Original Article
, Volume: 12( 7)Surface modification of biogenic hydroxyapatite particles with 2-thiophenecarboxaldehyde
- *Correspondence:
- Nail Uçyol , Faculty of Marine Science and Technology, Çanakkale Onsekiz Mart University, Turkey, E-mail: bayram342001@yahoo.com
Received: May 25, 2016; Accepted: June 20, 2016; Published: June 24, 2016
Citation: Kizilkaya B, Ucyol N, Tekinay AA. Surface modification of biogenic hydroxyapatite particles. Environ Sci Ind J. 2016;12(7):102.
Abstract
In present study, it was investigated whether biogenic hydroxyapatite is suitable to chemical modification. The chemical modification on the surfaces of bone particles was made on two step using silanization and shiff base reaction. Fish Bones were used as biogenic hydroxyapatite and it were cleaned in order to make them ready for chemical proceeding. Chemical modifications were performed on the surface of fish bone particles. Firstly, free amine (-NH2) group was obtained in molecule by silanization of the surfaces of fish bones with 3-amminopropyl-triethoksisilane (S1). Afterwards, amine groups that exist on the surface of bones were reacted with 2-thiophenecarboxaldehyde (B4) that includes aldehyde groups. The materials were analyzed with FT-IR, SEM-EDS, TGA and Zeta potential. In SEM-EDS analysis, the band of silisium (Si) and sulfur (S) elements of molecules for S1 and B4 can be clearly seen. The point of zero charge (Pzc) of unmodified bone apatite (H), modified bone 3-amminopropyl-triethoksisilane (HS1) and 2-thiophenecarboxaldehyde (HS1B4) was investigated and determined as 7.25, 7.72 and 7.57, respectively. Additionally, nitrogen for H, HS1 and sulfur for HS1B4 was detected as 8.031, 8.291 and 0.916 %, respectively. 2-thiophenecarboxaldehyde bonded on the surface of bone was calculated as 143.4 μmol g-1. The results showed that surface modification was performed successfully. BET surface area of the unprocessed apatite is 5.65 m2g-1 and the surface area of modified apatite HS1B4 is 2.95 m2 g-1. This study was shown that waste fish bones occurring waste and non-economic value as industrial is to be suitable for chemical modifications and it could be effectively converted to different materials.
Keywords
2-thiophenecarboxaldehyde; Fish Bone; Surface Modification; 3-amminopropyl-triethoksisilane
Introduction
It is known that waste products occur from many different areas. It is known to the measures to eliminate these wastes are inadequate and costly. Thus, many researchers develop many different studies to convert wastes to reclaim products in another field. The usage of biological apatite from bones is showed to be important converting meat and fish industry waste into a valuable sorbent material. Bones as biogenic hydroxyapatite generally consist of protein-collagen as organic composition (30%) and hydroxyapatite Ca10(PO4)6(OH)2 (HAP) (about 70%) as inorganic composition [1-4]. The composite poly(lactid-glycolide)(PLGA, LA/GA=80/20), polymerization (g-HAP) and modification were operated on the surface of HAP. The reaction was performed with the copolymerization of ring expansion in the catalytic tin octoat (SnOct2). It is observed that the sizes of HAP were between 30 nm and 40 nm and their mechanic properties were pretty developed [4,5]. The surface of HAP was firstly bonded with 3-aminopropil-trietoksisilan (APS) by silanization. Afterwards ring expansion polymerization was applied to amino groups in margin that belong to APS molecule benzil-glutamate N-carboxyanhidrite (BLG-NCA) on the surface of HAP. It is understood that composite materials acquired have different dispersion characteristics in dichloromethane [4,6]. Polymethylmethacrylate are used as antibiotic transmitter in bones. Hence, remarkable researches have been carried out in order to develop new strategies based on HA/composite materials for a controlled medicine distribution. Tetracycline is a therapeutic molecule and it is frequently used in the treatment of bacterial infections and bone diseases. In this respect using glycidylmethacrylate redox starter, polymerization and modification were applied on the surface of HAP. Glycidylmethacrylate can easily react with amino groups. Tetracycline medicine was added on HAP modified with poly-glycidylmethacrylate (PGMA). The results showed that HA/PGMA composite material can be used for medicine release as a biomaterial [7]. Bones are natural source materials as biogenic apatite with low cost when compared to other synthetic HAP. In order to remove fatty acids and other contaminations, the bones were treated with nitric acid, sodium hydroxide, hexane, alcohol, hydrogen peroxide, and water. It is determined that the bone powders cleaned with NaOH, hexane and ethanol are white and the bone powders cleaned with H2O, H2O2 and NHO3 are yellow. After adsorption, it is observed that in Cu+2, Co+2 and Ni+2 solutions white fish bones turned into blue, red, and yellow, respectively [1,2]. It were investigated adsorptions of cobalt [4], zinc [8], chrome [9], copper and nickel [10] using the animal bones. This study revealed a general investigation of the fish bones in the fish processing plant waste can be utilized in chemical processes. Fish bones were modified with amminopropyl-triethoksisilane (HS1) and 2-thiophenecarboxaldehyde (HS1B4). Chemical characterization of obtained materials was made.
