Original Article
, Volume: 15( 4)Therapeutic Potential of Chromones
- *Correspondence:
- Pandey J
Department of Chemistry, Amity University, Lucknow Campus, Lucknow, Uttar Pradesh, India
Tel: 9415325569
E-mail: jpandey@lko.amity.edu
Received: July 09, 2017; Accepted: September 27, 2017; Published: September 30, 2017
Citation: Kulshrestha A, Rupanwal R, Singh N, et al. Therapeutic Potential of Chromones. Int J Chem Sci. 2017;15(4):200.
Abstract
Chromones are found to be natural or natural origin. Several studies have been performed on the types of reagents utilized for the cyclization of chalcones to chromene-4-ones. Chromones are recognized as pharmacophores having a number of biological activities such as anticancer, antiviral, antifungal, antimicrobial, antioxidant, antidepressant and antiobesity etc. There are various synthetic methods reported. Therapeutic potential and brief discussion on synthesis of chromones are reviewed.
Keywords
Chromones; Anticancer; Antibacterial; Anti-HIV; Chalcones
Introduction
Chromone moiety forms the important component of pharmacophores of a number of biologically active molecules having synthetic or natural origin and many of them have useful medicinal applications. Chromones are the hetrocyclic compounds with benzopyron network with substituted keto group on pyron ring. Chromone is an isomer of coumarin. Chromone derivatives have a number of biological activities such as anticancer, antihypertensive, antiviral, antifungal, antimicrobial, antioxidant, antidepressant, anti-obesity. These derivatives also possess enzymetic inhibition properties towards different systems such as oxidoreductase, kinase, lipoxygenase and cycloxygenase.
Chemistry
The most conventional and common method for the cyclization is through an oxidative ring closure with bromine and a base or by refluxing with SeO2 in dioxane [1]. In the reaction of chalcone with bromine, besides bromine addition to the olefinic bond, nuclear bromination at position 5 also occurs resulting in the bromoflavone [2] Chalcone dibromide is converted into chromone by the action of pyridine [3]. Ray and Dutta converted chalcone dibromide into the corresponding chromone by heating above the melting point [4]. Rao et al. studied the cyclization of chalcones with DDQ and found that even a slight variation in reaction conditions led to the formation of a mixture of chromone and chroman-4-one [5].
Yao et al. synthesized nitro chromones through regioselective nitration of chalcones followed by cyclization catalyzed by I2-DMSO [6]. Huang et al. improved the total synthesis of baicalein, wogonin, and oroxylin from chalcones using I2-DMSO as the cyclizing agent [7] and Lokhande et al. also cyclized 2-allyloxychalcones to chromones using I2-DMSO [8]. Alam studied the conversion of methylenedioxy chalcones utilizing diphenyl sulphide, I2-DMSO or DDQ and found that cyclization with I2-DMSO improve the yields, while diphenyl sulphide gave the lowest yields [9].
Kumar and Perumal demonstrated the use of ferric chloride in the oxidative cyclization of o-hydroxychalcones to chromones [10]. Kumazawa et al. prepared a naturally occurring 6-C-glucosylated derivative and the 8-C-glucosylated compound via the cyclization of chalcones [11,12].
Ganguly et al. prepared 3-acylchromones through a modified Baker-Venkataraman rearrangement using DBU and pyridine via a triketo intermediate [13] these 3-acylflavones were then converted into chromones by refluxing with aqueous K2CO3. Similarly, Pinto et al. used K2CO3-pyridine under microwave assisted conditions to prepare β-diketone intermediates, which were cyclized to give 3-aroylchromones [14].
A synthesis of flavones from ortho-methoxy acetophenones via a two-step process, which utilizes only 1 equiv of LDA to form the lithium enolate has also been reported. The enolate is reacted with benzoyl cyanide to give β-diketone, which on treatment with HI yields the chromones [15]. Creuzet et al. conventionally prepared 3-Hydroxychromones from a methoxy- or benzyloxy-o-hydroxyacetophenone via Baker-Venkataraman rearrangement forming the b-diketones, which undergo cyclization [16,17].
Hageman et al. synthesized 3-Hydroxychromones from chalcones. In this method, o-hydroxychalcone was subjected to base catalyzed epoxidation of the conjugated double bond [Algar-Flynn-Oyamada (AFO)] oxidation followed by ring closure and aromatization to yield hydroxyl chromones [18].
Auwers et al. reported the synthesis of 3-hydroxychromones from aurone via a series of reactions commonly known as the Auwers synthesis [19]. Bromination of the alkene yields a dibromo adduct, which rearranges to hydroxychromone by treatment with KOH via various intermediates. The 4-O-alkyl-derivatives of 5, 7-dihydroxy-chromones were prepared by cyclization of the corresponding chalcones with sodium acetate followed by oxidation of the crude intermediates (chroman-4-ones) with a catalytic amount of iodine in pyridine [20].
7-O-alkyl derivatives of chromones have been prepared by various workers. Babu et al. prepared 7-O-alkylated derivatives of chrysin, (5, 7-dihydroxychromones) having antibacterial activity using alkyl halides and K2CO3 [21]. Shin et al. also attempted to synthesize regioselective 7-O-alkyl derivatives of chrysin using K2CO3 or KHCO3, but obtained a mixture of mono- and di-O-alkylated products [22]. They successfully tried to form 7-O-esters using DCC and DMAP. It was concluded that esterification occured primarily at C7 because of the shielding of the 5-hydroxy by the 4-keto group.
Jain et al. also attempted nuclear iso-prenylation of chrysin by refluxing it with prenyl bromide, but the results were not satisfactory due to poor yields [23] Comte et al. prepared C-prenylated chromones in low yields by microwave irradiation on a solution of chrysin and tetramethyl ammonium hydroxide in methanol containing tetraethylammonium iodide [24].
