Original Article
, Volume: 16( 1)Effective Solutions of Hardness by Using Adsorption Technique on Kaolinite Smectite Adsorbent from Aqueous Solution
- *Correspondence:
- Mahmoud Fathy Egyptian Petroleum Research Institute, Application Petroleum Department, Egypt, Tel: +(202) 227478474; E-mail: fathy8753@yahoo.com
Received Date: December 29, 2017 Accepted Date: January 04, 2018 Published Date: January 06, 2018
Citation: El-Naggar IM, Ahmed SA, Shehata N, et al. Effective Solutions of Hardness by Using Adsorption Technique on Kaolinite Semctite Adsorbent from Aqueous Solution. Int J Chem Sci. 2018;16(1):235
Abstract
The high hardness of ground water causes some problems in household and industrial use. Therefore, this research was carried out. Several parameters have an impact on the adsorption behavior of Ca2+ and Mg2+ onto clay mineral from aqueous solution, i.e., pH, exchange time, original ions concentration and temperature. All factors were carried out through batch technique. Optimum pH for Ca2+ and Mg2+ adsorption was found to be 6.0. As well as some thermodynamic parameters were calculated ΔG, ΔS and ΔH indicating adsorption was spontaneous, wantonness and endothermic. The isotherm modeling was investigated and the results were showed that Freundlich isotherm model more fitted than Langmuir, indicating the presence of heterogeneous sites for Ca2+ and Mg2+adsorption.
Keywords
Kaolinite smectite composite; Adsorption; Hardness; Softening; Calcium; Magnesium
Introduction
Some areas in the world like Egypt depend on ground water as the main source of water. The high hardness of ground water causes problems in household and in industrial applications by reacting of Ca2+, Mg2+ Mn2+ with soap anions during washing [1]. Decontamination of a number of impurities in drinking water, ground water and waterway is desirable for the Daily use and manufacturing uses. Along with the contaminants, Stiff water incorporate minerals such as calcium, magnesium, iron and manganese ions can reply with soap anions for the duration of washing, lessening the effectiveness of smooth. The minerals as well stimulate scaling problems and threatening disasters in tubes of reservoirs, warmness scattering in electrical machines consisting of washing machines, dishwashers and vapor irons. To eliminate the divalent ions, a number of manners to solve the trouble have been broadly carried out as a ways of capable water [2] softening like chemical precipitation, ion exchange process, nanofiltration, reverse osmosis and electro membrane techniques for instance electrodialysis, electrodialysis reversal and electro-deionization reversal, but Adsorption onto clay verified to be effective solutions for hardness specially natural clay such as kaolinite, smectite, montmorilite, bentonite, vermiculite and goethite comparing to other techniques [3].
Adsorption onto clay has many advantages such as low-cost, availability, profitability, ease of operation, efficiency and effectiveness. Adsorption process was happened through the layers of clay by swelling to accommodate the adsorbed water and ionic species. As well as clay have exchangeable cations and anions held to the surface that can exchange with Ca2+ and Mg+ ions from aqueous solution [4]. The materials that were used in this study are sedimentary rocks from Bayda, Egypt where oil is collected have porosity and can be exploited to remove metal ion from aqueous solution because they are safe on the environment.
These overlapping rocks are characterized by low permeability and high holes and gaps, so that the oil is released only under high temperature, high pressure or chemical treatment in the laboratories. These rocks are rich in smectite and small amount of kaolinite. Smectite and montmorilite appear to be the same minerals. It can be obtained by purification of bentonite. It is composed of Al2O3, SiO2, Fe2O3 in large quantities, CaO, NaO, MgO and K2O in minor quantities. This research aims to determine the adsorption behavior of Ca2+ and Mg2+ onto Kaolinite smectite composite (KSC) clay mineral from aqueous solution with examination the effect of pH, temperature and different concentrations on the adsorption of ions on KSC surface.
Experimental Methods
Materials and instrumentations
All chemicals without further purification then all materials had been present from BDH (England) yet Loba Chemie (India). In all experiments, deionizer water was used for analytical purposes and for washing of clay mineral from Bayda, Egypt. The instruments used in this study are Ultra Violet visible spectrophotometer, XRD, FTIR and pH meter that commonly used in laboratories.