Materials and Methods
Supply of fish bones and pretreatments
Tuna fish bones provided from Çanakkale Dardanel Factory were used as waste fish bones. Firstly purification and milling and then homogenization were applied to the waste fish bones provided. According to our previous experiences, the most efficient way for cleansing method is using alkali solutions [2]. Accordingly, unprocessed fish bones were treated with mechanic mixer in 60°C with NaOH solution until they were purified from contamination. The cleansing process is completed after the control whether they still had organic or any other residual by the analysis with FT-IR. Finally, the waste fish bones were dried in the incubator and pulverized with the aid of homogenizer and grinder.
Materials and chemicals
In the laboratory experiments, magnetic stirrer-heater with contact thermometer (WiseStir MSH-20D), centrifuge (Nüve NF400), ultrasonic bath, Ultrapure water (SG, Ultra Clear 2001-B), pH meter (InoLab pH 7110), and mechanic mixer (WiseStir HS-120A) were used. In the analysis of the products acquired, elemental analysis (LECO, CHNS 628), Scanning Electron Microscope (SEM-EDS, Jeol), FT-IR (Perkin Elmer, Spectrum One-ATR), Zetasizer (Malvern, Zetasizer Nano series Nano-ZS) were used.
Functionalization of bone surfaces with 3-aminopropil-trietoksisilan
Fish particles were silinised with the materials 3-amminopropyl-triethoksisilane (S1). Firstly in 50 mL ethanol/water (9/1), solution of 0.2 M silane compound (S1) was prepared for the reactions. It was taken into a 100 mL flask and stirred in room temperature (25°C ± 2) with magnetic stirrer for 30 minutes. Afterwards, 5 g H was added into the solution and stirred with magnetic stirrer in room temperature for 24 hours. Suspense mixture was centrifuged with technical ethanol in 2000 rpm and dried with solid phase, modified by washing 5 times, in 45°C in incubator. The material gained is named as HS1. Technical information about the silan compound used for the reaction is given in Table 1.
Modification with schiff base reaction in silanized surfaces
Firstly; preparing 25 mL ethanol solution of 0.2 M (5 × 10-3 mole) aldehyde compound (B4), 50 mL of it was taken into a flask. Afterwards, 2.5 g HS1 was added into the solution and refluxed in 70°C for six hours. After refluxing, it was kept in room temperature for 12 hours. The mixture was washed with technical ethanol 5 times and centrifuged in 2000 rpm. The modified solid phase was dried in 45°C. The materials gained are named as HS1B4. Aldehyde compounds used for the reactions and technical information about them are given in Table 1.
| Chemical | Molecules | MA | Molecular Formula | Code |
|---|---|---|---|---|
| 3-aminopropil-trietoksisilane | 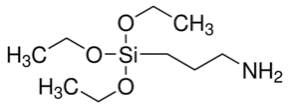 |
221.37 | C9H23NO3Si | S1 |
| 2-Thiophenecarboxaldehyde | 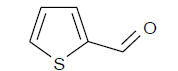 |
112.15 | C5H4OS | B4 |
Table 1: Thetechnical information of S1 and B4 molecules.
Determination of point of zero charge (Pzc)
Briefly, 0.01 M KNO3 solutions were firstly prepared in a 100 ml flask. The initial pH of this solution (PHB) between pH 4 and 10 was adjusted with 0.1 M HCl and NaOH. Then, H and HA5 products were added to the prepared solution. The solution was stirred in constant temperature with a magnetic stirrer for 48 hours. The pH of the final solution (PHS) was measured and recorded after 48 hours. It was taken the curve the initial pH (pHI) against pH difference (pH=pHI-pHF) between initial (pHI) and final pH (pHF). Pzc was determined the point cutting the x-axis of the curve [4].
