Review
, Volume: 19( 6)A Review on Titanium Carbide Synthesising Methods
Sangita Mohapatra*, A K Chaubey
Department of Scientific and Industrial Research, Institute of Minerals and Materials Technology, Bhubaneswar, India.
*Corresponding author: Mohapatra S, Department of Biotechnology, Uka Tarsadia University, Gujarat, India; E-mail: sunilysonu@gmail.com
Received: July 12, 2021; Accepted: July 26, 2021; Published: August 02, 2021
Abstract
Titanium carbide is the most common and widely used transition metal carbide. TiC, is an extremely hard refractory ceramic material, similar to tungsten carbide. Present article discuss about various synthesizing methods of TiC powder. However, the most widely used process for commercial production of TiC is carbothermal reduction of TiO2. The article focuses on carbo-thermal reduction of ilmenite (Fe2TiO3) for the synthesis of TiC as a low-cost industrial method. The importance of ilmenite to be used as a raw material for TiC synthesis has been discussed. Thermochemistry of carbothermal reduction of ilmenite has been briefly described.
Keywords
Titanium carbide; High temperature; Carbothermal reduction; Ilmenite; Ceramics
Introduction
Among various non-oxide metal carbides, Titanium Carbide (TiC) is one of the most important and potential metal carbide used in advanced ceramics. Because, it possesses many desirable properties such as high melting point, high hardness, high mechanical strength, good thermal and electrical conductivity and good corrosion resistance as well as low density, superb chemical & thermal stability, good wettability. Due to this, titanium carbide has become a promising candidate in cutting tools, grinding wheels, wear-resistant parts and coatings, high-temperature heat exchangers, magnetic recording heads, turbine seals, bullet-proof vests, forming dies, and light-weight amour pieces, etc [1]. TiC is a transition metal carbide of group IV with refractory in nature. Its crystal structure and bonding are somehow responsible for its superior properties for which titanium carbide (TiC) occupies a unique position in the spectrum of engineered ceramics offering many desirable advantages over other non-oxide ceramics in common usage.
Application of titanium carbide
Recently TiC has an excellent demand for its use in various applications, which require operations at high temperatures, long life, and weight savings of the components. Due to lower density, TiC is desirable for applications that demand materials of lightweight applications. TiC is also suitable for high-speed cutting tool applications for reducing thermal stresses and cracking. After tungsten carbide-cobalt, TiC-based ceramics have worldwide use in the cutting tools industry, as high-temperature ceramics, in the form of hard metal or cermet. TiC has effective use in cermet due to its low friction coefficient and higher oxidation resistance in comparison to cemented tungsten carbide.
Recently, out of a wide variety of available materials for hard, wear-resistant protective coatings, titanium carbide is well known in industrial applications. Thermal sprayed composite coatings of TiC bonded with different metals are assumed to be an exceptional substitute for the conventionally used WC or Cr3C2-based coatings, being potentially effective in many wear resistant applications. Another important application is in the area of plasma and flame spraying processes in air, where titanium carbide-based powders show higher-phase stability than tungsten carbide-based powders.
The superior properties of TiC have resulted in its extensive use as a reinforcing phase in composites and super alloys. Significant advantages of TiC over other metal powders and intermetallic compounds are not only its high electrical conductivity but also its oxidation resistance and chemical stability. It improves the various properties of composites like strength, hardness, and wear and corrosion resistance by controlling its microstructure and makes composites as value-added products. Fine-grained TiC can improve the yield strength of the composites through dispersion and grain size mechanisms, and enhance toughness by hindering crack propagation [2]. TiC has also several other important applications such as electrical discharge machining, in mechanical and microelectronics industry, for conducting diffusion barriers, additive to plastic and rubber parts etc.
Synthesis/fabrication techniques
The expansion of efficient and energy-saving technologies is of huge importance today. Wide variety of techniques are available for the production of transition metal carbides, which are very refractory and having melting temperatures up to 3950°C. One of the disadvantages in the commercialization of TiC for various applications is its difficulty in manufacturing. At present, the synthesis of TiC is one of the energy-intensive process and involves expensive high- temperature equipment. Most of the current production methods include reactions carried out at temperature well above the melting point of Titanium (1670°C). Due to this, titanium carbide is usually produced by high-temperature processes. Another major factor that hinders commercialization of TiC is the cost of high-quality powder. Recently almost all methods of synthesizing high-quality powders involve expensive steps that produce only minor quantities of products. Every approach has varying features of morphology, particle size and distribution, condition of agglomeration, chemical purity, and stoichiometry.
