Original Article
, Volume: 14( 13)Study on the Compatibilization Mechanism of Poly(Propylene Carbonate) /Thermoplastic Starch Composites
- *Correspondence:
- Hongguang Dai, College of Science, Inner Mongolia Agricultural University, Hohhot 010018, P.R. China, Tel: +86-0471-4308695; E-mail: imaudm@imau.edu.cn
Received: October 09, 2016; Accepted: November 07, 2016; Published: November 20, 2016
Citation: Dai H, Wang Q, Ding Z. Study on the Compatibilization Mechanism of Poly(Propylene Carbonate)/Thermoplastic Starch Composites. Mater Sci Ind J. 2016;14(13):108.
Abstract
Poly (propylene carbonate)/thermoplastic starch composites (PPC/TPS) are environment-friendly materials. Compatibilization of PPC and TPS in PPC/TPS using anhydrides as compatibilizers is reported, but the compatibilization mechanism of PPC and TPS is not studied. In the paper, succinic anhydride (SA) is used as compatibilizer for PPC/TPS (SAPPC/TPS) and succinic acid (S) is used as compatibilizer for PPC/TPS (SPPC/TPS) too. Compatibilization mechanism is analyzed by comparing SAPPC/TPS with SPPC/TPS. Scanning electron microscope demonstrates that compatibilization effect of S is better than that of SA. Thermal property of composites is characterized by differential scanning calorimetry. The mechanical properties of SPPC/TPS are better than those of SAPPC/TPS. The composites are extracted by methanol firstly, and then by acetone. The acetone extract products are characterized by attenuated total reflection-fourier transform infrared (ATR-FTIR). In the presence of acid or anhydride, transesterification of PPC and starch is the major reaction, which produces the starch-g-PPC graft copolymer. The production of starch-g-PPC copolymer improves the compatibilization of PPC and TPS.
Keywords
Compatibilization mechanism; Transesterification; Composites; Reactive extrusion; Graft copolymer
Introduction
Non-biodegradable plastics derived from petroleum play an important role in the human life, so they are used largely. But their decomposition needs hundreds of years, environment is polluted due to the improper disposal of them [1]. In order to solve the problem, much work is focused on biodegradable plastics. Poly (propylene carbonate) (PPC) is a biodegradable aliphatic polycarbonate with end-hydroxyl groups [2]. But it is expensive, which has limited its applications [3].
Thermoplastic starch (TPS) has received considerable research attention due to its low cost and biodegradability [4]. But its poor mechanical properties and sensitivity to humidity limit applications of TPS [5]. Blending of TPS and PPC lowers the cost and the humidity sensitivity of the material. Biodegradable composites of poly (propylene carbonate) (PPC) and unmodified corn starch have been studied by Peng et al. [6], Ge et al. [2], and Lu et al. [7]. However, the compatibility between hydrophobic PPC and hydrophilic starch is poor. Polymer compatibility is an important factor for polymer composites. It is critical in governing the morphology of the composites and, therefore, the eventual physical properties of the composites [3]. Due to the immiscibility of PPC with starch, the mechanical properties of composites are too poor to use.
To obtain material with desired mechanical properties by blending the two components, the compatibility of PPC and starch should be improved [8]. Ma et al. [9] prepare PPC/TPS composites by extrusion in the presence of succinic anhydride, the compatibility of PPC and TPS is improved in the presence of succinic anhydride. The compatibility of PPC and TPS could be improved in the presence of maleic anhydride, too [10].
Ma et al. [9,11] think that anhydride is prone to react with hydroxyl groups in starch, which introduced ester groups into starch and improved the compatibility between PPC and starch. Up to now, no designed experiments have aimed at proving the compatibility mechanism of PPC and TPS in the presence of anhydride during processing by reactive extrusion.
In the opinion, the in situ reaction between PPC and starch may form graft copolymers in the presence of anhydride during the extrusion procedure. The graft copolymer in situ generated at the interfaces can reduce the interfacial tension, increase the surface area of the dispersed phase, suppress particle coalescence and stabilize the dispersed phase morphology through interpenetration and entanglements at the polymer-polymer interface [12]. Interpenetration and entanglements at the polymer-polymer interface do favor to the improvement of the load transfer, mechanical properties are improved [13].