Marta Perro Neves et al. successfully synthesized dihydropyranochromones by one-pot synthesis, using Montmorillonite K10 clay as catalyst under microwave irradiation [25]. Ramesh Kamboj et al. synthesized 3-alkoxy-6-chloro-2-(3-methylthiophen- 2-yl)-4H-chromen-4-ones in methanol [26] with pyrex filtered UV-light led to the formation of tetracyclic compounds.
A series of bromo-substituted 3-aroyl flavanones and flavones have been synthesized [27]. The identities of the new compounds synthesized have been developed on the basis of usual chemical transformation and IR, NMR spectral studies. The diisochromenochromen-4-one has been prepared from the photocyclization reaction of bischromen-4-one. The later compounds are obtained from the O-alkylation of the suitable 3-hydroxy-2-aryl-4H-chromen-4-one with bischloromethyldiphenyl in dry acetone, anhydrous K2CO3, and PTC (Bu4N+I−) under refluxing conditions [28].
Liu Xin-Hua et al. synthesized ten novel 3-(2-(3-methyl-5-substituted-phenyl-4,5-dihydropyrazol-1-yl)-2-oxo-ethoxy)-2- substituted-phenyl-4H-chromen-4-one derivatives [29]. Eeda Venkateswararao et al, a series of (E)-5-alkoxy-3-(3-phenyl-3- oxoprop-1-enyl)-4H-chromen-4-ones and (E)-5-alkoxy-3-(3-hydroxy-3-phenylprop-1-enyl)-4H-chromen-4-ones were synthesized [30]. Qiao Ren et al. synthesized 6, 7-Dimethyl-3-[(methyl-2-(methyl-(1-3-trifluromethyl-phenyl)-1H- indol-3- yl-methyl)- amino)- ethyl)-chromen-4-one drug that prevent TNF-α binding to its receptor [31].
Biological activities of chromene-4-ones
Antibacterial and antifungal activities
Bingi et al. synthesized a number of 3-hydroxy-6-(hydroxymethyl)-2-(2-phenyl-4H-chromen-4-yl)-4H-pyran-4-ones in a one pot catalyst free reaction of 2-hydroxy chalcone with kojic acid in toluene at reflux temperature and studied their biological activities. The compounds showed potent antimicrobial activity against various strains of bacteria [32].
Hatzade et al. attempted a convenient route to synthesis 7-O-β-D-glucopyranosyloxy-3-(3-oxo-3-arylprop-1-enyl)-4Hchromene- 4-ones. These compounds were evaluated for antibacterial and antifungal activities [33].
Javed Sheikh et al. reported computational evaluation and experimental verification of 7-hydroxy-3-(1-phenyl-3-aryl-1Hpyrazol- 5-yl)-4H-chromen-4-ones and their O-β-D-glucopyranosides for their antimicrobial and antioxidant activity [34]. Palakuri Kavitha et al. synthesized tridentate 3-formyl chromone Schiff bases of Ni(II) and Zn(II) such as 3-((2- hydroxyphenylimino)methyl)-4H-chromen-4-one, 3-((3-hydroxypyridin-2-ylimino)methyl)-4H-chromen-4-one and 3-((2- mercaptophenylimino)methyl)-4H-chromen-4-one which exhibited pronounced activity against tested bacteria and fungi strains compared to the ligands [35].
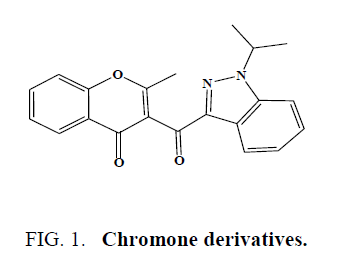
Kale et al. synthesized and characterized some of the chromone derivatives as antimicrobial agents (Figure 1) [36].
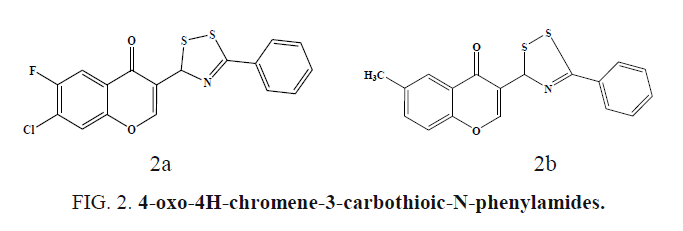
Chromone conjugated dithiazoles and 4-oxo-4H-chromene-3- carbothioic-N-phenylamides were synthesized and screened for antibacterial and antifungal by disk-diffusion assay. The dithiazole derivative (Figure 2a) bearing electron withdrawing (-F, - Cl) groups at C6 and C7 positions shows high antifungal activity in comparison to fluconazole. For Gram Positive bacteria S. aureus, maximum growth inhibition of 92.72% was observed for in Figure 2b [37].
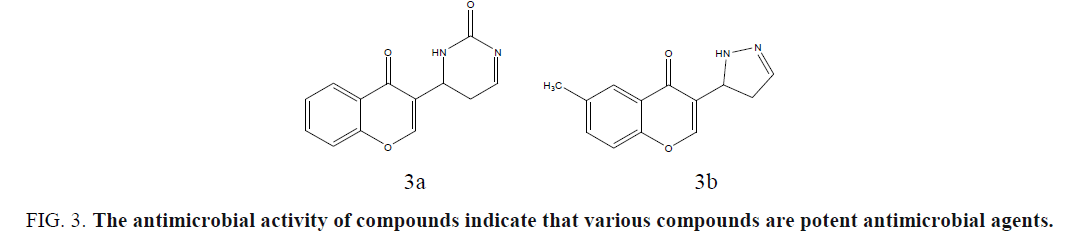
Musthafa et al. developed chromone fused pyrazolines, pyrazoles, dibromo derivatives and dihydropyrimidines, under microwave irradiation, and evaluated for in vitro antibacterial activity against an assortment of two Gram-positive bacteria, S. aureus, B. subtilis, and two Gram-negative bacteria, E. coli, Salmonella typhimurium, in vitro antifungal activity was tested against three fungal strains, C. albicans, A. niger and Aspergillus fumigatus. The antimicrobial activity of compounds indicates that various compounds are potent antimicrobial agents (Figure 3a and 3b) [38].
