Research
, Volume: 9( 1)Development and Electrochemical Characterization of Al2O3-ZnO Composite Electro Catalytic Material for Energy Synthesis from Electro Oxidation of Ethanol
- *Correspondence:
- Subir Paul Department of Metallurgical and Material Engineering, Jadavpur University Kolkata, India, E-mail: spaul.@metal.jdvu.ac.in
Received: July 30, 2018; Accepted: August 9, 2018; Published: August 14, 2018
Citation: Paul S, Molla AK. Development and Electrochemical Characterization of Al2O3-ZnO Composite Electro Catalytic Material for
Energy Synthesis from Electro Oxidation of Ethanol. Res Rev Electrochem. 2018;9(2):113.
Abstract
Energy synthesis through environment friendly process, using renewable fuels is needed for sustainable development. Ethanol which can be produced by pyrolysis and fermentation of fruits, vegetable and agriculture waste is a renewable fuel for clean energy in future. While chemical oxidation of this fuel produces energy through a series of steps of conversion of chemical energy to heat energy, mechanical energy, electrical energy; the electrochemical oxidation of the same fuel over an electro catalytic anode surface gives rise to straight electrical energy with higher efficiency and no polluting substances. The development of inexpensive high energetic electro catalytic material will put forward this energy technology route. The present investigation attempts to develop ZnO-Al2O3 composite electro catalytic material to electro catalytically oxide ethanol solution to electrical energy. The material has been synthesized by electro deposition technique, controlling deposition parameters such as electrolyte chemistry and applied deposition current to produce a high energetic 3D substrate to get the optimum current and hence energy delivered. The material was characterized by cyclic voltammetry (CV), Chronometry (CA), Potentiodynamic Polarization (PP) and electrochemical impedance spectroscopy (EIS). The constituents of the composite were analyzed by XRD and the morphology of the 3D structure were studied by SEM. It was found that electro catalytic property of the material is strongly influence by deposition current and concentration of depositing ions. The material with the best electro catalytic property could produce over 250 mA current over 1 cm2 electrode surface area on electro oxidation of ethanol.
Keywords
Composite oxide electrode ; Electro catalytic; Fuel cell; Ethanol; Voltammetry; Impedance ; XRD; SEM; Cell current
Introduction
The demand for energy is increasing with progress of lifestyle, automation, computation and advent of high tech cities. This increases the burning of more fossil fuels for energy by the conventional route which produces huge amount of polluting gases leading to increasing global pollution. To combat the situation, energy synthesis through alternative route, using renewable energy resources is the need of the hour. Biomass and biomass derived fuels are the good resources of energy synthesis [1-4] , by clean technology through electrochemical oxidation which delivers electrical energy with no polluting emerging gases. Ethanol which can be produced from fruits and agriculture waste [1,3] is a good green renewable fuel. The electrochemical oxidation of ethanol over an electro catalytic anode surface produces twelve electrons in the form of current as illustrated below.
Anode reaction: CH3CH2OH + 3H2O → 2CO2 + 12H+ + 12e- (Ea = 0.084Vvs SHE) (1)
Cathode reaction: 3O2 + 12H+ + 12e-. → 3H2O (Ec = 1.229Vversus SHE) (2)
Overall reaction: CH3CH2OH + 3O2→ 2CO2 + 3H2O (E cell = 1.145 V) (3)
The theoretical energy ΔG produced from one mole of ethanol can be computed as follows.
ΔG= nFE = 12 X 23061 X 1.145 = 316.86 kilocalorie = 368.26 watt-hour
(Where n=No of Electrons, F=Faraday’s constant in calorie/volt, E=Cell Potential)
This is quite a good energy. A 100 watt bulb can glow for three and half hours from one mole of ethanol in a fuel cell.
For energy conversion in a fuel cell, electrodes having high electro catalytic properties are needed to be developed. Platinum is already known as a good electro-catalytic material for many fuel cell applications, but due to its high cost, non-platinum based, inexpensive electrode is required to synthesize pure alternative energy at an economically viable cost. Extensive research has been carried on development of inexpensive metals, alloys and oxides, electrocatalytic materials in battery and fuel cell for future energy synthesis and storage [5-12]. Among the metallic systems Ni based alloys [9,10,13,14] have shown promising materials for future energy materials for low temperature fuel cells in cars and automobiles.