The synthesis of TiC powder generally requires the precursors containing Ti and C and high-temperature reaction between two precursors is the widely accepted method for synthesis of TiC. The Ti-containing precursors can be Ti metal, TiCl4, and TiO2. On this basis, the synthesizing methods for TiC powder can be broadly divided into three categories (i) direct reaction between metallic Ti and carbon, (ii) gas-phase reaction between TiCl4 and appropriate hydrocarbons or the chloride process, (iii) carbothermal reduction of TiO2. The direct reaction between metallic titanium and carbon may take place by self-propagation high-temperature synthesis (SHS) or by mechanical ball milling method [3]. There are also several other methods demonstrated for a laboratory scale synthesis. The most widely and popular amongst these methods is the carbothermal reduction of titanium
dioxide (TiO2) in the presence of carbon (C). Many research works have been carried out on these above-mentioned routes for synthesizing TiC powder.
Self-propagating high-temperature synthesis (SHS)
Self-propagating high-temperature synthesis (SHS) of powder compacts is also known as combustion synthesis and proceeds via exothermic reactions. Since the process occurs at high temperatures, this method can be used as an alternative to conventional furnace technology. Some other advantages of this process are the requirement of a comparatively low cost reactor with less power consumption, a lower environmental impact, and the probability of controlling the chemical and phase compositions and particle size of the products. Titanium carbide has been successfully prepared by this method by different researchers.
Prepared TiC powders by low temperature combustion synthesis at 530?C from the Ti-C system with polytetrafluoroethylene (PTFE) as a chemical activator. Their result shows that TiC powders with average particle size less than 100 nm are synthesized by adding 3wt% PTFE into the Ti-C system. These TiC particles consist of a single crystal as the result of a fast, low-temperature, solid-state synthesis process. They investigated that combustion synthesis mainly includes two reaction processes. Firstly, the initial reaction between titanium and PTFE particles releases a great amount of heat, and subsequently, the heat-induced combustion reaction between titanium and carbon particles.
In another approach, titanium carbide (TiC) and TiC-Al2O3 composite particles were obtained in situ SHS process of wood dust and TiO2 with Mg and Al, respectively under static argon gas. The reaction was carried out in a SHS reactor under static argon gas at the pressure of 0.5 MPa. The standard Gibbs energy minimization method was used to calculate the equilibrium composition of the reacting species. The effects of activated carbon used as precursor instead of wood dust were investigated. Their result shows a complete reaction of the precursors for both wood dust and activated carbon to yield TiC-MgO and TiC-Al2O3 as a product composite. These synthesized TiC-MgO composites were then leached with 0.1M HCl acid solution to obtain TiC particles as final products.
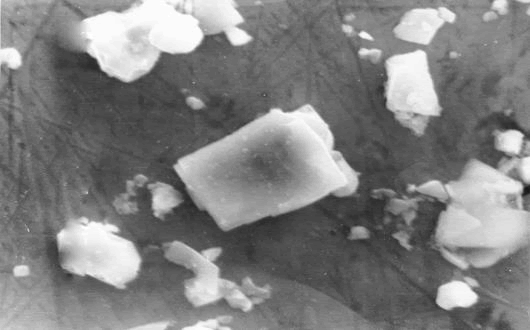
Recently SHS process is also studied to know the effect of pressure and shear on the quality of the synthesized titanium carbide powder. According to the authors, due to the larger specific surface area, the titanium carbide powder prepared by SHS comminution should show improved sinterability, which is very important in powder metallurgy. The apparatus and procedures that they have proposed can be used to assess the effect of shear deformation and pressure on structure formation processes during SHS. Applying shear loads to a partially formed material during synthesis may have a significant effect on the grain size and morphology, as well as on the mutual arrangement of the grains throughout the reaction system. Their results and proposed designs of the experimental setups can be used to develop technologies for the comminution of the SHS products, which are typically obtained in the form of a high-strength sinter. Below shows the TiC produced by them (Figure 1).
Synthesized TiC by combustion process using the magnesio-thermic reaction from a mixture of compacted powders of TiO2, Mg and charcoal (TiO2- 2 Mg- C) as starting materials (Figure 2).