Preparation of adsorbate and adsorbent
The clay was treated by contacting 50 g of oil shell natural composite with 500 mL of H2O2. The treated clay was left for two hours to get rid of organic material. After that the clay was further treated with 0.1 M HCL acid in a glass beaker for two hours to remove metal ions. Finally, the clay residue was washed with an excess of hot distilled de-ionized water, decanted and sundried. The samples were then pulverized and passed through mesh sieves of sizes 500 μm to 800 μm to obtain acid modified clay [5,6].
Adsorption procedure
Kinetic studies: The dynamic investigations were attained with analyze the impact of run through on the adsorption for Ca2+ Also Mg2+ on KSC previously, at pH 8. Stock solution of 1, 000 mg/L used to prepare solution of known primary concentration 500 mg/L, 250 mg/L, 125 mg/L, 75 mg/L. This was done by dissolving 2 g of adsorbent with 100 mL of particular contaminant solution at the pH=5. Batch adsorption tests were achieved at intervals contact time (0-150 min). At the wanted time, the tubes were centrifuged toward 4, 000 rpm to 20 min with make inspected toward utilizing UV-visible spectrophotometer.
Adsorption of ions as a function of pH
Batch adsorption of Ca2+ and Mg2+ at different pH was tested by shaking 1000 mg from KSC with 50 mL of 1000 mg/L Ca2+ and Mg2+ for 24 h. The pH of the adsorptive solution was adjusted using 0.01 N NaOH or HCl. The content was then centrifuged and absorbance was recorded by using UV-visible spectrophotometer [7]. The adsorption density (mg clay/g adsorbent) was determined from the equation:
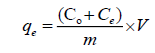 (1)
(1)
Where qe is the equilibrium adsorption capacity of clay adsorbed on unit mass of adsorbent (mg/g), Co and Ce are the initial and the final concentrations (mg/L), in that order, V is the volume of adsorbate solution (L) and m is the mass of adsorbent (g) [8].
The percentage elimination of KSC was calculated using the following relationship:
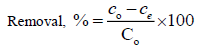 (2)
(2)
Effect of different weight of natural composite
For this study, different weights (1 g to 5 g) of natural composite clay were prepared and placed in the solution of calcium and magnesium ions at a concentration of 500 mg/L. The value of pH was adjusted to 8 for prepared solutions to ensure the low deposition of hydroxides. After 24 h to reach the chemical equilibrium of the solutions, we measure a sample of solutions about 10 mL to determine the concentration of the residual ions after the absorption process in the solution and calculated the adsorbed ions by using UV device.
Adsorption isotherms
The impact of the preliminary concentration of Ca2+ and Mg2+ changed into determined at pH 6 ranging from seventy-five- 500 mg/L at 278, 298 and 323 nm respectively in order to calculate the thermodynamic variables of the absorption reaction, also the adsorption experiments were done in 100 ml Erlenmeyer flasks with the aid of adding zero.1 g adsorbent clay in 50 ml of steel ion solution.
Results and Discussion
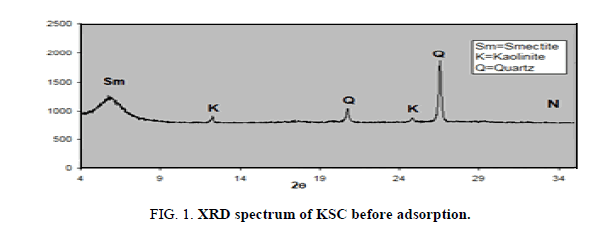
XRD analysis of the oil shell KSC
One sample was examined using XRD instrument and represented below. From examination, the XRD pattern of composite (77.52% of smectite and 22.48 of kaolinite) from Bayda, Egypt shows that it is a very small crystal that’s mainly X-ray amorphous. Some minerals could be identified with more or less certainty (Figure 1): quartz SiO2 7.61%, calcite 85.26%, hematite 2.47% and some trace elements as seen in following two Tables 1 and 2. Kaolinite smectite yield X-ray diffraction patterns characterized by basal reflections that vary with minerals composition that exposed to certain organic molecules, heat treatment and exchangeable cations [9].
| Mine name | Quartz | Calcite | Dolomite | Gypsum | Pyrite | Hematite | Apatite | Flour apatite | Clay |
|---|---|---|---|---|---|---|---|---|---|
| Bayda % | 62.76 | 26.09 | - | 3.35 | - | - | - | 2.71 | 5.1 |
Table 1. Results of XRD analysis of Bayda rock.
| Element name | Co | Cr | Cu | Ni | V | Zn |
|---|---|---|---|---|---|---|
| Conc. (ppm) | 118 | 188 | 58 | 136.7 | 258.4 | 487 |
Table 2. XRD results for trace elements in Bayda rock.