Result and Discussion
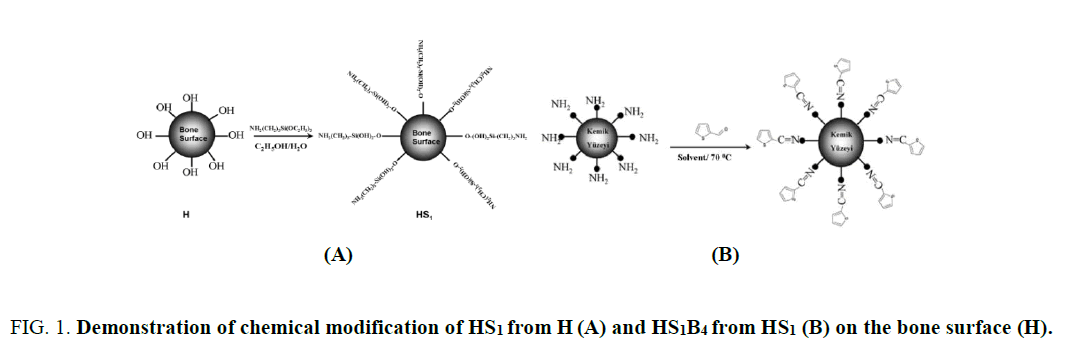
Modification scheme of HS1 and HS1B4 is shown in Figure 1. Silanization is known as the method of surface functionalizing. Silanization is performed with triethoxysisilane (C2H5)3-O-Si-R, silisium based organic molecule. Silanization is based on the reaction of active ethoxy silane groups (C2H5-Si-) with OH. These practices are successfully applied on silica, magnetic particles and surfaces of various materials [6,7]. The bone particles, whose surface is silanized with S1 (3-aminopropyl-triethoksisilan), are used in the reaction of Schiff base. Schiff base reactions were performed between amine groups (-NH2) existing on the surface of HS1 and aldehyde groups (-COH).
Figure 1: Evolution per year of water consumption.
Electro kinetics is the measurement of electricity load that is effective on colloidal dispersions or on the surface of particles and zeta potentials. It gives information about dispersion mechanism of particles on condition of repulsion and draw due to the surface loads of particles in colloidal systems. Measurement of zeta potential enables understanding important characteristics of colloidal, dispersion and aggregate systems, controlling and defining the potential or the load on particles. One of the most important reasons of determining is the measurement of the size of diffuse double layer around the particle. In Table 2, zeta potential load of all modified materials and surface conductivity values of these materials are given. Zeta potential of unmodified apatite (H) is determined as -20.4 mV. It is seen that zeta potential of HS1 and HS1B4 are 6.1 and is -6.9 mV. The HS1’s zeta potential is positive and this is due to the fact that there are the amine groups (-NH2) in the modified surface. The results of point of zero charge of H, HS1 and HS1B4 is given in Table 2. The experimental results of H, HS1 and HS1B4 were obtained as 7.2, 7.7 and 7.57, respectively. On the other hand, theoretical values of Pzc of H, HS1 and HS1B4 were found to be 6.6, 6.9 and 6.92. In Figure. 2, the curves between ΔpH and initial pH were given. It is obviously seen that each of H, HS1 and HS1B4 have different breakpoints in X- axis as Pzc. The best regression parameter is observed in H material as 0.99, and the lowest one is observed in HS1B4 material as 0.93.
| PzcD | PzcT | R2 | ZP (mV) | SC (mS cm-1) | BET | Langmuir | t-Plot | SPSA(m2g-1) | VP (cm3 g-1) | AWP (Å) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SA(m2g-1) | r | SA(m2g-1) | r | SA(m2g-1) | r | |||||||||
| H | 7.25 | 6,6 | 0,99 | -20,4 | 3,0×10-2 | 5,6 | 0,999 | 13,6 | 0,994 | 6,6 | 0,999 | 5,1 | 14,1×10-3 | 53,8 |
| HS1 | 7,72 | 6,9 | 0,96 | 6,1 | 3,1×10-2 | 3,0 | 0,997 | 7,5 | 0,997 | 3,5 | 0,999 | 2,8 | 5,0×10-3 | 35,8 |
| HS1B4 | 7,57 | 6,92 | 0,936 | -6,96 | 2,46×10-2 | 2,954 | 0,959 | 17,500 | 0,999 | 4,099 | 0,999 | 3,057 | 5,751×10-3 | 38,943 |
PZCD: Experimental PZC; PZCT: PZC and R2 values of experimental data calculated with linear regression; ZP: Zeta Potential; SC: Surface Conductivity; SPSA: Single Pointed Surface Area; VP: Volume of Pore; AWP: Average Width of Pore; SA: Surface Area.