Self-propagating high temperature synthesis produces TiC through exothermic reactions. During these reactions, when heated to reasonable temperatures fine particles of titanium become very reactive. For this process very fine, pure titanium is required, but quantities of TiC powder produced are very less. One of the potential drawbacks of this method is the high cost of pure metallic Ti that is required as a starting material [4]. During the process, the oxygen contained in the metal can hardly be reduced so that the product is generally characterized by high oxygen content. Another limitation of the SHS process is the possible porous nature of the final products.
Mechanical milling
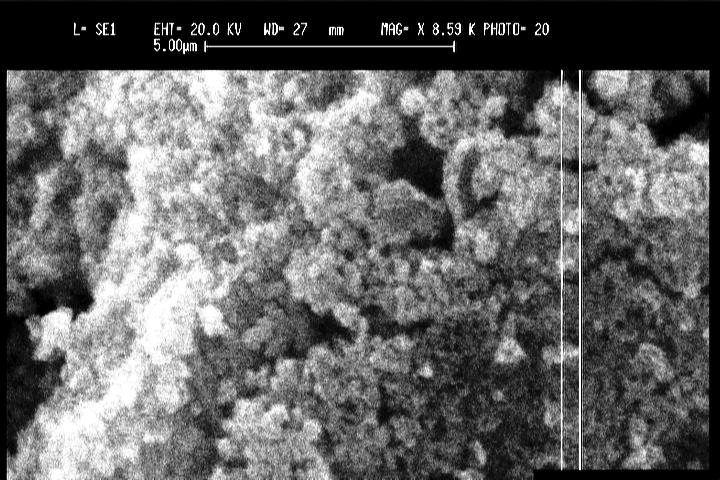
Mechanical milling is a solid-state powder processing technique. Different types of high-energy milling equipment are used to produce mechanically alloyed powders. This equipment differs in their capacity, efficiency of milling and the additional arrangements for cooling, heating,etc investigated the formation of TiC powder by the mechano-chemical reaction of titanium and graphite as starting materials during milling. They observed that prolonging the milling time up to 16 h resulted in a decrease of grain size to nanoscale along with increasing strain and a slight decrease in the lattice parameter of the TiC phase. They also found some iron impurity is coming during milling, but it did not have any significant effect on TiC formation. The TiC powder has been shown in (Figure 3).
Carbides of Ti have been synthesized directly from industrial ferrotitanium (Fe–Ti) for the first time, through high energy mechanical milling and heat treatment. They observed that the mechano-chemical process is occurring at a temperature far lower than those used in the conventional carbothermic reduction. The lowering of the synthesis reaction temperature is due to the enhanced activation energy of the reactants achieved from mechanical activation even after 10 hours milling which leads to the onset of formation and growth of crystallites of TiC at a lower temperature (1000?C). Along with TiC, carbides of iron also form simultaneously. At the ambient temperature, only Fe2C forms which get converted to Fe3C during heat treatment at 1000?C. The crystallite size of TiC decreases with increasing milling time but increases with an increase in heat treatment temperatures.
Investigated the preparation of TiC from Ti chips and carbon black by using a high-energy milled titanium and carbon elemental powder mixtures with compositions under a helium atmosphere using a magneto-ball mill. Titanium carbide (TiC) was formed rapidly via a highly exothermic mechanically induced reaction. Synthesized TiC via mechanical alloying by planetary ball milling of metallic Ti and carbon black powder. TiC forms at an instant via a mechanically induced self-propagating reaction.
Another fast and large-scale process in which TiC was synthesized by using a horizontal rotary ball mill within 60 min via a mechanically induced self-propagating reaction (MSR). The as-synthesized TiC particles have a well-defined crystals structure and a median diameter of∼1 μm with less impurity due to the short duration of milling. This method represents to be a great method due to large potential of high-speed horizontal rotary ball milling for fabricating fine and homogeneous ceramic powders.
Synthesis of TiC by this method from elemental Ti and C is challenging and has restricted applications. This method requires expensive ball milling equipment which makes the process highly energy intensive. This method generally requires a long time, such as 5-20 hours for completion, produces highly agglomerated powders that need massive post milling to yield fine powders, and requires chemical purification to remove impurities from mill wear. In addition, the high cost of the initial titanium powder of high purity leads to a relatively expensive product. During the mechanical milling process, also there is a possibility of the thermal explosion of the reactants.