The objective of this work is to present a detailed study on compatibilization mechanism of PPC and TPS during processing by reactive extrusion. Up to now, there is no report about using acid as compatibilizer for PPC/TPS, succinic acid is used as compatibilizer for PPC/TPS in the manuscript. PPC, starch, glycerol and succinic anhydride (or succinic acid) are mixed together, and then extruded by twin-screw extruder. Composite using succinic anhydride as compatibilizer is denoted by SAPPC/TPS, composite using succinic acid as compatibilizer is denoted by SPPC/TPS. The morphology, thermal properties and mechanical properties of the composites are examined. The composites are extracted by methanol firstly and then by acetone. The acetone extract products are characterized by attenuated total reflection-fourier transform infrared (ATR-FTIR). The compatibilization mechanism of PPC and starch is analyzed by comparing SAPPC/TPS with SPPC/TPS.
Materials and Methods
Materials
PPC was obtained from Mengxi group corporation (China). Corn starch was obtained from Zhangjiakou Yujing starch company (Zhangjiakou, Heibei, China). Glycerol and methanol (analytical grade) were obtained from Tianjin Fengfan chemical reagent company. Succinic anhydride, succinic acid and acetone (analytical grade) were purchased from Tianjin Guangfu fine chemical Co. Ltd. (Tianjin, China).
Preparation of the composites
Corn starch was dried at 130°C for 1.5 h to eliminate the moisture. PPC, dry starch, glycerol, succinic anhydride or succinic acid were mixed by using high speed mixer SHR-10A (made in Nanjing China), the formulations were shown in Table 1. The mixtures were fed in to the twin-screw plastic extruder PPT-3/SHJ2-25 (Screw ratio L/D=40:1, GuangZhou POTOP experimental analysis instrument Co. Ltd., China). The screw speed was 80 rpm. The temperature profile along the extruder barrel was 130°C, 135°C, 140°C, 140°C, 140°C, 140°C, 145°C (from feed zone to die). The die was a round sheet with the diameter 2.2 mm holes.
| Composites | PPC (g) | Dry starch (g) | Glycerol (g) | Succinic anhydride (g) (mol) | Succinic acid (g) (mol) |
|---|---|---|---|---|---|
| PPC/TPS | 700 | 300 | 120 | 0 | 0 |
| SAPPC/TPS | 700 | 300 | 120 | 8.2 (0.082) | 0 |
| SPPC/TPS | 700 | 300 | 120 | 0 | 9.7 (0.082) |
Table 1: Formulations used in the production of composites.
Scanning electron microscope (SEM)
Corn starch was investigated with the scanning electron microscope Phillips XL-3 (FEI Company, Hillsboro, USA), the experiment was operated at an acceleration voltage of 20 kV. Starch powders were suspended in acetone. The suspension drops were drawn on the glass slide, dried to remove the acetone, and then vacuum-coated with gold for SEM.
The cryo-fractured surfaces of extruded composites were examined with S-4800 scanning electron microscope (HITACHI company, Tokyo, Japan), operating at an acceleration voltage of 5 kV. The composites were cooled in liquid nitrogen, and then broken. The fracture surfaces were vacuum coated with platinum for SEM.
Differential scanning calorimetry (DSC) testing
DSC measurements were carried out in a DSC 4000 (PerkinElmer, USA). The DSC was calibrated with pure indium. An empty pan was used as reference. Samples of composites were scanned at a heating rate of 20°C/min in a sealed pan. Glass transition temperatures were determined from the resulting thermograms by the Pyris software.
Methanol and acetone soxhlet extractions
The composites were first subjected to a soxhlet extraction in methanol to remove glycerol from the composites. And then soxhlet extraction in acetone was carried out. The acetone soxhlet extract products were analyzed by attenuated total reflectance-fourier transformed infrared (ATR-FTIR). The residue remaining in the thimble was starch.
