) from June to September 2014.1
References
- Quesada-Penate I, Julcour-Lebiguea UJ, Jáuregui-Haza, et al. Degradation of paracetamol by catalytic wet air oxidation and sequential adsorption – Catalytic wet air oxidation on activated carbons. J Hazard Mater. 2012;221-222:131-8.
- Chon K, Sarp S, Sungyun Lee, et al. Evaluation of a membrane bioreactor and nanofiltration for municipal wastewater reclamation: Trace contaminant control and fouling mitigation. Desalination. 2011;272(1-3):128-134.
- Tungkananutuk K, Tungkananuruk N, Burns DT, et al. Cyclic voltammetric determination of acetaminophen in paracetamol tablets. KMITL Sci Tech J. 2005;5(3):547-51.
- Ozcan L, Sahin Y. Determination of paracetamol based on electropolymerized-molecularly imprinted polypyrrole modified pencil graphite electrode. Sensors and Actuators B. 2007;127:362-69.
- Babei A, Khalilzadeh B, Afrasiabi M, et al. A new sensor for the simultaneous determination of paracetamol and mefenamic acid in a pharmaceutical preparation and biological samples using copper (II) doped zeolite modified carbon paste electrode. J Appl Electrochem. 2010;40:1537-43.
- Shahrokhian S, Saberi RS. Voltammetric determination of acetaminophen in the presence of codeine and ascorbic acid at layer-by-layer MWCNT/Hydroquinone sulfonic acid-overoxidized polypyrrole modified glassy carbon electrode. Int J Electrochem. 2011;01-10.
- Babei A, Dehdashti A, Afrasiabi M. Development of a method for a sensitive simultaneous determination of dopamine and paracetamol in biological samples and pharmaceuticals preparations. Int J Electrochem. 2011;1-6.
- Ghoreishi SM, Behpour, Faezeh Saeidinejad. Electrochemical determination of acetaminophen in different pharmaceuticals forms with gold nanoparticules carbon paste electrode. Acta Chim Slovenica. 2011;58:69-74.
- Bahrampipur H, Jalali F. Sensitive determination of paracetamol using a grapheme-modified carbon-paste electrode. Afr J Pharmacy Pharmacol.2012;6(17):1298-1305.
- Zidan M, Tee TW, Halim Abdullah A, et al. Electrochemical oxidation of paracetamol mediated by nanoparticles bismuth oxide modified glassy carbon electrode. Int J Electrochem Sci. 2011;6:279-88.
- Li Y, Chen SM. The electrochemical properties of acetaminophen on bare glassy carbon electrode. Int J Electrochem Sci. 2012;7:2175-87.
- Chen TS, Huang KL. Electrochemical detection and degradation of acetaminophen in aqueous solution. Int J Electrochem Sci. 2012;7:6877-92.
- Xiong XQ, Huang KJ, CX Xu, et al. Glassy carbon electrode modified with poly-taurine/TiO2-graphene composite film for determination of acetaminophen and caffeine. Chem Ind Chem Eng. 2013;19(3):359-68.
- Calenda A, Bouchara JP. Microcapteurs électrochimiques pour des mesures durables en Environnement-Santé et Agroalimentaire, Book from C2i conference stated in Lyon (France) in 2013 and published by EDP Science Ed., Serie: Instrumentation Measure, Métrologie by CNRS. 2013;372-80.
- Roy E, Patra S, Madhuri R, et al. Gold nanoparticles mediated designing of non-hydrolytic sol-gel cross-linked metformin imprinted polymer network: A theoretical and experimental study Talanta. 2014;120:198-207.
- Song J, Yang J, Zeng J, et al. Graphite oxide film-modified electrode as an electrochemical sensor for acetaminophen. Sensors and Actuators B. 2011;155(1):220-5.
- Li W, Li S. Molecular imprinting: a versatile tool for separation, sensors and catalysis. Adv Polym Sci. 2007;206:191-210.
- Sharma PS, D’Souza F, W Kutner. Molecular imprinting for selective chemical sensing of hazardous compounds and drugs of abuse. Trends Analyt Chem. 2012;59-77.