Chloride process
The basic raw material for this method is TiCl4 and the process is generally based on the gas-phase reaction between TiCl4 and an appropriate compound or hydrocarbon such as C2H2, CCl4, CH4, and CaC2. Another method is based on the chemical reaction of TiCI4, H2, and CO. The reaction is driven in contact with an incandescent tungsten or carbon filament. This chloride method is quite complex. This method strictly limits the quantity and sometimes the quality of the resulting powder. The titanium-based halides, when treated with carbon-containing gases generate corrosive gases. In addition, special precautions have to be taken because the precursor TiCl4 and the product HCl are very corrosive. Titanium chloride that is used as a precursor in this process is very expensive on industrial scale.
The demerit of the process is that for initiating this chemical reaction, the mixture has to be heated to 2000oC under high vacuum to avoid oxidation. Some other experimental methods include direct carburization synthesis by multi-pulse laser treatment of elemental titanium in ambient CH4 gas. Reported successful carbidation of Ti in a layer forming on the surface of a Ti sample submitted to multi-pulse excimer laser treatment in CH4, at a slightly super atmospheric pressure. In this process, while its main part consists of fcc TiC, the surface layer is contaminated with oxygen. These methods use huge amounts of energy, are expensive and still do not produce powders with the required set of physical and chemical features [5].
Carbothermal reduction of Titanium dioxide
Among the various synthesizing methods available, carbothermal reduction is the most widely used route for the production of TiC. In this process, TiO2 is used as the titanium precursor while carbon sources generally include various materials like carbon black, activated carbon, and cellulose etc.In the Ellingham diagram, the slope of the C(s) +O2 (g) =2CO (g) line is reverse to those of the other oxides. Therefore, the C-CO line intersects most of the oxide lines. C thus can reduce these oxides, above the temperature at which their lines get intersected. Carbon being very cheap, available in the form of either charcoal or coke, is utilised for commercial production of these metals from their oxides. These reduction processes are carried out at high temperatures, hence they are known as carbothermal reduction process. Almost all commercial productions primarily use a carbothermal reduction process due to its low cost.
TiO2 is used as a raw material on an industrial scale due to its low cost and easy availability, which reduces the cost of production and makes the process economical. TiC powder is commercially produced primarily by the reduction of TiO2 by carbon, especially carbon black, in a temperature range between 1700-2100oC. Several authors have reported that TiC could be synthesized from polymeric precursors prepared by titanium alkoxides and organic compounds at lower temperatures compared to TiO2 with carbon. However, titanium alkoxides as raw materials are difficult to handle because they are prone to hydrolysis by moisture even in the air. If TiC is produced by carbothermal reduction on an industrial scale, it is favourable to utilize TiO2 as a raw material because of its cheap source and ease of handling. This process can be used for synthesizing TiC by reduction of TiO2 using a) carbon or b) carbonaceous organic materials. This process also involves c) carbothermal reduction of carbon-coated titanium dioxide, d) sol–gel and microwave carbothermal reduction methods at low temperature.
Carbothermal reduction of TiO2 using carbon
The carbothermal reduction process uses carbon for reduction at high temperatures. The chemical reaction in the carbothermal reduction process proceeds in the following way this chemical reaction proceeds thermodynamically at 1290°C when the partial pressure of CO gas is 1 atm. However, the production of TiC using the carbothermal reduction of TiO2 essentially needs high temperature (1700–2100°C) and long reaction times (10–24 h). The Gibbs free energy variation for reaction in equation (5) can be calculated at changing temperatures via the thermodynamic data. The Gibbs free energy change for reaction in equation (5) decreases as the partial pressure of CO gas (?CO) decreases from the standard value (1 atm). Therefore, the synthesis of TiC via reaction (5) is thermodynamically promising even at room temperature provided ?CO is adequately low. The decrease of ?CO can be achieved suitably via flowing an inert gas such as Ar over reaction sample. However, even under the flowing inert gas atmosphere, the synthesis of TiC via reaction (5) is usually conducted at the temperature range of 1700–2300oC for 10–24 h due to the kinetic barrier. The reaction behaviour of the titanium dioxide/carbon mixture, particularly titanium carbide formation, in the carbothermal reduction method is of great consequence to obtain an optimized titanium carbide powder. Many research works have been carried out on the carbothermal reduction of TiO2.