Composites were grounded into fine powder. Composites and cellulose extraction thimbles were dried at 70°C for 8 h before weighting [14]. After drying, the thimbles were weighed empty and about 3 g of dried sample was added into each extraction thimble. The thimbles containing the samples were then introduced into the soxhlet extractors, each was connected to a 150 mL flask containing 100 mL of the extraction solvent (methanol or acetone). The flasks were then heated to solvent reflux and extraction was allowed to continue for 3 days. At the end of the extraction, the thimbles were removed from the soxhlet and dried at 70°C for 8 h. The dried thimbles with the residues inside were weighed and the weight per cent of the residues was determined by equation (1).
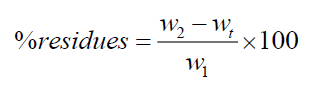 (1)
(1)
where, wt was the weight of the dried thimble measured before extraction; w1 was the weight of the dried sample introduced in the thimble before extraction; w2 was the weight of the dried thimbles with residues after acetone extraction. Extraction was replicated three times for each composite. The statistical significance was assessed by t-test (solver in Origin 6.1 software).
The acetone extraction solution was concentrated to evaporate acetone, leaving acetone extract products. The acetone extract products were analyzed by ATR-FTIR.
Attenuated total reflection-fourier transform infrared (ATR-FTIR)
ATR-FTIR spectra were acquired on a Spectrum 65 IR spectrum scanner (PerkinElmer, USA) equipped with a single reflection ATR system.
Mechanical testing
Mechanical testing (National Standard of China GB1040-79) of samples was determined in the TH-5000 materials testing machine (Jiangsu, China) at a speed of 10 mm/min. The data were averages of 5 specimens. The statistical significance was assessed by t-test (solver in Origin 6.1 software).
Results and Discussion
Scanning electron microscopy (SEM)
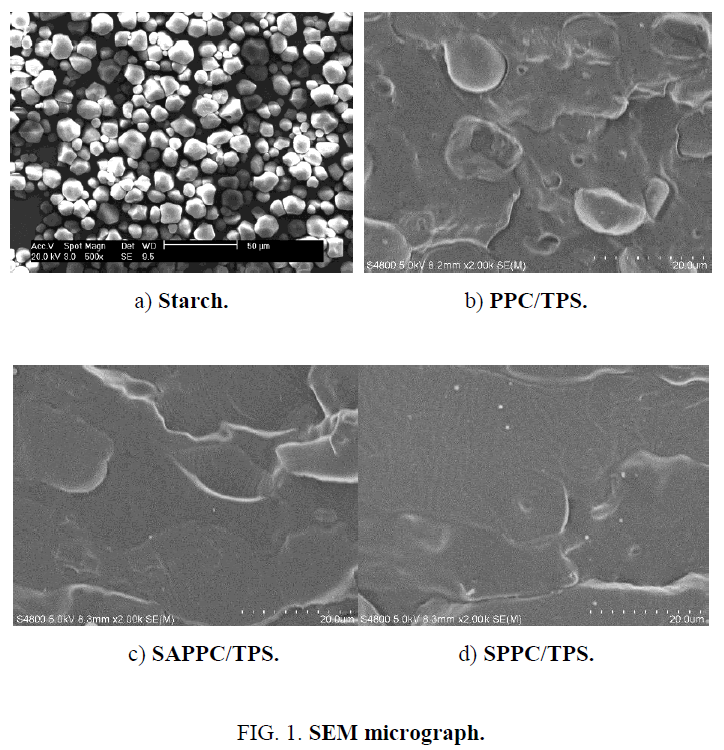
The morphology structure of composites is very important characteristic, because it has a great influence on the mechanical properties of the composites. The morphologies of native starch, extruded composites are shown in Figure 1. Corn starch shows the granular appearance ( Figure 1a). For PPC/TPS, there are many gaps, which are distinct interfaces between TPS and PPC ( Figure 1b). Therefore, their interfacial tension is large and their interfacial adhesion is low, the compatibility between hydrophobic PPC and hydrophilic TPS is deficient.
The morphology of SAPPC/TPS is shown in Figure 1c. In the presence of SA, it is more homogeneous. It demonstrates that interaction between TPS and PPC occurs, the compatibility of TPS and PPC is improved in the presence of SA.