XRD pattern of the composite represents a completely crystalline material. Figure 1 for smectite and kaolin composites shows clear reflections at the value of 12 and 25 which are characteristics for kaolinite and another reflection around 5 characteristics for smectite.
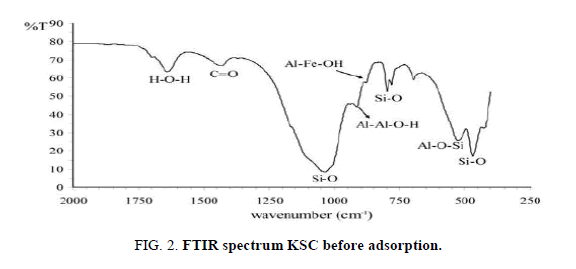
FTIR analysis of the oil shell KSC
It is essential to determine the distinctive effective groups on the exterior of the KSCs were answerable for adsorption of metal ions from solution. These functional groups were determined by the Fourier transfer infrared spectroscopy (FTIR) analysis. When KSCs were soaked with specific cations (Ca2+, Mg2+, Co2+ and pb2+), the positions and shapes of the IR bands of the Composites had been very comparable or almost equal to each other (Figure 2). The FTIR spectrum of KSC powder gave the records concerning the stretching and bending vibrations of those powerful companies [1].
The Si-O broaden vibration of smectite came about at inside the location of one, 1/2 cm-1 and or not it's capable of be affect via water adsorbed at the mineral. Water molecules had been incompletely excluded with the aid of the ions inside the composites as to be a sign of the dwindled water bands around 1, 645 cm-1 inside the IR spectra (e.g., Figure 2) and at approximately three, four hundred cm-1 (spectra now not shown). The reduced water environments might also cause the Si–O stretching bands to shift. however, numerous authors have discovered that, whilst moisture content decreased in Li+ and Na+ oversupplied montmorillonites, the 1, half cm-1 band become weaker. The 1, 044 cm-1 bands of the KSC studied at this study had been advanced but now not weakened.
The FTIR spectra of KSC powder before Ca2+ and Mg2+ adsorption are shown in Figure 2 shows well-defined peak at 713.66, 875.68, 1419.61, 2515.18, 2912.5 and 3410.15 (1/cm). The final one may be appointed to the existence of alcohol hydroxyl group (-OH) stretching. This result implies that Ca2+ and Mg2+ interact with metal oxides and-OH functional group in the KSC powder [10,11].
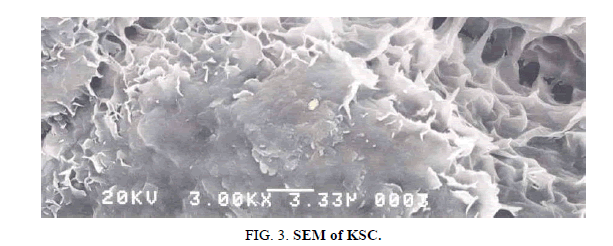
SEM micrographs and observations
Scanning electron microscope micrographs of KSC are represented in Figure 3. SEM micrographs of the composite clays present harmonized surfaces in the form of plates. This flocculated fabrics are typical of highly swelling soils with high salt concentrations which reduces the inter particle repulsion. The majority of the sample has unlocked structures (e.g., cornflake micro structure) formed by KS laminar particles which stay edge-to-edge and edge-to-face contacts, as in Figure 3 [1,12]. KSC is ultrafine-grained less than 2 micrometers in magnitude as regular particle size classifications and so may need particular analytical techniques for their recognition and study. The KSC is made of variable cluster of different sizes with a prevalence of the particles in spherules. Some porosity is observed on the surface of clay what predict interesting adsorbent properties. In universal it can be noticed that the total fabrics totally isotropic, created by unsystematic (non-oriented) group of atoms.