The preparation of TiC powders by carbothermal reduction of TiO2/ charcoal in vacuum at 1450 °C for 8 hours when the system pressure was about 1–60 Pa. Thermodynamic analysis indicates that it is easy to prepare TiC in a vacuum and the formation sequence of products are Ti4O7 (Magneli phase), Ti3O5, Ti2O3, TiCxO1-x and TiC, with the increase of reaction temperature. Experimental results demonstrate that TiC powders with single-phase are obtained with the molar ratio of TiO2 to C ranging from 1:3.2 to 1:6 at 1 550 °C for 4h when the system pressure is 50 Pa, and TiC1.0 is gained when the molar ratio of TiO2 to C is 1:4 and 1:5.
Carbothermal reduction of rutile (prepared in-house) and an amorphous carbon powder mixture to obtain spherical titanium carbide (TiC) at a temperature of 1350 oC with 1-hour holding in an argon atmosphere. The carbothermal reduction of anatase and rutile to Ti. Koc R. investigated the kinetics and phase evolution of the TiC formation process by carbothermal reduction of ultrafine titania/carbon mixture. The TiC powder synthesized at 1550 oC for 4 hour in a flowing argon atmosphere.
Carbothermal reduction of TiO2 using carbonaceous organic materials
Prepared titanium carbide (TiC) via the reaction of metallic magnesium powders with titanium dioxide (TiO2), carbon source and molten salt in an autoclave at 650°C. The carbon source (oxalic acid and citric acid) in this process was stable, low toxic and cheap. X-ray powder diffraction (XRD) patterns indicated that the products were cubic TiC. Scanning electron microscopy (SEM) images showed that the samples consisted of particles with an average size of 200 nm and 100 nm in diameter, respectively. Energy Dispersive Spectrometer (EDS) analysis of the samples suggested the products contained carbon and titanium elements. The product was also studied by the thermos-gravimetric analysis (TGA). It had good thermal stability and oxidation resistance below 350°C in the air. Investigated the formation of TiC particles during the carbothermal reduction of titanium dioxide (TiO2). The mixture of TiO2 and carbon resin was reacted at 1500oC for 0-45 min under flowing argon atmosphere.
Synthesized titanium carbide (TiC) from a composite constituted of nano-sized TiO2 particles and methyl cellulose (MC) via carbothermal reduction in an argon ?ow. They found that TiC can be prepared from the composite by heating above 1300°C, which is considerably lower temperature when compared to that employed in the conventional carbothermal reduction methods that use a mixture of TiO2 and carbon powders.
Carbothermal reduction of carbon-coated titanium dioxide
The synthesis of titanium carbide (TiC) by the carbothermal reduction of carbon-coated titanium dioxide (TiO2), a novel synthesis process, was investigated. A high surface area TiO2 powder consisting of anatase and rutile phases was used for starting powders. The carbon-coated method is a two-step process that utilizes a precursor derived from decomposing propylene (C3H6) and depositing carbon on the TiO2 particles. The carbon coating process provides a high contact area between the reactants which results in a TiC product with lower oxygen content (0.6 wt. %), finer particle size (0.1lm), and uniform shape when synthesized at 1550oC for 4 hours.
Sol–gel and microwave carbothermal reduction methods
Prepared titanium carbide ultrafine powders from tetrabutyl-titanate and sucrose by sol–gel and microwave carbothermal reduction method. They studied the influences of reaction temperature and molar ratio of Ti to C on the synthesis of titanium carbide. Their results show that excess amount of carbon plays a positive effect on the carbothermal reduction of TiO2 at low temperature. The inceptive carbothermal reduction temperature of TiO2 and formation of titanium oxycarbide was below 900 °C, and pure TiC can be prepared at 1,200 °C, which was considerably lower compared to that by conventional carbothermal reduction using a mixture of TiO2 and carbon powders as raw materials.
Why ilmenite
For the synthesis of TiC powder, TiO2 is favourably used as the raw material because of its cost effectiveness and ease of handling. It is of no importance to use commercially available TiO2 as there are lot of natural resources to have titanium dioxide. One abundantly available resource is the sea beach sand in which ilmenite is the main matrix.