Of oxides, MnO2 [5-7,15-17] has been found to act as good electro catalytic anode material for alcoholic based fuel cell. Modification of MnO2 with carbon, further enhancers the electro catalytic property. Carbon, being as good conductive electrode material has been found to be very effective. Addition of carbon in micro and nano size to metal alloys or oxide materials have been found to enhance their catalytic activities [6,18]. Carbon cloth or graphite paper, impregnated with gold nano particles is found to produce excellent electro catalytic performance on glucose oxidation in alkaline media [18]. Historically Zn and Al had been used as electrodes for battery due to their high electrical conductivity and availability at cheap price. The oxide of Zn, ZnO a good n type semiconductor, has a capacity to accept or release electron. Though Al2O3 is a refractory type oxide, introducing lattice and electronic defects into it can impart conductivity. A composite of ZnO-Al2O3 oxides produced by electro chemical deposition has been found [8] to act as very good electrode material for electro oxidation of methanol.
In the present investigation, ZnO-Al2O3 composite has been synthesized by electrodeposition technique, varying deposition current and concentration of depositing ions to control oxide defect structure so as to produce high energetic electro catalytic material. Electrochemical characterizations of the material ZnO-Al2O3 were performed by cyclic voltammetry (CV), Chronoammetry (CA), Potentiodynamic Polarization (PP) and electrochemical impedance spectroscopy (EIS). The constituents of the composite were analyzed by XRD and the morphology of the 3D structure was studied by SEM.
Experimental Methods
Pretreatment
The surface of the Aluminum sample (3cm X 1cm) prepared by the following sequential steps: Polished by emery papers, degreased by acetone. Electro polished in a solution of perchloric acid and ethanol washed by double distilled water dipped in 12.6% NaOH for 3 min for surface activity, rinsed in double distilled water and finally dried in hot air.
Electro Synthesis of Al2O3-ZnO
The synthesis of ZnO and Al2O3composite was done by electrodeposition in an electrolyte solution of ZnCl2 + KCl (TABLE 1) at a temperature 55° C by DC power source with a galvanostatic circuit , varying current and solution chemistry (TABLE 1) to achieve an optimum condition of producing the best Al2O3-ZnO, the electrocatalytic material for anodic oxidation of ethanol fuel.
| Electrode | Modifier | Limit of detection (μM) | Sensitivity (μA mM-1 cm-2) |
Linear range (mM) |
Detection method |
Ref. |
|---|---|---|---|---|---|---|
| PGE | polypyrrole | 0.79 | - | 0.005 –0.50 1.25–4.5 |
DPV | [4] |
| GCE | photosensitive polymers micelles | 1.0 | - | 0.01 – 8.0 | DPV | [20] |
| GCE/MWCNT | o-phenylenediamine | 0.5 | - | 2×10-4-4×10-2 | LSV | [21] |
| CFME | o-phenylenediamine and aniline | 1.5 | 180 | 6.5×10−3 – 2 | SWV | [29] |
| GCE | p-phenylenediamine | 0.21 | - | 6.6×10−4 – 3.3 | CV + SWV | This work |
TABLE 1. Comparison of the efficiency of different electrochemical MIP sensors used in the analysis of acetaminophen.
Electrochemical Testing
The developed electro catalytic electrode, Al2O3-ZnO, was electrochemically characterized in ethanol solution (1MCH3CH2OH + 1M KOH), by studying Cyclic Voltammetry (CV), Chronoamperometry (CA), Potentiodynamic polarization (PP) and Electrochemical Impedance Spectroscopy (EIS), in 3 electrode system electrochemical cell, with sample as working electrode, Graphite rod as counter electrode and saturated calomel electrode (SCE) as reference electrode, in the potentiostat, AMETEK Versa STAT 3:Model-500 and GAMRY Instruments for EIS.
For CV the Potential was scanned -1V to 1V vs. SCE with scan rate 0.05 mV/second to find out the Imax (current amplitude). The CA was carried out at different fixed potentials, selected, between -0.5V and -1.0V vs. SCE. The current I was monitored as a function of time t, to find out, how long the steady state current is delivered from the cell. The Potentiodynamic polarization (PP) was conducted in the same Instrument with a different software. The potential was scanned between -2.5V and -0.5V vs. SCE at a scan rate of 0.5 mV/second.