The morphology of SPPC/TPS is shown in Figure 1d. Compared with SAPPC/TPS, the structure of SPPC/TPS is more compact. This shows that S acted more efficiently than SA in compatibilizing PPC and TPS. The improvement of compatibility between PPC and TPS is due to the improved interfacial adhesion between PPC and TPS.
The improved interfacial adhesion attributes to the strong interaction of PPC and starch. In the presence of SA or S, the starch-g-PPC graft copolymer in situ generated at the interfaces can reduce the interfacial tension through interpenetration and entanglements at the PPC-TPS interface [12]. The compatibility of SPPC/TPS is better than that of SAPPC/TPS, because the amount of starch-g-PPC graft copolymer generated in situ in the presence of S is larger than that in the presence of SA. It can be proven by the analysis of extraction and ATR-FTIR later.
Therefore, the compatibilization effect of S is better than SA, producing a more uniform and compact material, which is also indicated by the improvement of their mechanical properties discussed later.
DSC analysis
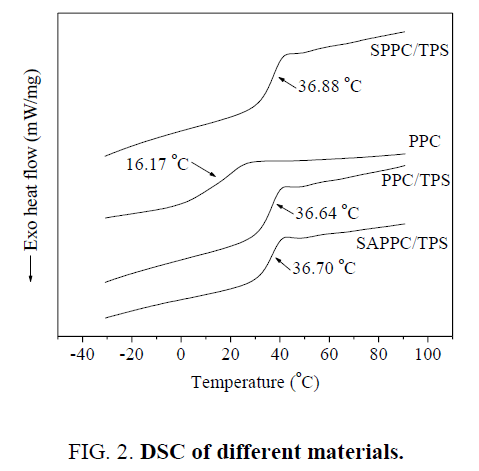
The DSC measurements serve to determine the relaxational transitions of the materials. As shown in Figure 2, the transitions of materials could be linked to their glass transitions. The Tg of PPC, PPC/TPS, SAPPC/TPS, SPPC/TPS are 16.17°C, 36.64°C, 36.70°C and 36.88°C, respectively. The Tg of composites is higher than that of PPC. The Tg of PPC limits its application fields, the improvement of Tg contributes to its applications. But the Tg of composites are so close that they are same, which demonstrated that the influence of compatibility of PPC and TPS upon Tg is little.
Mechanical properties
The mechanical properties are shown in Table 2. The tensile strengths of PPC/TPS, SAPPC/TPS, SPPC/TPS are 12.89 MPa, 15.87 MPa and 20.16 MPa, respectively. The tensile strength of SPPC/TPS is better than that of SAPPC/TPS, the tensile strength of SAPPC/TPS is better than that of PPC/TPS. The elongations at break of PPC/TPS, SAPPC/TPS, SPPC/TPS are not significantly different by t-test analyzing at the 0.05 level (using Origin 6.1 software). The Young's modulus of SAPPC/TPS is larger than that of PPC/TPS, the Young's modulus of SPPC/TPS is larger than that of SAPPC/TPS. Generally, the mechanical properties of SPPC/TPS are better than those of SAPPC/TPS, the mechanical properties of SAPPC/TPS are better than those of PPC/TPS.
| Composites | Tensile strength (MPa) | Elongation at break (%) | Young's modulus (MPa) |
|---|---|---|---|
| PPC/TPS | 12.89±0.32 | 8.60±2.69 | 757.52±97.62 |
| SAPPC/TPS | 15.87±0.78 | 10.70±2.66 | 1022.60±223.61 |
| SPPC/TPS | 20.16±0.82 | 11.40±5.35 | 1628.22±269.97 |
Table 2: Mechanical properties of composites.
Interfacial interaction between PPC and TPS is an important factor affecting the mechanical properties of the composites. In the case of no adhesion, the interfacial layer cannot transfer stress, the mechanical properties are poor.
SA or S is added to promote the reaction of PPC and TPS to produce starch-g-PPC copolymer. The copolymer does favour to interpenetration and entanglements at the polymer-polymer interface, improves the compatibility between PPC and TPS [15]. The mechanical properties of SPPC/TPS are better than those of SAPPC/TPS, because the compatibility of SPPC/TPS is better than that of SAPPC/TPS. The amount of starch-g-PPC graft copolymer generated in situ in the presence of S is larger, which could be evidenced by soxhlet extraction experiments and ATR-FTIR analysis later. The larger the amount of starch-g- PPC is, the better the compatibility of composites is.