Ilmenite (FeO.TiO2) and rutile (TiO2) are the two chief minerals of titanium. Rutile has a higher titanium dioxide content than ilmenite, so it was the traditional choice for the production of TiO2 and titanium metal. In rutile, titanium dioxide is in an impure form, whereas, in ilmenite, titanium is present as a compound oxide with iron. Rutile deposits are very scarce in nature and they are becoming increasingly more expensive to mine. In addition, the cost of natural rutile is many times higher compared to ilmenite. Due to scarcity and the high cost of natural rutile, it is being replaced. Hence, TiO2 can be derived from ilmenite (FeTiO3), which is abundantly available in nature. In the world, nearly all titanium dioxide is produced from ilmenite as it is naturally occurred in high concentrations, accessible and in a form, which allows the preparation of the synthetic rutile.
However, extraction of the iron from the ilmenite involves metallurgical processes that require high-temperature processing and hot acid leaching, with associated environmental problems. This makes these processes expensive. The involved leaching process of leaching out FeO from FeTiO3 appears to be more difficult than that of leaching out Fe from TiC. Many research groups have proved that Fe-TiC can be synthesized in-situ by carbothermal reduction of ilmenite. As ilmenite is a chief source of TiO2, it would be more economical to use ilmenite as a precursor for TiC production. These favourable factors have made ilmenite a competitive raw material for the world's producers. Consequently, wide research has been carried out on the carbothermal reduction of ilmenite.
Carbothermal reduction of ilmenite
The reduction of ilmenite concentrate plays an important role in the titanium industry. Ilmenite concentrate usually needs higher reduction temperatures or needs additives to improve its reactivity when it is directly reduced. Carbothermal reduction of ilmenite at temperatures below ~1200 °C produces metallic iron and reduced form of titanium oxides. The reactivity of ilmenite was also be improved by using the pre-oxidization process, increasing the rate of ilmenite reduction and the rate of leaching.
Investigated the phase evolution during the carbothermal reduction process of Panzhihua ilmenite concentrate under the argon atmosphere. This ilmenite concentrate briquette with graphite powder was reduced at 1200 oC - 1500 oC, with the molar ratios of C to FeTiO3 being 4:1 and 5:1. They investigated the phase transformation of the briquette reduced at different temperatures. The authors found that during the carbothermic reduction process from 1200oC - 1500oC, the main phases were Fe, Ti3O5, Ti2O3, and TiCxOy. The lowest temperature for the generation of TiCxOy was (1300 oC) for both kinds of briquettes with different C contents. The rate-controlling step for the carbothermic reduction above 1300 oC obeyed the diffusion model. The reduction degree of the ilmenite was increased by increasing the temperature. With the increase of reaction temperature and reaction time, the TiCxOy phase would be reduced to the TiC phase.
Investigated the carbothermal reduction of ilmenite with coal in laboratory-scale experiments. Reactions were performed in nitrogen atmospheres in the temperature range of 1,225–1,325 °C with a residence time of 6 h. It was investigated that temperature increase leads to an increase of reduction degree for constant bed depth, but the reduction degree decreases with an increase of bed depth. They obtained a reduction degree of 42.2% with a bed depth of 25mm, at 1,350°C after 6 hour of residence time. The reaction was initiated with the reduction of ilmenite to titania, followed by the reduction of titania to suboxides. Titania was reduced to Ti3O5; later it was converted to titanium nitride or titanium oxycarbonitride depending on element reactivity and temperature. It was found that Ti2O3 does not appear as an intermediate reaction product due to its instability.
Mechanical activation is a very effective technique of inducing reaction. Ball- milled a mixture of ilmenite and graphite with a molar ratio of 1 to 3-4 at room temperature and subsequently annealed it for the production of TiC.
Studied a systematic effect of milling conditions on the low-temperature carbothermal reduction of the mineral ilmenite. They found that after ball milling of an ilmenite-carbon mixture at room temperature, the ilmenite was reduced to rutile and metallic iron during subsequent low-temperature annealing (760 °C for 30 minutes). A longer milling time results in a lower reduction temperature and a higher reduction rate.
That ball milling ilmenite and coal together before thermal processing leads to a decrease in both temperature and time of reaction compared with powders milled separately then mixed investigated the carbothermal reduction of Ilmenite in a plasma reactor to analyze the effect of various process parameters such as the amount of reducing agent and composition of plasma gen gas on the degree of reaction to recover titanium and iron. Experimental conditions suitable for preparing a TiC-rich Fe-TiC master alloy and TiC-reinforced cast-iron composites have been determined. Investigated the preparation of titanium carbide powder, using ilmenite and methane as raw materials, in a thermal plasma reactor. The plasma gases used during the operation of the plasma torch were argon helium and argon-nitrogen mixtures.