Effect of pH
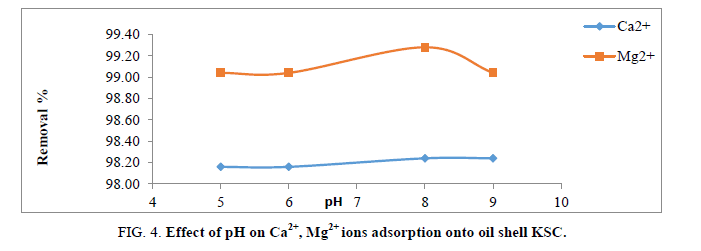
The pH solutions greatly influence the ionization of functional groups of the sorbet due to the fact that protons are strong competing sorbets in the solution. Additionally, the pH value controls the chemical speciation of metal ions in the solution and also determines the surface charge of the adsorbent.
The pH of solution was inspected at different pH values from 5-9 for Ca2+ and Mg2+ removal on the adsorbent. The removal efficiency depends on pH as shown in Figure 4. Greatest adsorption obtained at pH 8 for both ions. At pH<5.0, H+ ions compete with Ca2+ and Mg2+ ions for the surface of the adsorbent which would hinder metal ions from reaching the binding sites of the sorbet caused by the repulsive forces. At pH>9, the Ca2+ and Mg2+ will precipitate due to forming calcium and magnesium hydroxide precipitate with hydroxide anions [11,13]. For this cause, the greatest pH esteem was 8. At pH=8. The uppermost elimination effectiveness was observed at 98.24% and 99.24% of Ca2+ and Mg2+, in that order.
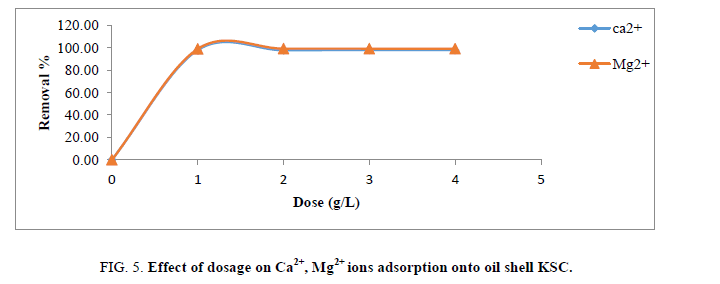
Effect of dosage
Figure 5 presents elimination efficiency of Ca2+ and Mg2+ ions as indication of adsorbents dosage which it turned into numerous among 1 g/L and 4 g/L, other operational variables (pH, contact time, initial ion concentration and particle length) have been saved steady agitation speed were kept at 25°C and two hundred rpm, inside the same order. From the consequences in Figure 5, growing dose of the adsorbents greater removal performance of cited ions. This is predicted because greater joining websites for ions are available at high-stage of adsorbents dose. This indicates that a smaller amount of adsorbents dosage causes more competition for the active site while a greater availability of exchangeable sites can be found with higher amounts of adsorbents dosage. Furthermore, at positive dose of adsorbent, adsorption top is reached and subsequently, no increase inside the elimination performance above 1 g of the adsorbent because the amount of loose ions in answer stays constant [14].
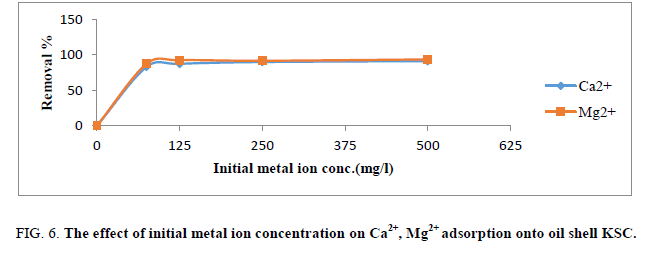
Effect of initial metal ion concentration
The proportion elimination of Ca2+ and Mg2+ by the adsorbent at first rose speedily with growing concentration and reduced speed until reached 125 mg/L (Figure 6). At lesser concentrations, Ca2+ and Mg2+ ions within the answer might engage with the joining sites. At excessive-level concentrations; extra ions are loose in answer have a tendency of entirety of binding sites. This point to those actively a lesser quantity of favorable sites included with rising ion concentrations within the aqueous solution.
Figure 6: The effect of initial metal ion concentration on Ca2+, Mg2+ adsorption onto oil shell KSC.
Different mechanisms of ion alternate processes further to the adsorption method for the duration of the ion-change method, ions undergo the pores of the adsorbent, in addition to passages of the arrange lattice, moreover they needed to displace interchangeable cations [1,15]. Propagation was quicker via the pores and was not on time while the ions moved thru the smaller diameter channels. In this case, the ion sorption may want to specifically be lead to ion-change reactions in the micro pores of the adsorbents proceeds.