Extraction analysis
Glycerol is soluble in methanol, but PPC and starch are not. Soxhlet extraction in methanol is used to remove glycerol from the composites. PPC and starch-g-PPC are soluble in acetone, but starch is not. Soxhlet extraction in acetone is used to solve PPC and starch-g-PPC in acetone, the leaving solid is starch. In the same extraction conditions, the per cent residues of different composites are different. As shown in Table 3, per cent residues of SPPC/TPS is less than that of SAPPC/TPS, per cent residues of SAPPC/TPS is less than that of PPC/TPS. The less per cent residues is, the larger amount of starch-g-PPC produces. The amount of starch-g-PPC generated in SPPC/TPS is the largest; the amount of starch-g-PPC generated in SAPPC/TPS is larger than that in PPC/TPS.
| Sample | Per cent residues (%) |
|---|---|
| PPC/TPS | 28.70±0.40 |
| SAPPC/ TPS | 27.66±0.17 |
| SPPC/ TPS | 22.48±0.19 |
Table 3: Residues of different composites.
ATR-FTIR analysis
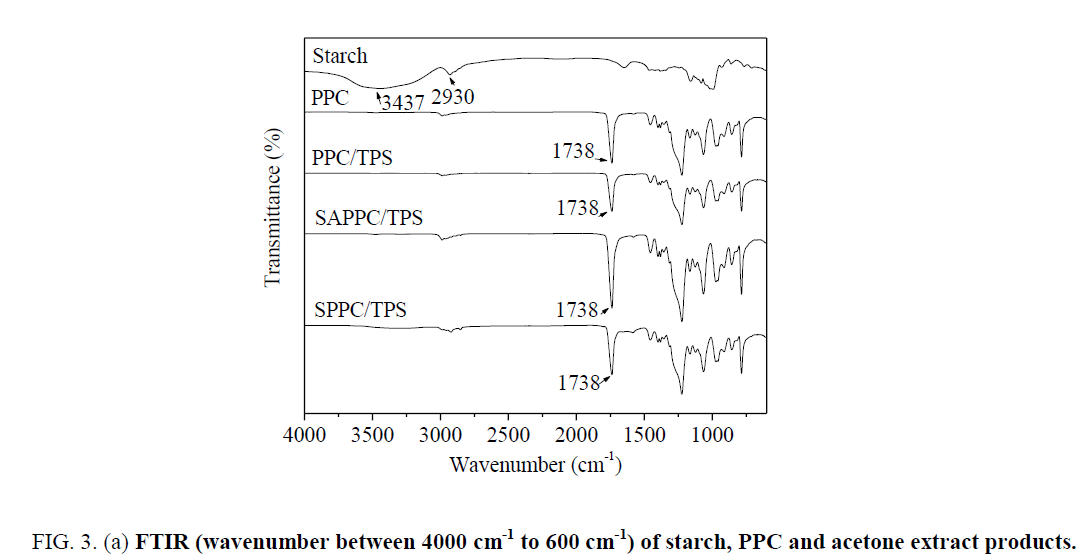
A special attention is directed to the analysis of the acetone extract products by ATR-FTIR. The spectra are presented in Figure 3. From the spectrum of starch, the strong and broad peak at 3437 cm-1 is attributed to O-H bond stretching vibration, 2930 cm-1 is ascribed to C-H bond stretching vibration.
Figure 3a: FTIR (wavenumber between 4000 cm-1 to 600 cm-1) of starch, PPC and acetone extract products.