Figure 3: The antimicrobial activity of compounds indicate that various compounds are potent antimicrobial agents.
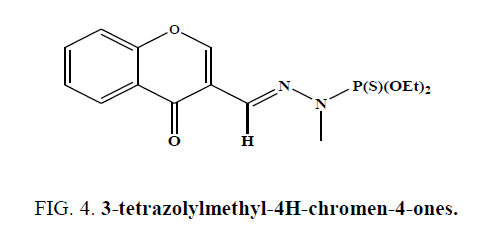
Nawrot-Modranka et al. synthesized chromone derivatives and studied there in vitro antibacterial activity (Figure 4) [39].
Pedro et al. synthesized novel 3-tetrazolylmethyl-4H-chromen-4-ones via multicomponent reaction and studied their biological evaluation against Entamoeba histolytica, Giardia lamblia and Trichomona vaginalis [40].
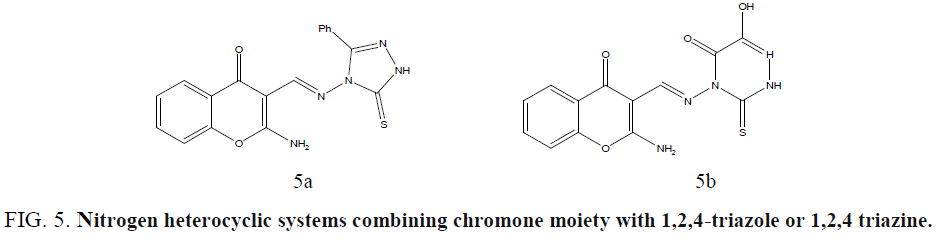
Ibrahima coworkers synthesized new nitrogen heterocyclic systems combining chromone moiety with 1,2,4-triazole or 1,2,4 triazine in one molecular frame through an azomethine linkage and evaluated in vitro for their antimicrobial activities, using the disc-agar diffusion method, against S. aureus and Streptococcus pyogenes as Gram positive bacteria, Pseudomonas fluorescens and Pseudomonas phaseolicola as Gram-negative bacteria, and the fungi F. oxysporum and A. fumigatus [41]. Compounds showed high activity toward the tested fungi. (Figure 5a and 5b).
Figure 5: Nitrogen heteroycclic systems combining chromone moiety with 1,2,4-triazole or 1,2,4 triazine.
Anti-cancer activity
Liu T et al. synthesized chromone analogues bearing heterocyclic thioether moiety and were assayed for their antitumor activity. Out of these, IC50 of 3- (benzothiazole-2-ylsulfanyl)-chromen-4-one against MDA-MB-435S was found out to be 17.2 μM. They concluded that the presence of heterocyclic thioether or cyclic tertiary amine will benefit the antitumor activities of chromones [42].
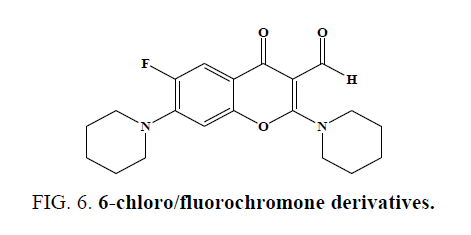
Three methylated quercetins and a series of O-3-substituted, tetra-O-methylated quercetin derivatives have been synthesized by Jian Yuan et al. and studied their anticancer activity [43]. He Huang et al. successfully prepared a new type of quercetin derivatives, the novel compounds show higher selectivity as inhibitors against Src tyrosine kinase. (IC50 values ranging from 3.2 mM to 9.9 mM) than against EGFR tyrosine kinase. Molecular docking revealed that both hydrophobic and hydrogen bonding interactions are important to the selectivity [44]. Guo-Biao Liu et al. synthesized (5, 7-dihydroxy-4-oxo-4Hchromen- 3-yl) methyl esters and evaluated LPS-activated murine macrophages cell culture systems and cytotoxicity of these compounds was checked using MTT assay [45]. Liu Xin-Hua et al. synthesized ten novel 3-(2-(3-methyl-5-substitutedphenyl- 4,5-dihydropyrazol-1-yl)-2-oxo-ethoxy)-2-substituted-phenyl-4H-chromen-4-one derivatives and screened for their anticancer activity (Figure 6). The bioassay tests show that compounds exhibited potentially high activity against human gastric cancer cell SGC-7901 [46]. Ishar et al. Synthesized and evaluated novel 6-chloro-/fluorochromone derivatives as potential topoisomerase inhibitor anticancer agents [47].
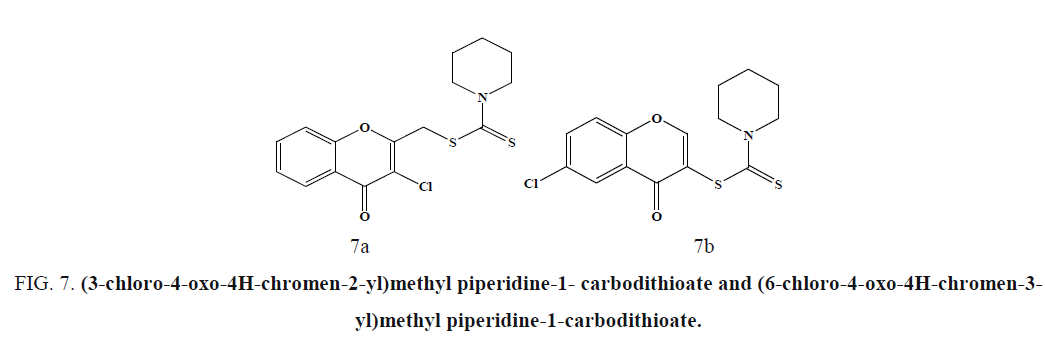
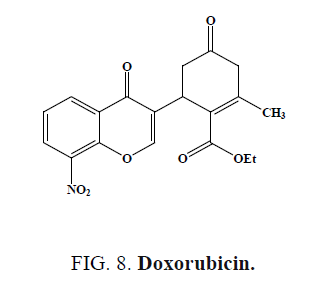
The compounds (3-chloro-4-oxo-4H-chromen-2-yl)methyl piperidine-1-carbodithioate (Figure 7a) and (6-chloro-4-oxo-4Hchromen- 3-yl)methyl piperidine-1-carbodithioate (Figure 7b), showed the most promising antitumor activity against SW-480 cells and MDA-MB-435 cells [48]. Compound exhibited cytotoxicity against human neuroblastoma cell line compared to the standard drug Doxorubicin (Figure 8) [49].