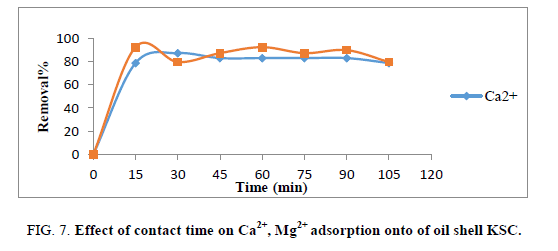
Effect of contact time
Contact time is an important parameter because this factor can reflect the adsorption kinetics of an adsorbent for a given initial concentration of the adsorbate. The impact of contact time was tested for starting Ca2+ and Mg2+ ions concentration (100 mg. L1) and adsorbent mass 1 g. Figure 7 shows the beginning adsorption rate of Ca2+ and Mg2+ ions till 15 min of contact time, then, the adsorption rates became practically constant and then reached equilibrium point after 60 min.
Isotherm modeling
In this paper, equilibrium relationship between adsorbent and adsorbate became defined by the usage of the Langmuir and Freundlich isotherm equations.
The Langmuir model: The Langmuir isotherm of adsorption was described by rearranged equation in the following linear form:
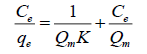 (3)
(3)
Where, Qe (amount (mg) of cation for each g of adsorbent) is the adsorption at the equilibrium cations concentration Ce, Ce is the equilibrium concentration of cations in solution (mg/L), Qm (amount (mg) of solute adsorbed for each g of adsorbent) is the highest adsorption capability subsequent to one sheet coverage a K is the Langmuir constant correlated to energy of the adsorption (L of adsorbate for each mg of adsorbent) [16].
The linear outline can be obtained by plotting Ce/Qe versus Ce. The Langmuir constants Qm and K can be calculated from the slope and intercept of the linear equation. Langmuir isotherm is relying on three states: Adsorption cannot carry out away from mono sheet coverage, all surface sites are the same and can house at most one adsorbed atom, or the capability of a molecule to adsorb at a given site is independent of the occupation of adjacent [17].
The Freundlich model: The Freundlich isotherm is often specified by a linear equation in logarithmic model as following:
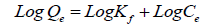 (4)
(4)
Where Qe is the amount of cations took by adsorbent at equilibrium (adsorption density: mg of adsorbate per g of adsorbent), Ce is the concentration of cations at equilibrium Kf, and n are the empirical constants dependent on many variables and n is greater than one [18].
The outline of LnQe vs. LnCe giving a straight line shows the verification of the Freundlich isotherm for adsorption. The constants n and K can be resolved from the slope and the intercept, in that order. These two forms are broadly used, the previous supposing that highest adsorption takes place when the surface is coated by one sheet of adsorbate; and the latter being purely empirical [19]. The results recommended the studied ions (Ca2+ and Mg2+) on KSC clay is favorable for Freundlich isotherm more than Langmuir Isotherm for the following reasons:
The correlation coefficient esteems represented form Freundlich isotherm for studied ions is much higher than Langmuir was shown in Table 3. Besides, the Freundlich example 1/n gives a sign for adsorption idealness. The estimation of n>1.0 speaks the positively adsorption conditions. This result indicated the sorption process was dominated by physisorption (i.e., physical adsorption) on structurally heterogeneous adsorbent with minimum interaction between the adsorbed ions. From the above discussion, Ca2+ and Mg2+ are physically adsorbed. So, it can be concluded that adsorbent surface in both micro and nano dimensions are heterogeneous; and surface adsorption is often done in the form of multilayer.