- Sharma PS, Dabrowski M, Souza FD, et al. Surface development of molecularly imprinted polymer films to enhance sensing signals. Trends in Analytical Chemistry. 2013;15:146-57.
- Wang X, Luo J, Yi C, et al. Paracetamol sensor based on molecular imprinting by photosensitive polymers. Electroanalysis. 2013;25(8):1907-16.
- Peng Y, Wu Z, Zhigang Liu. An electrochemical sensor for paracetamol based on an electropolymerized molecularly imprinted o-phenylediamine film on a multi-walled carbon nanotube glassy carbon electrode. Anal Methods. 2014;6:5673-81.
- Pontié M, Gobin C, Pauporté T, et al. Electrochemical nitric oxide microsensors: sensitivity and selectivity characterisation. Anal Chim Acta. 2000;41:175-85.
- Tapsoba I, Bourhis S, T Feng, et al. Sensitive and selective electrochemical analysis of methyl-parathion and 4-nitrophenol by a new type p-NiTSPc/p-PPD coated carbon fiber microelectrode (CFME). Electroanalysis. 2009; 21(10):1167-76.
- Cot A, Lakard C, Dejeu J et al. Electrosynthesis and characterization of polymer films on silicon substrates for application in micromanipulation. J Synthetic Metals. 2012;162(24):2370-6.
- Li S, Du D, J Huang, et al. One-step electrodeposition of a molecularly imprinting chitosan/phenyltrimethoxysilane/ AuNPs hybrid film and its application in the selective determination of p-nitrophenol. The Analyst. 2013;138:2761-8.
- Zang Z, Luo L, Chen H, et al. polypyrrole-Imprinted electrochemical sensor based on nano-SnO2/Multiwalled carbon nanotubes film modified carbon electrode for the determination of oleanolic acid. Electroanalysis. 2013; 23(10):2446-55.
- Que X, Liu B, Fu L, et al. Molecular imprint for electrochemical detection of streptomycin residues using enzyme signal amplification. Electroanalysis. 2013;25(2):531-7.
- Tan X Y, Zhou Z, Wang P, et al. A study of a bio-mimetic recognition material for the BAW sensor by molecular imprinting and its appliction for the determination of paracetmol in the human serum and urine. Talanta. 2001;55: 337-47.
- Gomez-Caballero YA, Goicolea MA, Barrio RJ. Paracetamol voltammetric microsensors based on electrocopolymerized-molecularly imprinted film modified carbon fiber microelectrodes. The Analyst.2005;130:1012-8.
- Sbaï M, Essis-Tome H, Gombert U, et al. Electrochemical stripping analysis of methyl-parathion (MPT) using carbon fiber microelectrodes (CFME) modified with combinations of poly-NiTSPc and Nafion® films. Sensors and Actuators B. 2007;124:368-75.
- Pontié M, Sikpo L, Thouand G, et al. Direct electroanalysis of-p-nitrophenol in estuarine and surface waters by a high sensitive type C/p-NiTSPc coating carbon fiber microelectrode. Electroanalysis. 2011;23(2):433-41.
- Lakard B, Herlen G, Lakard S et al. Ab initio study of the polymerization mechanism of poly(p-phenylediamine). J Mol Struct (Theochem). 2003;638:177-87.
- Kauffmann JM, Van Antwerpen P, Ahmad Sarakbi, et al. Utility of screen printed electrodes for in vitro metabolic stability assays: application to acetaminophen and its thioconjugates. Electroanalysis. 2011;23(11):2643-50.
- Pontié M, Thouand G, Nardi D, et al. Antipassivating electrochemical process of glassy carbon electrode dedicated to the oxidation of nitrophenol compounds. Electroanalysis. 2011;23(7):1579-84.
- Pontié M, Bédioui F. Evaluation of the nonbiofouling behaviour of nitric oxide electrochemical sensor materials by using sessile drop contact angle measurements and free enthalpy of adhesion calculations. Materials Science & Eng C. 2002;2SSSSSF1:69-73.