Figure 7: (3-chloro-4-oxo-4H-chromen-2-yl)methyl piperidine-1- carbodithioate and (6-chloro-4-oxo-4H-chromen-3- yl)methyl piperidine-1-carbodithioate.
Anti-inflammatory activity
Hasan and researcher synthesized 6-Aminomethyl-2-aryl-1-benzopyran-4-one derivatives and tested for anti-inflammatory, analgesic, ulcerogenic, and lipid peroxidation actions. Among the tested compounds, two compounds showed higher degree of anti-inflammatory activity [50]. Khan et al. effectively synthesized and characterized 3-formylchromone and its derivatives. Anti-inflammatory activities were studied of these compounds [51].
Anti-HIV activity
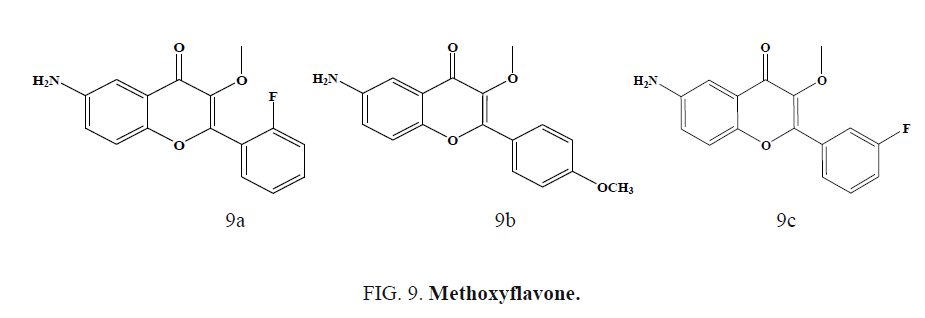
Casano et al. developed and synthesized a series of methoxy flavones and studied their antiproliferative activity on Plasmodium falciparum parasites and anti-HIV activity. Methoxyflavone (Figure 9a) was active in both P. Falciparum and HIV-1 whereas compounds (Figure 9b) and (Figure 9c) were specific inhibitors of the HIV-2 multiplication. Author suggested that para substitution on the B ring is needed to promote antiplasmodial activity and increase HIV-2 potency [52].
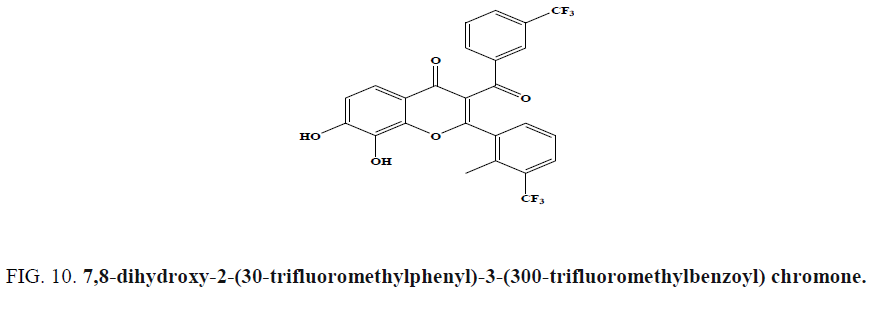
Among a series of benzopyran-4-one scaffolds synthesized by Ungwitayatorn et al. via one pot cyclization reaction, 7,8- dihydroxy-2-(30-trifluoromethylphenyl)-3-(300-trifluoromethylbenzoyl) chromone (Figure 10) showed HIV-1 protease inhibition in vitro [53].
Antioxidant activity
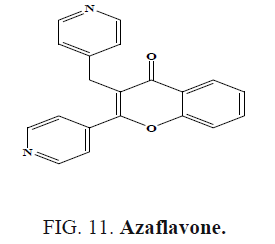
Yasar et al. synthesized azaflavone (Figure 11) and evaluated their antioxidant activities and antimicrobial activities [54].
Anti-malarial activity
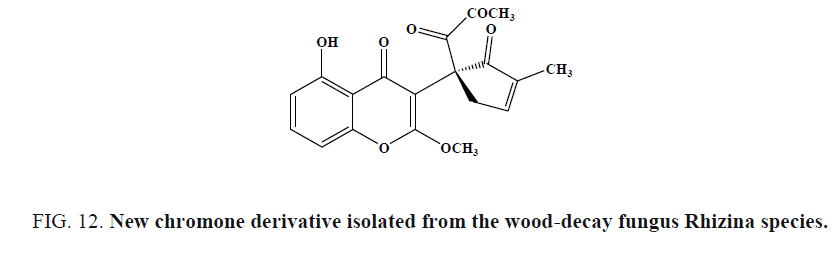
A new chromone derivative (Figure 12) was isolated from the wood-decay fungus Rhizina species by Isaka et al. and evaluated for antimalarial activity against P. falciparum K1. This derivative exhibited antimalarial activity with an IC50 of 5.1 mg/mL.