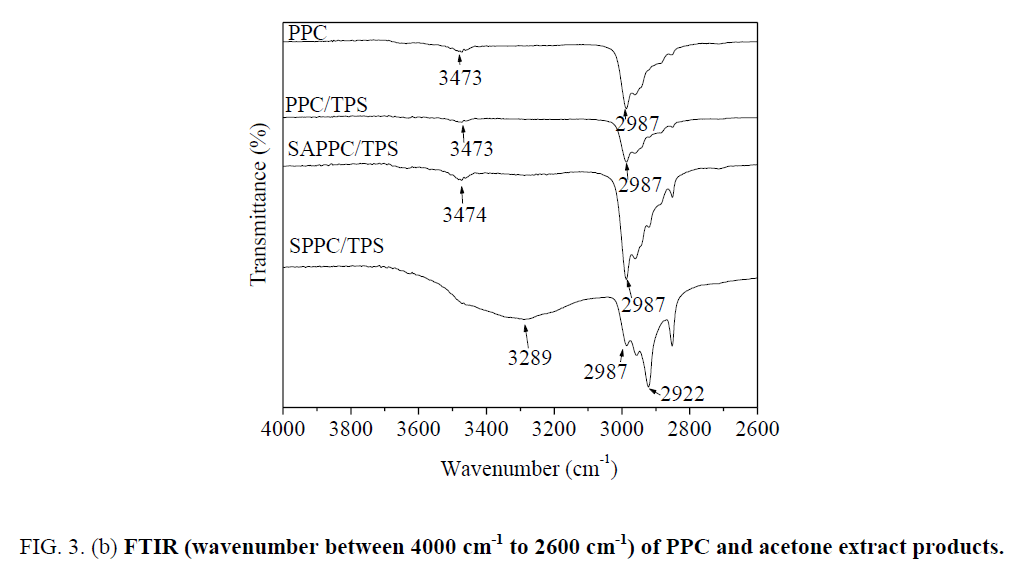
In spectra of PPC, the peak at 3473 cm-1 is very weak; it is attributed to the stretching vibration of O-H in end-hydroxyl groups. The peak at 2987 cm-1 is attributed to C-H bond stretching vibration, the peak at 1738 cm-1 is attributed to C=O bond stretching vibration.
For the spectra of acetone extract products of PPC/TPS, the peak at 1738 cm-1 is due to C=O bond stretching of PPC. The spectra of acetone extract products of PPC/TPS are very similar to those of PPC; there are not apparent characteristic peaks of starch. It demonstrated that the acetone extract product is PPC. There is no starch-g-PPC produced in PPC/TPS. In the simple PPC/TPS melt-blend, no reaction between starch and PPC occurred.
The spectra of acetone extract products of SAPPC/TPS are different from those of PPC. The peak at 3474 cm-1 is stronger than that of PPC ( Figure 3b), which demonstrates that the amount of hydroxyl groups increases. It is due to the presence of starch hydroxyl groups in the acetone extract products. PPC is soluble in acetone, but starch is insoluble. Since starch is insoluble in acetone, the presence of starch segment in the acetone extracted soluble fraction clearly indicates that starch-g-PPC generated. The graft copolymer is formed by covalent bonds through reactions between PPC and starch. Starch-g-PPC produces in SAPPC/TPS. But the amount of starch-g-PPC is small.
For the spectra of acetone extract products of SPPC/TPS, the peak at 3289 cm-1 is strong and broad; it is due to O-H bond stretching of starch segments. The peak at 2922 cm-1 is attributed to C-H bond stretching of starch segments, the peak at 1738 cm-1 is due to C=O bond stretching of PPC segments. Starch-g-PPC produces in SPPC/TPS. The starch characteristic peaks 3289 cm-1 and 2922 cm-1 are apparent; the amount of starch-g-PPC in SPPC/TPS is larger than that in SAPPC/TPS.