Table 2: Determination of atomic fluorescence or atomic absorption of Fe and Mn (mg/kg).
FT-IR spectrums of H, HS1B1 and HS1B4 compounds are given in ( Figure 3). According to FT-IR data of the compounds, in the structure of natural hydroxyapatite (H) the peaks are observed as characteristic weak –OH in 3243 cm-1, aliphatic –CH2 in 2984 cm-1, Carbon yl (–C=O) in 1655 cm-1, CO3-2 in 1407 cm-1, intensive PO4-3 in 1038 cm-1 and weak C-H in 870 cm-1. In each HS1 compound vibration bands of CH2 groups, weak –OH in 3261 cm-1 and aliphatic in 2988 cm-1, were observed. In HS1 compound, the reason of the increase in the intense of peaks in 1643 cm-1 and 1557 cm-1 can be explained with the overlap of vibration stretches of Carbon yl group (–C=O) and imines (C=N–), and the increase in the intense of peak in 1018 cm-1 can also be explained with the overlap of vibration bands of Si-O and PO4-3 group.
Additionally, the reason of the rise in the intense of peak of PO4-3 in 1028 cm-1 can be explained with the overlap of vibration bands of PO4-3 and Si-O. In HS1B4 compound, it was observed that the intense increased due to unsaturated C–C bond stretches (because of aromatic circle) in 1651 cm-1, C=N stretch vibrations, N–H curve vibrations, and the overlap of Si–O vibration bands with the peak of PO4-3 group in 1026 cm-1.
In this study, surface areas and other data were determined by doing BET analyses from 11 different points. Measurement of surface area and porosity were performed with the method of BET in 77 K (-196°C) and in liquid Nitrogen thanks to the technique of Nitrogen gas (N2) adsorption. Using the volume of the gas adsorbed, parameters of multi-pointed surface area (with the method of BET), Langmiur surface area, t-Plot surface area, single-pointed surface area, average width of pore, volume of pore were automatically calculated. Calculated with different methods, surface areas of the materials are given in Table 2.
According to BET results, the highest surface area is determined as 5.6 m2/g in H. In Langmiur, t-plot and single-pointed models; the highest surface area is determined as 13.6, 6.6 and 5.1 m2/g in H. The fact that the surface area of HS1 and HS1B4materials is lower than those in H can be explained with the filling of pores on the surface of particle with modification materials. It is seen that the best regression parameter is in t-plot model, generally. In Table 2, volume of pore and average width of pore results of H, HS1 and HS1B4 are given. The volume of pore of H, HS1 and HS1B4 is found as 14.1, 5.0 and 5.75 × 10-3 cm3/g, respectively. The highest average width of pore is determined as 53.8 Å in H and the lowest one is determined as 35.8 Å in HS1.
Thermogravimetric analysis of the obtainment materials were made. For this purpose thermogravimetric analysis of unmodified bone (H) and each modified materials were made to investigation of mass loss versus temperature and compared. Changes were investigated with determining of the temperatures of each one of the materials, which belong to the 20% and 30% mass loss and materials are compared with each other. Main aim of determining of the temperatures of each one of the materials, which belong to the 20% and 30% mass loss is compare the temperatures in this percentages and to see if there are any differences or not. At the same time it was intended to determining of residual amount was from the modified molecules to the material or not. The amount of residue that may derive from modified molecule is usually Carbon residue. Thus, in the light of this obtained data, it was intended to provide information about whether there are modifications or not; depending on the loss mass of modified materials against the temperature. Thermal analysis results of modified materials are given in Table 3. According to the thermal data of the compound H, temperatures which belong to the 20% and 30% mass loss were obtained as 354°C (BS1) and 503°C (BS2), respectively. The total mass loss at 800°C was observed as 33.88% (RA) so the residual was 66.22%. According to the thermal data of the compound HS1, temperatures which belong to the 20% and 30% mass loss were obtained as 369°C and 586°C, respectively. The total mass loss at 800°C was observed as 32.35% so the residual was 67.65%. According to the thermal data of the compound HS1B4, temperatures which belong to the 20% and 30% mass loss were obtained as 375°C and 612°C, respectively. The total mass loss at 800°C was observed as 31.81% so the residual was 68.19%.