Anti-convulsant activity
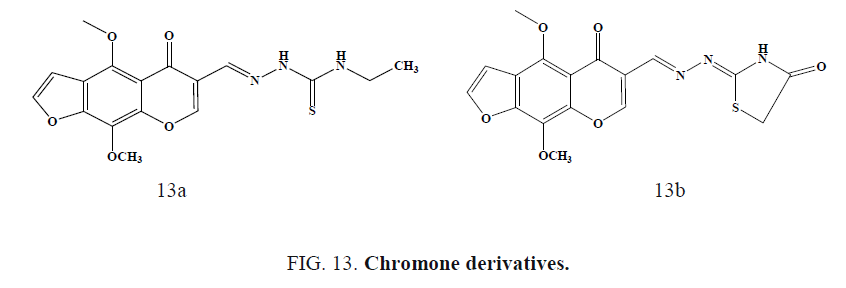
Following Figure 13a and 13b chromone showed 100% protection of 300 mg/kg in scPTZ test. In the MES test, all the tested compounds were inactive showing no protection against the seizures induced even up to a dose of 300 mg/kg body weight.
Anti-platelet activity
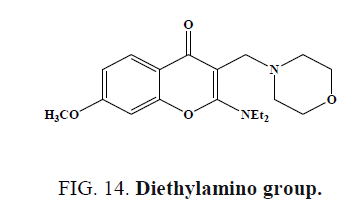
Highest activity was found, when the 2-amino substituent of tested chromones (Figure 14) was a diethylamino group Activity was increased, when the presence of electron releasing substituents (-OH, -OCH3, -CH3) was at position 7, whereas a decrease occurred when an electron withdrawing substituent was present in position (3-NO2) or (6-NO2, 6-Cl).
Gastroprotective activity
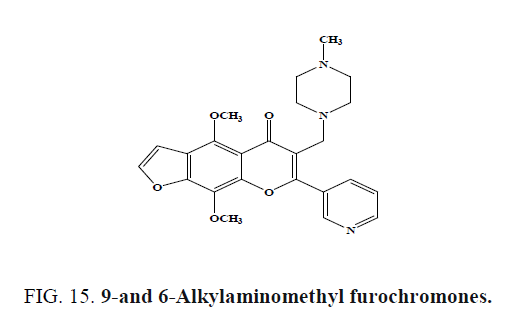
9-and 6-Alkylaminomethyl furochromones (Figure 15) synthesized from the naturally occurring chromones visnagin and khellin were screened for gastroprotective activity in the rat ethanol-induced damage model. The presence of methoxy group (either in 4, 9 or 7-position as methoxyphenyl) and through the appropriate substitution in 6-position with alkylaminomethyl group, furochromones exhibited good gastroprotective activity in the ethanol damage model.
H1 Anti-histaminic activity
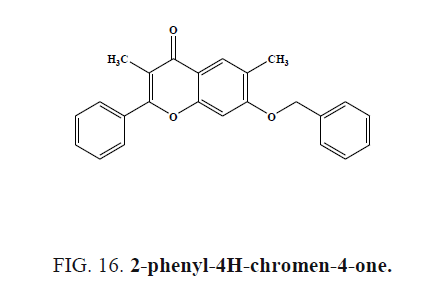
2-phenyl-4H-chromen-4-one (Figure 16) analogs were evaluated for the H1 antihistaminic activity computational method, the compounds showed highest antihistaminic activity.
Antihypertensive activity
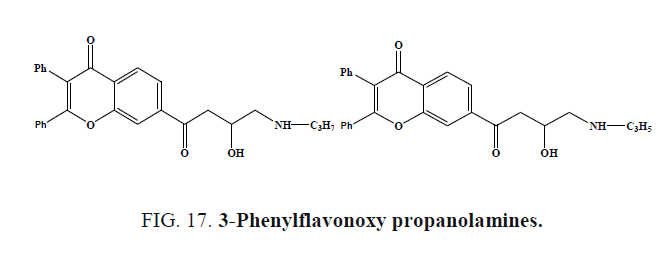
Wu et al. synthesized 3-Phenylflavonoxy propanolamines (Figure 17a and 17b) and evaluated for potential antihypertensive activity in spontaneously hypertensive rats as well as for in vivo and in vitro evidence of β-adrenoceptor antagonism. Compounds were active in lowering blood pressure at 8 mg/kg.
m-calpain inhibition activity
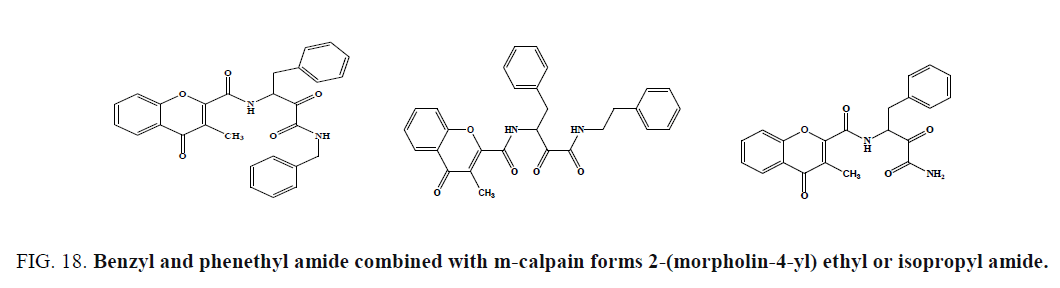
Lee et al. prepared chromone carboxamide derivatives and evaluated for m-calpain inhibition using a casein-coomassie blue microplate assay. Compound (Figure 18c), was the most potent calpain inhibitor of this series (IC50¼ 0.24 mM), exhibited 14.4% and 22.4% inhibition, demonstrating high selectivity of chromone derivatives for m-calpain. The introduction of dioxane ring in the chromone ring generally resulted in the decreased inhibitory activity of the parent compound, irrespective of amide; however, amide substituents were also important in the activity.
Figure 18: Benzyl and phenethyl amide combined with m-calpain forms 2-(morpholin-4-yl) ethyl or isopropyl amide.