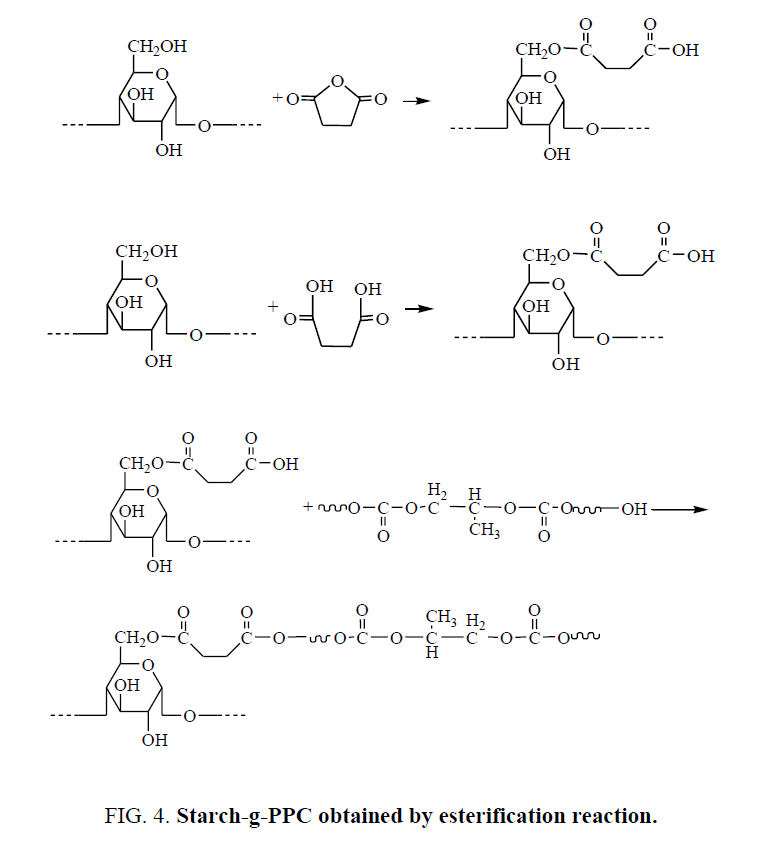
Starch-g-PPC may be obtained by two ways. The first way, hydroxyl groups of starch react with S or SA by esterification reaction to obtain esterified starch, there are carboxylic groups in esterified starch, then carboxylic groups in esterified starch react with PPC hydroxyl groups to obtain starch-g-PPC by esterification reaction. The reactions are shown in Figure 4.
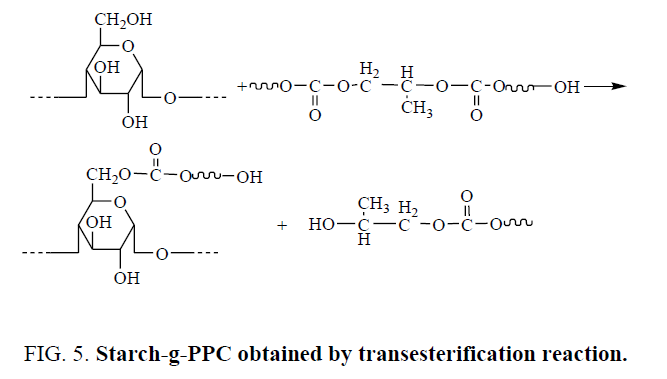
The second way, hydroxyl groups of starch react with SA by esterification reaction to obtain esterified starch with carboxylic groups. Starch-g-PPC graft copolymers generate by transesterification reactions between PPC ester groups and starch hydroxyl groups, the transesterification reactions are promoted by SA derived acidic moieties grafted onto the starch backbone. Starch-g-PPC in SPPC/TPS could generate by the transesterification reaction of PPC ester groups and starch hydroxyl groups under the catalyzing of S. The reaction is shown in Figure 5.
For esterification reaction of starch, SA is more reactive than S. If starch-g-PPC was mainly obtained by the first way, the amount of starch-g-PPC in SAPPC/TPS should be larger than that in SPPC/TPS. But the amount of starch-g-PPC in SAPPC/TPS is less than that in SPPC/TPS. So, starch-g-PPC does not generate mainly by the first way.
Compared with S, SA is more reactive in esterification reaction with starch to produce more esterified starch. So, the amount of carboxylic group in SAPPC/TPS is less than that in SPPC/TPS. Transesterification reaction could be catalyzed by acid. Transesterification reaction between starch and PPC could occur more easily in SPPC/TPS. The amount of starch-g-PPC generated in SPPC/TPS is larger than that in SAPPC/TPS. So, starch-g-PPC generates mainly by the second way.