In EIS study, the electrochemical cell was connected to an impedance analyzer (EIS300 controlled by E chem analyst software) for electrochemical impedance spectroscopy. The electrochemical impedance spectra were obtained at frequencies between 100 kHz and 0.1 Hz. The results and information obtained from the EIS experiments were, Polarization resistance (Rp), electrolyte resistance (Rs), double layer capacitance (Cdl), capacitive load or constant phase element (CPE ,Y0), and α which is defined from the capacitive impedance equation Z=1/C(jw)–α. Capacitors in EIS experiments often do not behave ideally. Instead, they act like a constant phase element (CPE Y0). The exponent α is close to 1 for ideal capacitance (Cdl). For a constant phase element, Y0, the exponent α is much less than one.
Physical characterization
The developed electro catalytic material was physically characterized by XRD and SEM. The crystalline structure and crystalline phase of the electrode was characterized by XRD, using Rigaku Ultima III X-ray diffraction for recording the diffraction traces of the samples with monochromatic Cu Kα radiation at room temperature, at a scan rate of 20/min. The crystallographic planes of X-ray diffraction were obtained from the inbuilt software of the X-Ray machine. The surface morphology and particle distribution of the electrodeposited composite coatings were performed by Scanning Electron Microscopy (SEM) (JEOL-JSM 6360).
Results and Discussions
Al2O3-ZnO and NiO materials are synthesized by electrodeposition by controlling the chemistry of the electrolyte and the current density. The electrolyte of compositions and the variations of different parameter are illustrated in TABLE 1. The electrocatalytic properties of the above electro synthesized composite oxide are discussed in the following sections
Electro Chemical Characterization
Electrochemical characterizations, viz. Cyclic voltammetry (CV), Chronoammetry (CA), potentiodynamic polarization (PP) were carried out in 1M ethanol and 1M KOH solution at 25°C. KOH was added to increase the conductivity of the ethanol solution.
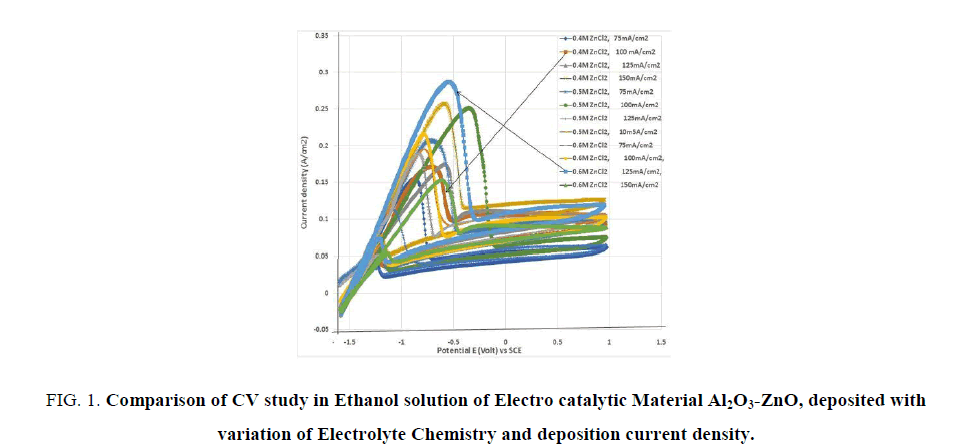
FIG. 1 shows a branch of CV curves for differently electrodeposited Al2O3-ZnO in ethanol fuel cell solution. It is seen that the peak current density is varying depending on electrodeposition condition. The material electrodeposited in the electrolyte solution of 0.6 M ZnCl2 and 0.2 M KCl at 55°C and at a current density of 125 mA/cm2, gives the highest peak current density with very good energy content (the area enclosed by the curve). While the material electrodeposited in the electrolyte solution of 0.4M ZnCl2 and 0.2M KCl at 55°C and at a current density of 100mA/cm2 , produces electrode with much less electro catalytic activity. This indicates the influences of deposition current and electrolyte composition. The higher the concentration of Zn++ ions and charge density (current) at the electrode surface adjacent to the electrolyte, the electro catalytic property of the material is enhanced.