The compounds (Figure 18) possessing benzyl and phenethyl amide showed good inhibition of m-calpain, while the potencies were decreased about 10-fold when these substituents were replaced by 2-(morpholin-4-yl) ethyl or isopropyl amide.
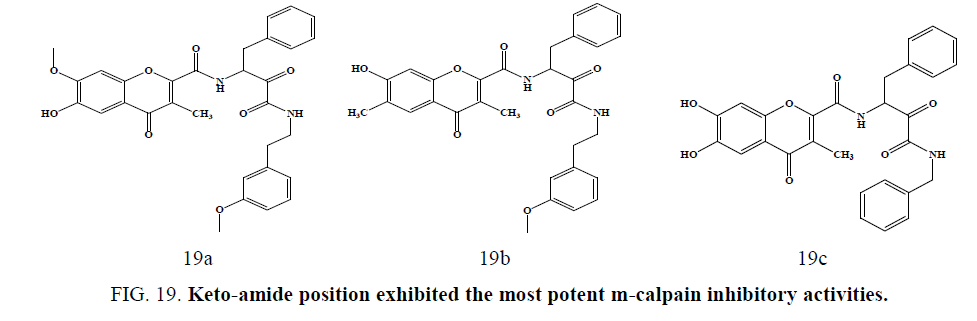
New chromone carboxamide derivatives were synthesized and evaluated using human calpain I isolated from erythrocytes. Compounds with 4-methoxyphenethyl group at the keto-amide position, i.e., (Figure 19a) and (Figure 19b) exhibited the most potent m-calpain inhibitory activities (IC50¼ 0.09e0.10 mM), and compound (Figure 19c) showed both potent m-calpain inhibitory activity (IC50¼ 0.28 mM) and antioxidant activities in DPPH scavenging and lipid peroxidation inhibition assay reported by Kim et al. [54].
Glutathione reductase activity
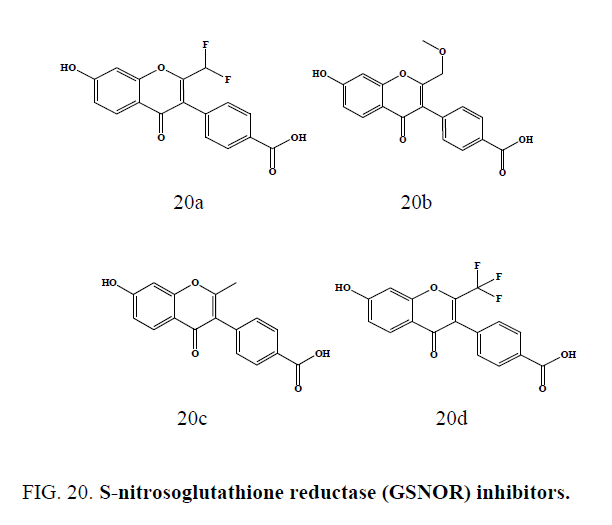
A series of chromone compounds (Figure 20a-20d) were synthesized as S-nitrosoglutathione reductase (GSNOR) inhibitors by Sun et al. These GSNOR inhibitors can be utilized in any pharmaceutically acceptable dosage form, including but not limited to injectable. Some of the compounds (Figure 20a to 20c) have showed IC50 less than 0.5 mM and compound (20d) showed IC50 less than 0.1 mM against the GSNOR inhibitors [55].
Anti-allergic activity
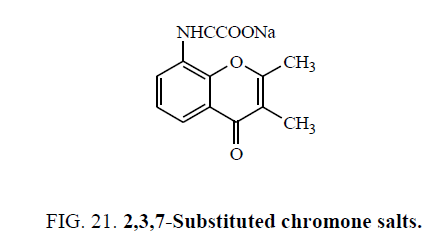
Abram et al. [56] synthesized 2,3,7-Substituted chromone salts (Figure 21) and screened for their antiallergic activity. All compounds tested exhibited oral antiallergic activity when ad ministered at a concentration of 30 mg/kg.
Conclusion
Therapeutic potential and synthetic brief of chromones are reviewed. There have been several synthetic methods developed. Noticeably, cyclization of chalcones to chromene-4-ones is one of the most utilized synthetic tools used in the synthesis of chromones. These moieties are recognized as pharmacophores having a number of biological activities such as anticancer, antiviral, antifungal, antimicrobial, antioxidant, antidepressant, anti-HIV and antiobesity etc.
Acknowledgement
The authors are thankful to the managements of Dr. Ambedkar College, Nagpur and Amity University, Lucknow campus for supporting this research work. Two of the authors, JP and AK are thankful to DST, New Delhi for financial support (Grant registration# CS-236/2013).
References
- Rao YK, Fang SH, Tzeng YM. Synthesis, growth inhibition, and cell cycle evaluations of novel flavonoid derivatives. Bioorganic & medicinal chemistry. 2005 Dec 15;13(24):6850-5.
- Yao N, Song A, Wang X, et al. Synthesis of flavonoid analogues as scaffolds for natural product-based combinatorial libraries. Journal of combinatorial chemistry. 2007 Jul 9;9(4):668-76.
- Huang WH, Chien PY, Yang CH, et al. Novel synthesis of flavonoids of Scutellaria baicalensis Georgi. Chemical and pharmaceutical bulletin. 2003;51(3):339-40.
- Lokhande PD, Sakate SS, Taksande KN, et al. Dimethylsulfoxide–iodine catalysed deprotection of 2′-allyloxychalcones: synthesis of flavones. Tetrahedron letters. 2005 Feb 28;46(9):1573-4.
- Alam S. Synthesis, antibacterial and antifungal activity of some derivatives of 2-phenyl-chromen-4-one. Journal of Chemical Sciences. 2004 Nov 1;116(6):325-31.