The advantage of producing TiC rich Fe-based composites through carbothermal reduction of ilmenite is the reduction of several steps, which helps in reducing the cost of production. Enough heat is always required for the system to supply for the carbothermal reaction besides melting the charge. Energy is always a costly input in any process route, so while choosing the route; an attempt is always made to minimize energy input. Carbothermal reduction of ilmenite has been carried out by various researchers by using different methods for the preparation of TiC or TiC reinforced Fe composites. TiC powder has also been successfully prepared directly from ilmenite by using a tubular furnace. The carbothermal reduction in a tubular furnace takes several hours because attaining 1800 °C takes a long time.
The mechanical milling method used reduces the reaction temperature, but this is also a time-consuming method involving high-energy intensive ball mills. However, the high temperature of 1800oC can be easily achieved in a thermal plasma process. The thermal plasma method appears to be an appropriate method for carbothermal reduction of ilmenite.
With the above unique advantage, several researchers have investigated on the synthesis of ceramics by the thermal plasma method. Thermal plasma synthesis of titanium carbide has been investigated based on the direct carburization of metallic titanium and methane. Presented a thermodynamic analysis for predicting the conditions for the plasma synthesis of TiC powders developed a novel method capable of sufficient mixing of titanium powder and methane of carbon source in the synthesis of titanium carbide by induction plasma reactive spray. The experimental results show that both primary carburization of the titanium particles inside the plasma flame and secondary carburization of the growing deposit on high- temperature substrate contribute to the forming of titanium carbide. For the first time, Taylor et al. investigated the formation of fine titanium carbide powder directly from the carbothermal reduction of ilmenite by using thermal plasma. They have used ilmenite concentrate and methane as feed in a thermal plasma reactor. The plasma torch was operated using argon-helium and argon-nitrogen mixtures as plasma gases. However, the non-transferred arc torch type plasma reactors used for this purpose are very expensive.
TiC powder has been prepared directly by carbothermal reduction of ilmenite using a thermal plasma reactor. An indigenously developed extended transferred arc DC thermal plasma reactor using less expensive graphite electrode has been used for carbothermal reduction, eliminating a number of intermediate process steps as compared to conventional carbothermal reduction methods. The TiC powder produced has a very good application as a reinforcement in composites.
Thermo chemistry
The feasibility of different reactions and temperatures needed for reduction of ilmenite by C can be readily determined by the knowledge of thermodynamics.
Conclusion
The majority of the available literature on the synthesis of TiC powders have reported on carbothermal reductions of TiO2. Carbothermal reduction takes several hours because attaining a high temperature takes a long time. Only a few have reported on methods like mechanical milling and SHS methods. Mechanical milling method reduces the reaction temperature, but this is also a time-consuming method involving high-energy intensive ball mills. In the discussion between various types of synthesis techniques available for the synthesis of TiC reveals that carbothermal reduction of ilmenite can be an inexpensive technique for large mass production of TiC. This is possible if high temperature like the one 1700-2100oC, is easily attainable within a short period.
Acknowledgement
Author Sangita Mohapatra is thankful to CSIR for providing Senior Research Associateship (Pool scheme: Pool No- 9001 A) of the Government of India. The authors are thankful to director, CSIR-IMMT, Bhubaneswar, Odisha for providing research facility for conducting works.
References
- Rahaei MB, Rad RY, Kazmzadeh A, et al. Mechanochemical synthesis of nanoTiC powder by mechanical milling of titanium and graphite powders. Pow Technol. 2012;217:369-376.
- Shi-Bo L, Wei-Hua X, Hong-Xiang Z, et al. Formation of TiC hexagonal platelets and their growth mechanism. Pow Technol. 2008;185:49-53.
- Benoit C, Ellen H, Nikhil K, et al. TiC nucleation/growth processes during SHS reactions. Pow Technol. 2005;157:92-99.
- Yunhong L, Qian Z, Zhihui Z, et al. Preparation and characterization of TiC particulate locally reinforced steel matrix composites from Cu?Ti?C system with various C particles. J Asian Cer Soc. 2014;2:281-288.
- Niyomwas S. Synthesis and characterization of TiC and TiC?Al2O3 composite from wood dust by self-propagating high temperature synthesis. Energy Procedia. 2011;9:522-531.