FIG 1: Comparison of CV study in Ethanol solution of Electro catalytic Material Al2O3-ZnO, deposited with variation of Electrolyte Chemistry and deposition current density.
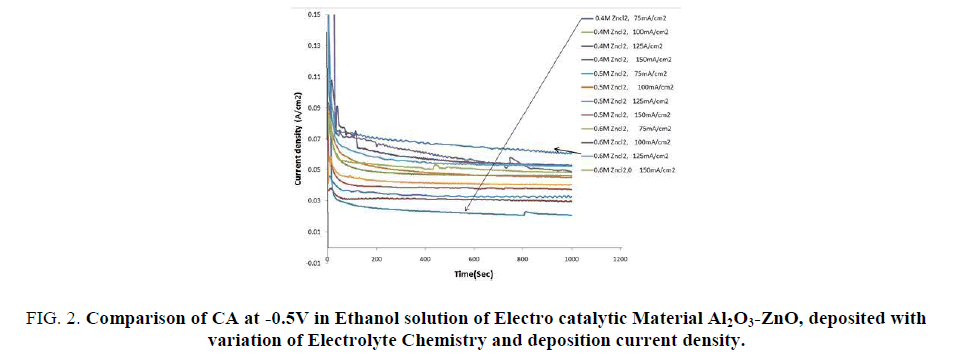
The CA study at 0.5V vs. SCE oxidation potential of the above materials electrodeposited under different conditions are shown in FIG. 2. It is seen all the curves reach a steady state current density within 50 seconds and the highest steady state current occurs for the same electrodeposited material, as that of CV study that is produced by electrodeposition in the electrolyte solution of 0.6 M ZnCl2 and 0.2 M KCl at 55°C and at a current density of 125 mA/cm2.
FIG 2: Comparison of CA at -0.5V in Ethanol solution of Electro catalytic Material Al2O3-ZnO, deposited with variation of Electrolyte Chemistry and deposition current density.
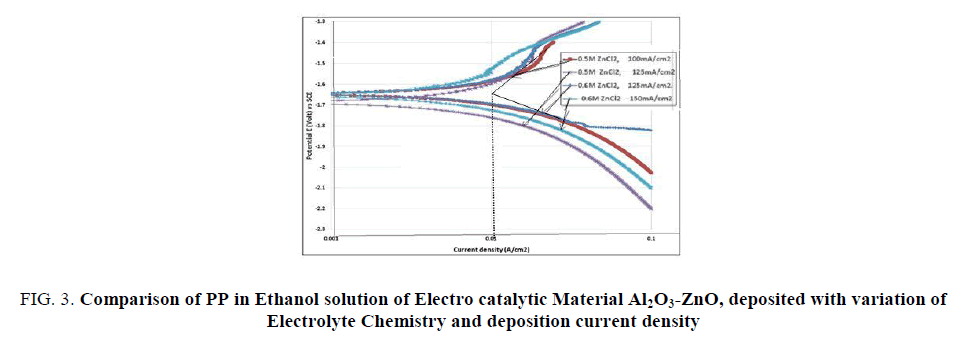
Having obtained a good electrocatalytic material of Al2O3-ZnO in CV and CA studies, the potentiodynamic polarization (PP) studies are done only for those electrodeposited materials which performed good in CV and CA investigation. FIG. 3 shows PP studies of Al2O3-ZnO electrodeposited in 0.5 and 0.6 molar solutions ZnCl2 in the current density range of 100-150 mA/cm2. The curve shifts to the right with material of higher electro catalytic activity. The materials, Al2O3-ZnO produced in 0.5 M ZnCl2 C and at a current density of 100 mA/cm2 and 0.6M ZnCl2 and at a current density of 125mA/cm2 performed better with much less polarization resistance than that of others. The maximum reversible cell current comes out around 10 mA/cm2 which is very satisfactory, considering a cell with 100 cm2 electrode surface area can deliver 1 amp current.