- Kumar KH, Perumal PT. A novel one-pot oxidative cyclization of 2′-amino and 2′-hydroxychalcones employing FeCl 3· 6H 2 O–methanol. Synthesis of 4-alkoxy-2-aryl-quinolines and flavones. Tetrahedron. 2007 Sep 17;63(38):9531-5.
- Ganguly AK, Kaur S, Mahata PK, et al. Synthesis and properties of 3-acyl-γ-pyrones, a novel class of flavones and chromones. Tetrahedron Letters. 2005 Jun 6;46(23):4119-21.
- Pinto DC, Silva AM, Cavaleiro JA. Baker-venkataraman rearrangement under microwave irradiation: A new strategy for the synthesis of 3-aroyl-5-hydroxyflavones. Synlett. 2007 Jul;2007(12):1897-900.
- Lee JI, Son HS, Jung MG. A novel synthesis of flavones from 2-methoxybenzoic acids. Bulletin of the Korean Chemical Society. 2005;26(9):1461-3.
- Fukui K, Nakayama M, Horie T. Synthetic studies of the flavone derivatives. XVII. Synthesis of 5, 7-dihydroxy-6-methoxyflavone derivatives. Bulletin of the Chemical Soc of Japan. 1970 May;43(5):1524-9.
- Van Acker FA, Hageman JA, Haenen GR, et al. Synthesis of novel 3, 7-substituted-2-(3‘,4‘-dihydroxyphenyl) flavones with improved antioxidant activity. J Med Chem. 2005;43(20):3752-60.
- Lee YJ, Wu TD. Total synthesis of kaempferol and methylated kaempferol derivatives. Journal of the Chinese Chemical Society. 2001;48(2):201-6.
- Babu KS, Babu TH, Srinivas PV, et al. Synthesis and biological evaluation of novel C(7) modified chrysin analogues as antibacterial agents. Bioorg and Med Chem Let. 2006;16(1):221-4.
- Shin JS, Kim KS, Kim MB, et al. Synthesis and hypoglycemic effect of chrysin derivatives. Bioorg & Med Chem Let. 1999;9(6):869-74.
- Jain AC, Sharma BN. Synthesis of isopentenylated chrystins. The J Org Chem. 1974;39(8):1149-52.
- Comte G, Daskiewicz JB, Bayet C, et al. C-Isoprenylation of flavonoids enhances binding affinity toward P-glycoprotein and modulation of cancer cell chemo resistance. J Med Chem. 2001;44(5):763-8.
- Neves MP, Cidade H, Pinto M. Prenylated derivatives of baicalein and 3, 7-dihydroxyflavone: synthesis and study of their effects on tumor cell lines growth, cell cycle and apoptosis. Eur J Med Chem. 2011;46:2562-74.
- Kamboj RC, Sharma G, Kumar D, et al. Photocyclisation of 3-alkoxy-6-chloro-2-(3-methylthiophen-2-yl)-4H-chromen-4-ones. Comptes Rendus Chimie. 2012;15(4):311-6.
- Mahalle PR, Khaty NT. Synthesis of some bromo-substituted 3-aroyl flavanones and flavones. J Chem. 2010;7(4):1359-61.
- Yusuf M, Solanki I, Jain P. Photoinduced synthesis of new diisochromenochromen-4-ones and their antimicrobial activities. The Sci World J. 2012.
- Liu XH, Li J, Wu FR, et al. Novel 3-(2-(3-methyl-5-substituted-phenyl-4, 5-dihydropyrazol-1-yl)-2-oxoethoxy)-2-substituted-phenyl-4H-chromen-4-one: Synthesis and anticancer activity. Medicinal Chemistry. 2011;7(6):605-10.
- Venkateswararao E, Kim MS, Sharma VK, et al. Identification of novel chromenone derivatives as interleukin-5 inhibitors. Eur J Med Chem. 2013;59:31-8.
- Ren Q, Siau WY, Du Z, et al. Expeditious assembly of a 2‐amino‐4H‐chromene skeleton by using an enantioselective mannich intramolecular ring cyclization–tautomerization cascade sequence. Chem-a Euro J. 2011;17(28):7781-5.
- Seo S, Lee HY, Park M, et al. Fluorescein‐functionalized silica nanoparticles as a selective fluorogenic chemosensor for Cu2+ in living cells. European Journal of Inorganic Chemistry. 2010 Feb 1;2010(6):843-7.
- Hatzade KM, Taile VS, Gaidhane PK, et al. Synthesis and biological activities of new 7-O-D-glucopyranosyloxy-3-(3-oxo-3-arylprop-1-enyl)-chromones. Ind J of Chem 2009: 1548-57.
- Sheikh J, Hatzade K, Bader A. et al. Computational evaluation and experimental verification of antibacterial and antioxidant activity of 7-hydroxy-3-pyrazolyl-4H-chromen-4-ones and their o-glucosides: Identification of pharmacophore sites. Med Chem Res. 2014;23:243-51.
- Kavitha P, Reddy LK. Synthesis, structural characterization, and biological activity studies of Ni (II) and Zn (II) complexes. Bioinorg Chem App. 2014;2014.
- Kale SB, Karale BK. Synthesis and characterization of some important indazolyl derivatives. J Het Chem. 2007;44(2):289-301.
- Raj T, Bhatia RK, Sharma RK, et al. Mechanism of unusual formation of 3-(5-phenyl-3H-[1,2,4] dithiazol-3-yl) chromen-4-ones and 4-oxo-4H-chromene-3-carbothioic acid N-phenylamides and their antimicrobial evaluation. Eur J Med Chem. 2009;44:3209-16.
- Siddiqui ZN, Musthafa TM, Praveen S. Solvent-and catalyst-free synthesis of bis-adducts of 3-formylchromone as potential antimicrobial agents. Med Chem Res. 2013;22(1):127-33.
- Nawrot-Modranka J, Nawrot E, Graczyk J. In vivo antitumor, in vitro antibacterial activity and alkylating properties of phosphorohydrazine derivatives of coumarin and chromone. Eur J Med Chem. 2006;41(11):1301-9.