| C | N | S | TGA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 (%) |
C2 (mmol.g-1) |
CACC (μmol.g-1) |
N1 (%) |
N2 (mmol.g-1) |
CACN (μmol.g-1) |
S1 (%) |
S2 (mmol.g-1 |
CACS (μmol.g-1) |
%20 BS1 (°C) | %30 BS2 (°C) | RA (%) (800°C) | |
| H | 14,174 | - | - | 8,031 | - | - | 0,458 | - | - | 354 | 503 | 66,22 |
| HS1 | 14,816 | 0,535 | 178,33 | 8,291 | 0,186 | 185,85 | 0,457 | - | - | 369 | 586 | 67,65 |
| HS1B4 | 15,730 | 0,762 | 152,33 | 8,262 | - | - | 0,916 | 0,143 | 143,42 | 375 | 612 | 68,19 |
C1(%) ve N1(%):Percentage rate of carbon and nitrogen amount in material totally, C2(mmol/g) ve N2(mmol/g):Millimole amount of total carbon and nitrogen bonded on the surface per each bone (gram), BCAC, BCAN and BCAS (μmol/g):Micromole amount calculated over element C, N and S of compound amount bonded on the surface of HAP per each bone (gram); DT1 and DT2: Decomposition Temperature at 20% and 30% of mass loss; RA: Residue amount
Table 3: Elemental analysis and TGA Results of H, HS1 and HS1B4.
SEM was analyzed with using Carbon coating method. The magnification and accelerating voltage of instrument are between 40x and 300.000, 0.2 kV and 30 kV, respectively. Imaging analyses were carried out on powder samples. The magnification was between 25x and 1200x. EDS analysis of the all materials which obtained in this study were performed too. Images were taken with using the secondary electrons in SEM instrument. Carbon was excluded in EDS results because of the materials were coated with Carbon. SEM images and EDS analysis of H, HS1 and HS1B4 compounds are given in Figure 4. It is seen that surfaces of HS1 and HS1B4 vary according to H, when SEM images are examined. According to SEM image of H, the surface seems as if a tough rock. On the other hand, the surfaces have different layering due to the result of modification of H with S1 and HS1 with B4. The element compositions of H, HS1 and HS1B4 are given in EDS spectrums of Figure. 4. As it is seen in EDS analysis, N for HS1 and S for HS1B4 was observed on the surface, clearly. It is seen that Nitrogen (N) and Sulfur (S) elements that is determinant in S1 and B4 molecules have increased distinctively. EDS spectrums show that they occur on the surface of apatite with the existence of elements that are determinant in modification.
The percentage amount of total Carbon (C1), Nitrogen (N1) and Sulfur (S1) in the material, bonded micromole and millimole amount of compound using the percentage of Carbon and Nitrogen on the bone surface per g are given in Table 3. C1 (%), N1 (%) and S1 (%) expressions which are given in Table 3 symbolize the percentage amount of analysed Carbon , Nitrogen and Sulfur in the material. The micromole amounts that calculated using the percentage of Carbon , Nitrogen and Sulfur elements which were bonded on the bone surface per g were mentioned as CACC, CACN and CACS (μmol/g). The values represented by abbreviations in Table 3 are calculated as follows.
C2, N2 or S2 = [(ER – EM) × 1000]/(100 × MMa)
C2, N2 or S2: The millimole amount of compound using the percentage of Carbon (C) and Nitrogen (N) on the bone surface per g (mmol.g-1)
ER: Amounts of Carbon and Nitrogen in unmodified bone
ER: Amounts of Carbon and Nitrogen in unmodified bone
EM: Amounts of Carbon and Nitrogen in modified bone
1000: Conversion factor from mole to milimole
100: Conversion factor of elemental analysis results to gram material in terms of percentage
MMa: The molar mass of Carbon, Nitrogen and Sulfur (g/mol)
CACC, CACN and CACS=(C2, N2 or S2 1000x)/EM
Calculated amount of compound (CACC, CACN and CACS): The micromole amount of compound that calculated over total Carbon and Nitrogen elements in molecules which bonded on surface per bone (μmol.g-1)
1000: Conversion factor from micromole to milimole
EM: The number of Carbon or Nitrogen in the compound that bonded on the surface
According to the data in the Table 3, it was determined 14.2% Carbon (C), 8.0% Nitrogen (N) and 0.458% Sulfur (S) in unmodified bone (H). On the other hands, it is seen that total percentage of Carbon (C1) is 14.8% for HS1 and 15.73% for HS1B4. Considering the elemental analysis results of HS1 material that obtained after silanization process; it was determined that the percentage of total Carbon (C1) 14.81% was rising and besides this the amount of compound bonded on the surface per each bone (gram) that calculated over N element (BCAN) was obtained as 185.85 μmol/g in HS1 material. The percentage of total Carbon (C1) 15.73% were rising in HS1B4 material which obtained with reaction of HS1 material and 2-Thiophenecarboxaldehyde was determined. The percentage of Nitrogen and Sulfur were obtained as 8.26% and 0.91%, respectively. The amount of Sulfur in material that modified with 2-Thiophenecarboxaldehyde is an indicator of whether the compound bonded on surface or not. The amount of Sulfur was raised from 0.45 to 0.91 and also the amount of bonded compound on the surface of bone per gram with using percentage of Sulfur was calculated as 143.4 μmol/g. Overall, it is seen that the amount of bonded 2-Thiophenecarboxaldehyde compound was close to the amount of compound which bonded on HS1 material that obtained with silanization.