FIG 3: Comparison of PP in Ethanol solution of Electro catalytic Material Al2O3-ZnO, deposited with variation of Electrolyte Chemistry and deposition current density
Electrochemical Impedance Study
All the above studies show that the Al2O3-ZnO can make a very good electrocatalytic anode material for direct ethanol fuel cell. Now for better understanding of the fundamental aspects of oxidation of the fuel on the electrolytic substrate, EIS study is carried out. It will throw light on the electrochemical phenomena occurring at the metal-electrolyte interface and compute R-L-C circuit. In general, the interface consists of layer of (+ve) charge and a layer of (-ve) charge which is called electrical double layer, which produce a capacitance or pseudo-capacitance. In addition, there are resistance load for polarization resistance (Rp), solution resistance (Rs). There may also be induction or more capacitance due to coating. The phenomenon can be interpreted by Nyquist and Bode plot, which are depicted and discussed in the following section for various electro coated electro catalyst materials.
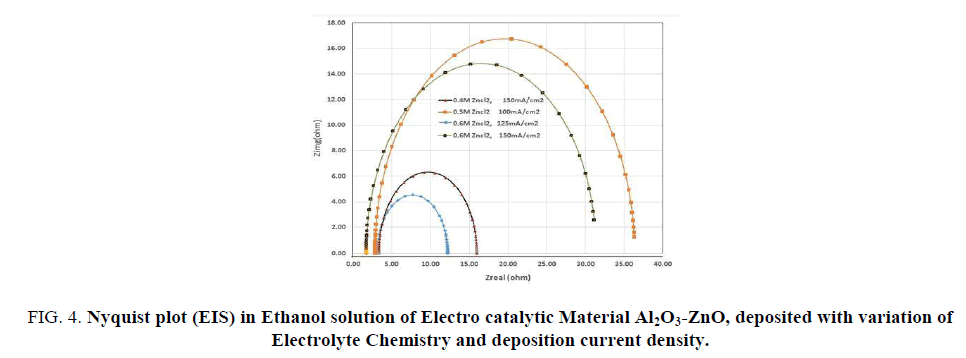
EIS studies of Al2O3-ZnO, electro synthesized under different conditions are shown in FIG. 4 (Nyquist plot) and FIG. 5 and 6 (Bode plots). The Nyquist shows a branch of semicircles, the diameter of each represents the polarization resistance. The lower the diameter of the semicircle, the better is the electro catalytic property. It is interesting to find out that material electro synthesized in 0.6 M ZnCl2 solution and at a current density of 125 mA/cm2 has the minimum polarization resistance and hence high electrocatalytic material. So the EIS investigation supports the CV, CA and PP findings. It is seen that the higher the concentration of ZnCl2 solution and current, the less is the polarization resistance. High concentration solution has more Zn++ ion available at the interface and also the charge density is more at higher current density, the effect will favour more nucleation of deposits than the growth. This will produce fine deposit substrate with more electrode surface area available for charge-discharge reaction in fuel cell on electro oxidation of the fuel ethanol.
FIG 4: Nyquist plot (EIS) in Ethanol solution of Electro catalytic Material Al2O3-ZnO, deposited with variation of Electrolyte Chemistry and deposition current density.
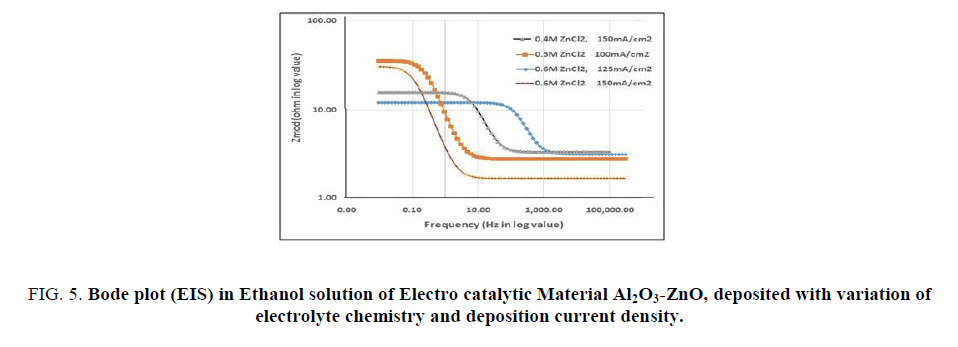
FIG 5: Bode plot (EIS) in Ethanol solution of Electro catalytic Material Al2O3-ZnO, deposited with variation of electrolyte chemistry and deposition current density.