- Cano PA, Islas-Jácome A, González-Marrero J, et al. Synthesis of 3-tetrazolylmethyl-4H-chromen-4-ones via Ugi-azide and biological evaluation against Entamoeba histolytica, Giardia lamblia and Trichomona vaginalis. Bioorg Med Chem. 2014;22(4):1370-6.
- Ibrahim MA, El-Mahdy KM. Synthesis and antimicrobial activity of some new heterocyclic Schiff bases derived from 2-amino-3-formylchromone. Phosphorus, Sulfur, and Silicon. 2009;184(11):2945-58.
- Liu T, Xu Z, He Q, et al. Nitrogen-containing flavonoids as CDK1/Cyclin B inhibitors: Design, synthesis, and biological evaluation. Bioorg and Med Chem Lett. 2007;17(1):278-81.
- Yuan J, Wong IL, Jiang T, et al. Synthesis of methylated quercetin derivatives and their reversal activities on P-gp-and BCRP-mediated multidrug resistance tumour cells. Eur J Med Chem. 2012;54:413-22.
- Huang H, Jia Q, Ma J, et al. Discovering novel quercetin-3-O-amino acid-esters as a new class of Src tyrosine kinase inhibitors. Eur J Med Chem. 2009;44(5):1982-8.
- Liu GB, Xu JL, Geng M, et al. Synthesis of a novel series of diphenolic chromone derivatives as inhibitors of NO production in LPS-activated RAW264. 7 macrophages. Bioorg & Med Chem. 2010;18(8):2864-71.
- Liu XH, Li J, Wu FR, et al. Novel 3-(2-(3-methyl-5-substituted-phenyl-4, 5-dihydropyrazol-1-yl)-2-oxoethoxy)-2-substituted-phenyl-4H-chromen-4-one: Synthesis and Anticancer Activity. Med Chem. 2011;7(6):605-10.
- Ishar MP, Singh G, Singh S, et al. Design, synthesis, and evaluation of novel 6-chloro-/fluorochromone derivatives as potential topoisomerase inhibitor anticancer agents. Bioorg & Med Chem Lett. 2006;16(5):1366-70.
- Huang W, Ding Y, Miao Y, et al. Synthesis and antitumor activity of novel dithiocarbamate substituted chromones. Eur J Med Chem. 2009;44(9):3687-96.
- Raju BC, Rao RN, Suman P, et al. Synthesis, structure–activity relationship of novel substituted 4H-chromen-1, 2, 3, 4-tetrahydropyrimidine-5-carboxylates as potential anti-mycobacterial and anticancer agents. Bioorg and Med Chem Lett. 2011;21(10):2855-9.
- Hasan SM, Alam MM, Husain A, et al. Synthesis of 6-aminomethyl derivatives of benzopyran-4-one with dual biological properties: Anti-inflammatory-analgesic and antimicrobial. Eur J Med Chem. 2009;44(12):4896-903.
- Khan KM, Ambreen N, Mughal UR, et al. 3-Formylchromones: Potential anti-inflammatory agents. Eur J Med Chem. 2010;45(9):4058-64.
- Casano G, Dumètre A, Pannecouque C, et al. Anti-HIV and antiplasmodial activity of original flavonoid derivatives. Bioorg and Med Chem Lett. 2010;18(16):6012-23.
- Ungwitayatorn J, Wiwat C, Samee W, et al. Synthesis, in vitro evaluation, and docking studies of novel chromone derivatives as HIV-1 protease inhibitor. J Mol Stru. 2011;1001(1):152-61.
- Yaşar A, Akpınar K, Burnaz NA, et al. Microwave‐assisted synthesis of 4′‐azaflavones and their n‐alkyl derivatives with biological activities. Chem and Biodiv. 2008;5(5):830-8.
- Isaka M, Sappan M, Auncharoen P. Chromone derivatives from the wood-decay fungus Rhizina sp. BCC 12292. Phytochem Letters. 2010;3(3):152-5.
- Ragab FA, El-Sayed NA, Eissa AA, et al. Synthesis and anticonvulsant activity of certain substituted furochromone, benzofuran and flavone derivatives. Chem and Pharmaceu Bull. 2010;58(9):1148-56.
- Mazzei M, Balbi A, Roma G, et al. Synthesis and anti-platelet activity of some 2-(dialkylamino) chromones. Eur J Med Chem. 1988;23(3):237-42.
- Ragab FA, Hassan GS, Yossef HA, et al. Synthesis of 6-and 9-alkylaminomethyl furoflavones as gastroprotective agents. Eur J Med Chem. 2007;42(8):1117-27.
- Dave SS, Rahatgaonkar AM. Computational evaluation of 2-phenyl-4H-chromen-4-one analogues as antihistamines: Potential histamine N-methyltransferase (HMT) inhibitors. J Chem. 2009;6(4):1009-16.
- Wu ES, Cole TE, Davidson TA, et al. Synthesis and antihypertensive activity of (3-phenylflavonoxy) propanolamines without beta-adrenoceptor antagonism. J Med Chem. 1987;30(5):788-92.
- Lee KS, Seo SH, Lee YH, et al. Synthesis and biological evaluation of chromone carboxamides as calpain inhibitors. Bioorg and Med Chem Lett. 2005;15(11):2857-60.
- Kim SH, Lee YH, Jung SY, et al. Synthesis of chromone carboxamide derivatives with antioxidative and calpain inhibitory properties. Eur J Med Chem. 2011;46(5):1721-8.
- Sun X, Qiu J, Wasley J. Chromone inhibitors of s-nitrosoglutathione reductase. Patent. US 8481590, 2013.
- Abram TS, Norman P, Warren BT. Anti-allergic chromone or thiochromone-s-oxamic acid derivatives, compositions, and method of use there for. Patent. US 4571405, 1986.