Conclusion
It is known that, today, environmental pollution reaches to dangerous levels for the people and environment because of the growing world population, technological development and uncontrolled industrialization. Besides this, it is seen that natural sources consume too quickly to meet the needs of rapidly growing world population. Most of the human, industrial and technological wastes are thrown to the environment without eliminating in a healthy way. Therefore many researches study about converting the waste materials to be usable materials in many different areas. This study revealed a general investigation of the fish bones in the fish processing plant waste can be utilized with a chemical processes. The physical and chemical properties of materials that obtained with surface modification are expected to be different. –NH2 groups in the materials of HS1 are found as free groups, and also –NH2 groups are reacted easily with especially aldehyde groups. For this purpose, the material obtained after silanization was reacted with 2-Thiophenecarboxaldehyde. According to the elemental analysis results, in the HS1 material which is silanizated bone surfaces and in the HS1B4 material which is modified with 2-Thiophenecarboxaldehyde, the amounts of bonded compounds per gram were calculated as 185 μmol for HS1 and 143 μmol for HS1B4. On the thermal analyses data, the residual amounts of H, HS1 and HS1B4 materials in 800°C were obtained as 66.2%, 67.6% and 68.1%, respectively. As a result, this study was shown that waste fish bones occurring waste and non-economic value as industrial is to be suitable for chemical modifications and it could be effectively converted to different materials. The converted to useful, economic, effective and qualified materials of waste products will contribute to the environment and economy with the development of research in this area.
Acknowledgements
The authors acknowledge TUBİTAK; this study was supported by TUBITAK Project No: 213M200.
References
- Kizilkaya B, Tekinay AA. Comparative study and removal of Co and Ni (II) ions from aqueous solutions using fish bones. Science of Advanced Materials. 2011;3(6): 949?61.
- Kizilkaya B, Tekinay AA, Dilgin Y. Adsorption and removal of Cu (II) ions from aqueous solution using pretreated fish bones. Desalination. 2010;264(1-2): 37?47.
- Dimovic S, Smiciklas I, Plecas I. et al. Comparative study of differently treated animal bones for Co2+ removal. Journal of HazardousMaterials. 2009;164 (1): 279?87.
- Tan E, Kizilkaya B, Üçyol N. Surface modification with P-aminohippuric acid on biogenic apatite (fish bones) particles Mar Sci Tech Bull. 2014;3(2):45-50.
- Hong Z, Zhang P, Liu A, et al. Composites of poly(lactide-co-glycolide) and the surface modified carbonated hydroxyapatite nanoparticles. Journal of Biomedical Materials Research Part A. 2007; 81(3):515-22.
- Wei J, Liu A, Chen L, The surface modification of hydroxyapatitenanoparticles by the ring opening polymerization of g-Benzyl-L-glutamate N-carboxyanhydride. MacromolBiosci. 2009;9(7): 631?38.
- Murugan R, Ramakrishna S. Coupling of therapeutic molecules onto surface modified coralline hydroxyapatite. Biomaterials. 2004;25(15): 3073?80.
- Banat F, Asheh SA, Mohai F. Batch Zinc removal from aqueous solution using dried animal bones. 2000; Separation and Purification Technology. 21: 155-64.
- Chojnacka K. Equilibrium and kinetic modeling of Chromium(III) sorption by animal bones. Chemosphere 2005;59 (3): 315?20.
- Alasbeb S, Banat F, Mobai F. Sorption of Copper and Nickel by spent animal bones. Chemosphere. 1999; 39(12): 2087-96.