Such observations attest for the formation of a starch-g-PPC graft copolymer derived from PPC and TPS, covalently linked to each other mainly through acid-promoted transesterification reactions between the ester groups from the PPC backbone and the hydroxyl groups from starch [16].
Conclusion
SEM demonstrates that the compatibility of PPC and TPS in SPPC/TPS is better than that in SAPPC/TPS. The Tg of composites is higher than that of PPC. Compared with SAPPC/TPS, SPPC/TPS has better mechanical properties. The soxhlet extraction experiment and soxhlet extract products ATR-FTIR analysis illustrate that transesterification reactions between PPC and starch in the presence of S or SA is the main reaction to produce starch-g-PPC. Starch-g-PPC copolymer improves the compatibility of PPC and TPS, the improvement of PPC and TPS compatibility contributes to the improvement of the composites mechanical properties.
Acknowledgment
The work was supported by Natural Science Foundation of Inner Mongolia Autonomous Region of China (2014BS0510) and the Inner Mongolia Agricultural University Research initiation funds for Doctor (BJ09-38).
References
- Silva IFE, Yamashita F, Müller CMO, et al.How reactive extrusion with adipic acid improves the mechanical and barrier properties of starch/poly (butylene adipate-co-terephthalate) films. Int J Food Sci Tech. 2013;48(8):1762-9.
- Ge XC, Li XH, Zhu Q, et al. Preparation and properties of biodegradable poly(propylene carbonate)/starch composites. Polym Eng Sci.2004;44(11):2134-40.
- Wang XL, Li RKY, Cao YX, et al. Essential work of fracture analysis for starch filled poly(propylene carbonate) composites. Mater. Design.2007;28(6):1934-9.
- López OV, Ninago MD, Lencina MM, et al. Thermoplastic starch plasticized with alginate-glycerol mixtures: Melt-processing evaluation and film properties. Carbohydr Polym.2015;126:83-90.
- Taghizadeh A, Sarazin P, Favis BD. High molecular weight plasticizers in thermoplastic starch/polyethylene blends. J Mater Sci.2013;48(4):1799-811.
- Peng SW, Wang XY, Dong LS. Special interaction between poly(propylene carbonate) and corn starch. Poly Compos.2005;26(1):37-41.
- Lu XL, Du FG, Ge XC, et al. Biodegradability and thermal stability of poly(propylene carbonate)/starch composites. J Biomed Mater Res. Part A. 2006;77A(4):653-8.
- Zeng S, Wang S, Xiao M, et al. Preparation and properties of biodegradable blend containing poly (propylene carbonate) and starch acetate with different degrees of substitution. Carbohydr Polym.2011;86(3):1260-5.
- Ma X, Chang PR, Yu J, et al.Preparation and properties of biodegradable poly(propylene carbonate)/thermoplastic dried starch composites. Carbohydr Polym.2008;71(2):229-34.
- Yu H, Li L, Wang H, et al.Study on Truly Biodegradable Material of PPC/Corn Starch Blends. Journal of Shenyang Institute of Chemical Technology.2005;(3):188-92.
- Ma X, Yu J, Zhao A. Properties of biodegradable poly(propylene carbonate)/starch composites with succinic anhydride. Compos Sci Technol.2006;66(13):2360-6.
- Li J, Sun CR, Zhang XQ. Preparation, thermal properties, and morphology of graft copolymers in reactive blends of PHBV and PPC. Poly Compos.2012;33(10):1737-49.
- Joshi SS, Mebel AM. Computational modeling of biodegradable blends of starch amylose and poly-propylene carbonate. Polymer.2007;48(13):3893-901.
- Hablot E, Dewasthale S, Zhao Y, et al. Reactive extrusion of glycerylated starch and starch–polyester graft copolymers. Eur Polym J.2013;49(4):873-81.
- Olivato JB, Grossmann MVE, Bilck AP, et al. Effect of organic acids as additives on the performance of thermoplastic starch/polyester blown films. Carbohydr Polym.2012;90:159-64.
- Guo M, Brittain WJ. Structure and Properties of Naphthalene-Containing Polyesters. 4. New Insight into the Relationship of Transesterification and Miscibility. Macromolecules.1998;31(21):7166-71.