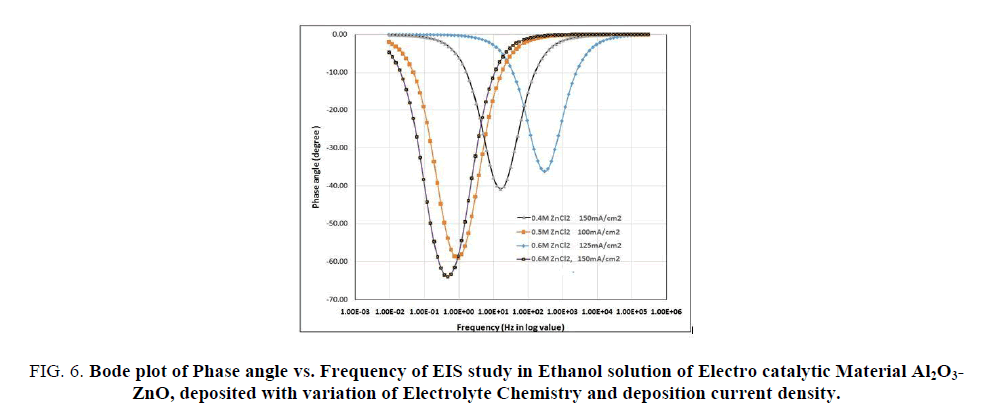
FIG 6: Bode plot of Phase angle vs. Frequency of EIS study in Ethanol solution of Electro catalytic Material Al2O3-ZnO, deposited with variation of Electrolyte Chemistry and deposition current density.
The Bode plots also show FIG. 5, the similar trend, impedance is less for the material electro synthesized at higher concentration solution and current density of 125 mA/cm2. The phase angle vs. frequency plot (FIG. 6) indicates one capacitance for each of the curve. It follows a Randle circuit (inscribed in FIG. 6) and the phase angle lags -35 degree to -65 degree which indicates that the capacitance is not a pure one but a pseudo capacitance. The computed EIS parameters are shown in TABLE 2. In R-L-C circuit, the lower the Rp and higher the Cp or Y0 (pseudo capacitance), the more is the current flow, since the reactance due to capacitance is the reciprocal (1/2πfC) of the capacitance. It is seen (TABLE 2) that the material electro synthesized in 0.6M ZnCl2 solution and at a current density of 125 mA/cm2, has the minimum Rp and the maximum Cp values, which indicates that it is the best electro catalytic material for electro oxidation of ethanol over it as electrode. So the EIS findings support CV, CA and PP studies.
| Doliprane®500 | RSD (%) | ||
| Mass tablet weighted after powdered usual acetaminophen mass in a Tablet | 582 ± 2 | 1.5 (n=3) | |
| Acetaminophen mass calculated in the weighted tablet (mg) | 483 ± 1 | ||
| Acetaminophen mass determined with MIP-GCE in the weighted tablet (mg) | 480 ± 10 | 2.5 (n=3) | |
| Acetaminophen calculated in the initial mass tablet and determined with MIP-GCE (mg) | 497 ± 10* | ||
| Recovery range (%) | 97.4-101.4 |
*(3% error admitted by the European drug regulation for a mass value range admitted equal to 500 mg ± 15 mg)
Table 2. Real applicability of the proposed MIP for commercial tablets.
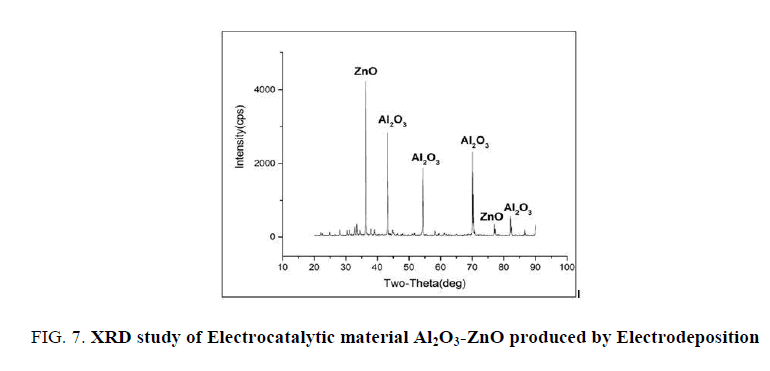
XRD studies of Al2O3-ZnO
FIG. 7 shows the XRD graph of Al2O3-ZnO electrocatalytic material. It clearly reveals presence of ZnO and Al2O3. This indicates the Al base electrocatalytic material developed is a composite of Al2O3-ZnO which produces very good electrocatalytic effect for electro oxidation of ethanol giving high current.