| cation | Reaction temperature (K) | Freundlich isotherm | Langmuir isotherm | ||||
|---|---|---|---|---|---|---|---|
| Kf | n | R2 | Qm | b | R2 | ||
| Ca2+ | 303 | 0.12 | 0.72 | 1.00 | 32.13 | 9.76 | 0.85 |
| 313 | 0.01 | 0.48 | 0.99 | 4.75 | 0.62 | 0.99 | |
| 328 | 10.21 | 2.26 | 1.00 | 2.15 | 1.06 | 0.99 | |
| 338 | 11.99 | 2.04 | 1.00 | 1.92 | 0.61 | 0.99 | |
| 348 | 7.10 | 3.10 | 0.99 | 2.08 | 0.99 | 0.99 | |
| Mg2+ | 303 | 0.77 | 1.12 | 1.00 | 122.90 | 75.81 | 0.91 |
| 313 | 1.01 | 1.14 | 1.00 | 100.95 | 84.06 | 0.93 | |
| 328 | 0.01 | 0.40 | 1.00 | 2.99 | 0.29 | 0.99 | |
| 338 | 0.25 | 0.86 | 0.98 | 9.38 | 2.07 | 0.85 | |
| 348 | 0.05 | 0.54 | 1.00 | 11.65 | 3.17 | 0.99 | |
Table 3. Langmuir and Freundlich isotherms parameters for the adsorption of Ca2+ and Mg2+ onto oil shell KSC.
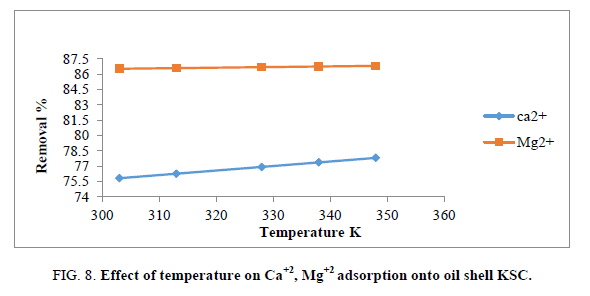
Effect of temperature
The adsorption behavior of Ca2+ and Mg2+ on the composite increased with increasing the reaction temperature from 303 to 348 ± 1 K. These results revealed the adsorption system was endothermic in nature. This trend may be attributed to the mobility of these studied ions with increasing reaction temperature. Well-known thermodynamic parameters like free energy change (ΔG), enthalpy change (ΔH) and entropy change (ΔS) had been applied to assess attainability of the adsorption technique via the following equations:
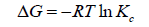 (5)
(5)
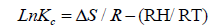 (6)
(6)
Where Kc is the distribution coefficient of the adsorption, R is the universal gas constant (8.314 J/mol-1 K) and T is the absolute temperature (K) [18,20]. The positive values of (ΔH) indicate the endothermic nature of the adsorption process, while the positive values of ΔS for Ca2+ and Mg2+ indicate the increased randomness at solid-solution interface during the adsorption of these cations in oil shell on KSC (Figure 8 and Table 4).
| Cation | TK | 1/T (E-03) | Kc | LnKc | ?G (E+03) (kJ/mol K) |
?H (kJ/mol K) |
?S (E+01) (kJ/mol K) |
|---|---|---|---|---|---|---|---|
| Ca2+ | 303 | 3.30 | 1.54 | 0.50 | -1.26 | 2.10E+04 | 2.71 |
| 313 | 3.19 | 2.15 | 0.70 | -1.82 | |||
| 328 | 3.05 | 3.38 | 1.10 | -3.00 | |||
| 338 | 2.96 | 3.69 | 1.30 | -3.65 | |||
| 348 | 2.87 | 4.86 | 1.58 | -4.57 | |||
| Mg2+ | 303 | 3.30 | 6.50 | 1.83 | -4.61 | 1.01E+03 | |
| 313 | 3.19 | 6.54 | 1.84 | -4.79 | |||
| 328 | 3.05 | 5.32 | 1.86 | -5.07 | |||
| 338 | 2.96 | 7.55 | 1.87 | -5.26 | |||
| 348 | 2.87 | 6.68 | 1.88 | -5.44 |
Table 4. Thermodynamics parameters of Ca2+, Mg2+ adsorption of onto oil shell KSC.
The negative values of the free energy change (ΔG) for the investigated metal ions indicate that the adsorption process procedure is spontaneous and imply the superior adsorption of those cations on KSC clay in comparison to H+ ion [20].
Conclusion
The composite adsorbent revealed efficiency in the removal of Ca2+ and Mg 2+ aqueous solutions. The removal efficiency depended on pH, initial ion concentration and contact time of solution where equilibrium time was 15 minutes and adsorbent concentration about 1 g for both studied ions. The study of the thermodynamic variables appeared that the adsorption was thermodynamically spontaneous and endothermic process under usual circumstances. The equilibrium data fitted Freundlich isotherm, indicating heterogeneity in the sorption sites and adsorption process is physical adsorption.