Scanning Electron Microscopes (SEM) study
Having obtained the constituent of electrocatalytic materials by XRD studies, it will be interesting to know the morphology of coated electrodes under scanning electron microscope. FIG. 8 shows the scanning images of Al2O3-ZnO electro catalytic material at lower magnification and higher magnification. It is seen the electro deposited Al2O3-ZnO composite is distributed throughout the surface in spherical and sub spherical globular forms with channels and inter channels. The nano channels and inter channels with micro to nano grain enhance the effective surface area for charge-discharge reactions by many times more than the geometrical electrode surface. This makes huge charge density and the current delivered is very high. So this morphology of the scanning images support the high current obtained in CV, CA and Pd studies.
FIG 8: SEM micrographs of the coated sample of Al2O3-ZnO at different magnification (a) 1000; (b): 4000.
Mechanism of Al2O3-ZnO Electrodeposition
Al2O3-ZnO oxide is formed at cathode by the following possible reactions. It is known that the water reduction potential is much nobler than that the Zinc electro deposition. Water reduction takes place with the formation of OH- ion as follows
O2 + 2H2O + 4e- = 4OH- (4)
Zn2+ ions in the electrolyte will react with OH- ions to form metal hydroxide. This metal hydroxide further dissociates to more stable form ZnO at temperature above 35, 27, 28 .
The reactions are shown below:
2Zn2+ + 4OH- = 2Zn(OH)2 (5)
2Zn(OH)2= 2ZnO + 2H2O (6)
Overall reaction can be written as
2Zn2+ + 5O2 + 2e- = 2ZnO (7)
The proposed mechanism for the formation of Al2O3 oxide is given here. At the time of electrodeposition, aluminum (Al) goes into solution through the dissolutions of ZnCl2. Al3+ ions readily combine with OH- ion to form aluminum hydroxide, Al(OH)3. This hydroxide decomposes to Al2O3 oxide through the following reactions.
2Al + 3Zn2+ = 3Zn + 2Al3+ (8)
Al3+ + 3OH- = Al(OH)3 (9)
2Al (OH)3 = Al2O3 + 3H2O (10)
Control of Al2O3-ZnO structure
Electrodeposition method influences the structure and quality of coating 19. Graded coatings can be manufactured by changing the process variables, like current density, applied potential, chemistry of the electrolyte, temperature, PH, stirring and particle loading in the bath. Among these parameters, the imposed current is one of the most important ones, having a great influence on particle content in the composite coating and consequently on coating properties.
The relative rates of nucleation RN and growth RG, decide the particle size of the electrodeposited material and therefore nanomaterials structure. If RN is much greater than RG, more nuclei will be formed from the ions than the growth of the already formed nuclei, giving rise to nanodeposit.
Current density is probably the most important parameter, defining the quality of the deposited material. Once a nucleus has been formed, the current flowing to the cathode causes a local deformation of the current density distribution in the vicinity of the growing center. New nuclei are therefore expected to form only at a given distance from the initial nucleus. This results in a temporary drop of current efficiency, a decrease of cluster superficial density and an increase in cluster apparent surface. At low current density, coarse deposits are formed, because the speed of nucleation centers creation is much lower compared to crystal growth of the existing seeds. For high current density, quite often, a decrease of ions concentration close to the deposited surface is observed, so high concentration of depositing ions will favour more nucleation. Thus, it is seen from electrochemical characterizations studies (FIG. 1-6) that very good electrocatalytic Al2O3-ZnO was formed at higher current density and the concentration of the depositing ions
Conclusion
Al2O3-ZnO composite oxide has been found to act as good electro catalytic electrode for electro oxidation of ethanol fuel to deliver current in fuel cell. The structure Al2O3-ZnO composite, synthesized by electrodeposition is strongly influence by the deposition parameters such as applied current density and the chemistry of the electrolyte solution. The material produced at an optimum electrodeposition conditions was to be highly electro catalytic energy material, delivering high current and energy on electro oxidation of ethanol.
References
- Paul S. Characterization of bioelectrochemical fuel cell fabricated with agriculture wastes and surface modified electrode materials. J Fuel Cell Sci Technol ASME. 2012;9(2): 021013.
- Paul S, Jana A. Study on Bioelectrochemical Fuel Cell with Algae. J Inst Eng Interdisciplinary Div. 2007; 88: 27-30.
- Paul S, Mondal P. Pyrolysis of Forest Residue for Production of Bio. Fuel Int Energy journal. 2007;7:221-5.
- Paul S, Mondal P. Fabrication and Characterization of Bioelectrochemical Fuel Cell With Pyrolysed Produced Bio Oil and Hydrolysed Biomass By Fermentation. J Inst Eng Interdisciplinary. 2009;90:40-5.
- Paul S, Ghosh A. Electrochemical Characterization of MnO2 as Electrocatalytic Energy Material for Fuel Cell Electrode. J Fuel Chem Tech. 2015;43(3):344-351.
- Paul S, Chatterjee R. Development of Nano Carbon-MnO2 Energy Material for Glucose Fuel Cell Electrode. Nanomat Energy ICE Pub. 2015;3:1-9.
- Paul S, Ghosh A. MnO2, A High Electrocatalytic Energy Material to Synthesis Energy from Oxidation of Methanol in Fuel Cell. Energy Env Focus. 2015;5:1-7.
- Guchhait S, Paul S. Synthesis and Characterization of ZnO-Al2O3 Oxides as Energetic Electrocatalytic Material for Glucose Fuel Cell. J Fuel Chem Tech. 2015;43(8):1004-10.
- Paul S, Naimuddin S K, Ghosh A. Electrochemical characterization of Ni-Co and Ni-Co-Fe for oxidation of methyl alcohol fuel with high energetic catalytic surface. J Fuel Chem Tech. 2014;42:87-95.
- Paul S, Naimuddin S K. Electrochemical Characterization of Synthesized Ni–Co and Ni–Co–Fe Electrodes for Methanol Fuel Cell. J Fuel Cell SciTechnol ASME. 2014;l(11):18-25.
- Paul S. Materials and Electrochemistry: Present and Future Battery. J Electrochem Sci Technol. 2016;7(2):1-17.
- Paul S. Book: Electrochemical Energy from Battery and Fuel Cell. Amazon Book Store. 2017.
- Mohamedi M, Hisamitsu Y, Kihara K, Kudo T, et al. Ni-Al alloy as alternative cathode for molten carbonate fuel cells. Original Research Article. J Alloys Compd. 2001;315:224-33.
- Suresh Kumar K, Haridoss Prathap, Seshadri S.K. Synthesis and characterization of electrodeposited Ni–Pd alloy electrodes for methanol oxidation. Original Research Article. Surface and Coatings Technology. 2008;202:1764-70.
- Turkusic E, Kalcher K, Schachl K, et al. Amperometric Determination of Glucose with an MnO2 and Glucose Oxidase Bulk-Modified Screen-Printed Carbon Ink Biosensor. Anal Lett. 2001;34:2633-47.
- Xu JJ, Feng JJ, Zhong X, et al. Low-Potential Detection of Glucose with a Biosensor Based on the Immobilization of Glucose Oxidase on Polymer/Manganese Oxide Layered. Nanocomposite. Electroanalysis. 2008;20:507-12.
- Xu JJ, Luo XL, Du Y, et al. Application of MnO2 Nanoparticles as an Eliminator of Ascorbate Interference to Amperometric Glucose Biosensors. Electrochem Commun. 2004;6:1169-73.
- Pasta1 M, Ruffo R, Falletta1 E, et al. Alkaline glucose oxidation on structured gold electrodes, Gold Bulletin. 2010;43.Paul S. Electrochemical Energy Synthesis and Storage in Battery and Fuel Cell. Amazon Books. 2016;153: ISBN-13: 978-1520321929, https://www.amazon.com/dp/1520321929.