References
- Farrag AE, Moghny TA, Mohamed AM, et al. Abu Zenima synthetic zeolite for removing iron and manganese from Assiut governorate groundwater, Egypt. App Water Sci. 2016;7(6):3087-94.
- Fathy M, Moghny TA, Allah AE, et al. Cation exchange resin nanocomposites based on multi-walled carbon nanotubes. App Nanosci. 2014;4(1):103-12.
- Zhang X, Guo Z, Zhang C, et al. Exploration and optimization of two-stage vacuum membrane distillation process for the treatment of saline wastewater produced by natural gas exploitation. Desalination. 2016;385:117-25.
- El-Maghrabi HH, Hosny R, Ramzi M, et al. Novel mesoporous silica (MCM-41) and its characterization for oil adsorption from produced water injected in water injection projects using fixed bed column processes. Desalin Water Treat. 2017;60:70-7.
- El-Sayed M, Ramzi M, Hosny R, et al. Breakthrough curves of oil adsorption on novel amorphous carbon thin film. Wat Sci Technol. 2016;73(10):2361-9.
- Zhou X, Xu Y. Solar updraft tower power generation. Solar Energy. 2016;128:95-125.
- Mahmoud Fathy TAM, Awadallah AE, Abdou MM, et al. Development of sulfonated nanocomposites ion exchange resin for removal of sodium ions from saline water. Int J Mod Org Chem. 2015;4(1):62-9.
- Zare V. Exergoeconomic analysis with reliability and availability considerations of a nuclear energy-based combined cycle power plant. Energy. 2016;96:187-96.
- Fathy M, Moghny TA, Mousa MA, et al. Absorption of calcium ions on oxidized graphene sheets and study its dynamic behavior by kinetic and isothermal models. App Nanosci. 2016;6(8):1105-17.
- Yin S, Zhang Y, Tian W, et al. Simulation of the small modular reactor severe accident scenario response to SBO using MELCOR code. Progress in Nuclear Energy. 2016;86:87-96.
- Moghny TA, Keshawy M, Fathy M, et al. Preparation of sorbent materials for the removal of hardness and organic pollutants from water and wastewater. World Academy of Science, Engineering and Technology. International Journal of Environmental, Chemical, Ecological, Geological and Geophysical Engineering. 2017;11(5):461-8.
- Bao S, Tang L, Li K, et al. Highly selective removal of Zn (II) ion from hot-dip galvanizing pickling waste with amino-functionalized Fe3O4@SiO2 magnetic nano-adsorbent. J Coll Int Sci. 2016;462:235-42.
- Angelo JM, Lenhoff AM. Determinants of protein elution rates from preparative ion-exchange adsorbents. J Chromatography A. 2016;1440:94-104.
- Mahmoud Fathy, Ahmed TA, Awadallah M. Adsorption kinetics of sulfates by anion exchange resin containing pristine multiwalled carbon nano tubes. Global J Res Eng. 2014;1(14).
- Arancibia-Miranda N, Baltazar SE, García A, et al. Nanoscale zero valent supported by zeolite and montmorillonite: template effect of the removal of lead ion from an aqueous solution. J Haz Mat. 2016;301:371-80.
- Fathy M, Moghny A, Abdou MM, et al. Study the adsorption of Ca (II) and Mg (II) on high cross-linked polystyrene divinyl benzene resin. Int J Mod Chem. 2015;7(1):36-44.
- Alizadeh T, Sabzi RE, Alizadeh H. Synthesis of nano-sized cyanide ion-imprinted polymer via non-covalent approach and its use for the fabrication of a CN--selective carbon nanotube impregnated carbon paste electrode. Talanta. 2016;147:90-7.
- Wassel MA, Hosny MFR, Desouky AM, et al. Study of the removal of cupper ions from textile effluent using cross linked chitosan. 8th International Conferences of Textile Research Division. 2017.
- Akl ZF, El-Saeed SM, Atta AM. In situ synthesis of magnetite acrylamide amino-amidoxime nanocomposite adsorbent for highly efficient sorption of U (VI) ions. J Ind Eng Chem. 2016;34:105-16.
- Agullo-Lopez F, Climent-Font A, Munoz-Martin A, et al. Ion beam modification of dielectric materials in the electronic excitation regime: Cumulative and exciton models. Progress in Mat Sci. 2016;76:1-58.