Research
, Volume: 15( 2) DOI: 10.21767/0974-7532.1000152Plasma Level and Correlation of Leptin and Prolactin with Fertility Hormones in Women with Infertility in Southwest Nigeria
- *Correspondence:
- Akande JO
Department of Chemical Pathology
College of Health Sciences
Bowen University Ogbomoso
Oyo State, Nigeria
Tel: +234 7066377491
E-Mail: joel.akande@bowen.edu.ng
Received: April 30, 2020; Accepted: May 29, 2020; Published: June 5, 2020
Citation: Oke EO, Oke OF, Akande JO, et al. Plasma Level and Correlation of Leptin and Prolactin with Fertility Hormones in Women with Infertility in Southwest Nigeria. Res Rev Biosci. 2020;15(2):152.
Abstract
Background: Reproduction is a complex process relying on the interactions between the anatomical structure and the male and female normal reproductive function. Abnormal leptin levels can lead to infertility, obesity, anxiety, depression, hypertension and other cardiovascular diseases. The complex interactions between leptin, fertility hormones and various factors play important role in human fertility.
Objective: This study was designed to determine the plasma level of leptin and prolactin and correlation with fertility hormones in women with infertility.
Methods: This study was a case-control study conducted in 2016 between January and May at Gynaecology and Family Planning clinics of LAUTECH Teaching Hospital, Ogbomoso and Osogbo. A total number of 106 women each recruited as cases and controls after categorizing them as infertile and fertile groups respectively. Total sample of 212 respondents was obtained. Approval was obtained from the Ethics and Research Committee of the institution while informed consent was taken from the study participants. On the third day of the menstrual cycle, venous blood sample was taken from the respondents for analysis. Leptin, Follicle Stimulating Hormone (FSH), Luteinizing Hormone (LH), estradiol and prolactin were measured. The data obtained were analyzed using the IBM Statistical Package for the Social Sciences (SPSS) software version 20.0. Level of significance for the study was set at p-value less than 0.05.
Results: Weight and Body Mass Index (BMI) were higher in the subjects than controls and the differences were statistically significant (weight; p=0.029), (BMI; p=0.034) FSH (=0.005), estradiol (p=0.000) and prolactin (p=0.007). The plasma level of leptin was lower in subjects compared to controls and the difference was statistically significant, (p=0.000). The study found a high level of prolactin among 56.6% of the cases and 30.2% of the controls respectively. BMI had negative significant correlation with plasma level of estradiol (r=-0.197, p=0.044). FSH had strong and highly significant positive correlation with LH (r=0.875, p=0.000), while leptin shows weak and negative significant correlation with both FSH (r=- 0.246, p=0.011) and LH (r=-0.229, p=0.018). BMI had negative significant correlation with plasma level of estradiol (r=-0.197, p< 0.05).
Conclusion: There was correlation between plasma level of leptin and prolactin and fertility hormones in women with infertility but the relationship is non-linear and depends largely on the underlying causes of the infertility.
Keywords
Leptin; Prolactin; Infertility; FSH; LH; Estradiol
Abbreviations
FSH: Follicle Stimulating Hormone; LH: Luteinizing Hormone; SPSS: Statistical Package for the Social Sciences; BMI: Body Mass Index; LEP: Leptin; IVF: In-Vitro Fertilization; PCOS: Polycystic Ovarian Syndrome; ELISA: Enzyme Linked Immunosorbent Assay
Introduction
Infertility is defined as the failure to conceive, with no contraception, after one year of regular intercourse in women <35 years and after 6 months in women >35 years. Globally, epidemiological data suggest that about 10% to 15% of couples are infertile [1,2]. In Nigeria, infertility prevalence is about 12.1% and is the most frequent reason for gynecological consultations [3,4]. Usually, infertile couples are offered conventional treatment like ovulation induction, tubal surgery, with not too encouraging outcomes due to limited availability and high cost of modern technologies like in vitro fertilization [5].
Reproduction is a complex process relying on the interactions between the anatomical structure and the male and female normal reproductive functions; any factor that disrupts either of the function has the potential to cause infertility [6].
Leptin is hormone produced by Leptin (LEP) gene of white adipocytes to modulate satiety and energy homeostasis, it is also a product of many other tissues like gastric mucosa, mammary epithelial cell, human placenta and uterine endometrium [7,8]. The enhanced levels of leptin are associated with the advent of reproductive maturity and fertility [9,10]. Serum leptin plays a role in the maintenance of body weight, associated with several other regulatory functions in endocrine systems including the hypothalamic-pituitary-gonadal axis [11]. Studies have also shown the involvement of leptin in reproduction and fertility in both humans and animals [12,13]. Leptin can be associated with how well an embryo implants in the uterus, amenorrhea, irregular cycles, egg quality and In-Vitro Fertilization (IVF), IVF success rates. Abnormal leptin levels can lead to infertility, obesity, anxiety, depression, hypertension and other cardiovascular diseases [14,15].
In humans, serum leptin concentration correlates with the adiposity measured either as the Body Mass Index (BMI) or as percentage of the body fat [16]. Leptin receptors have been identified in hypothalamus, gonadotropic cells of the anterior pituitary, granulose, theca and interstitial cells of ovary as well as in the endometrium, all of which are concerned with fertility [17,18]. The central and peripheral signals of the hypothalamic-pituitary-gonadal axis seem to play an important role in the action of leptin in the menstrual cycle. Although the physiological impact of the alteration in the plasma leptin during the menstrual cycle is uncertain, it has been established that the lowest threshold of the circulating leptin is essential for a normal ovulatory function [6]. Therefore, leptin clearly appears to be linked to the reproductive system, probably relaying information regarding the adequacy of body energy homeostasis to the prospective event of pregnancy, demonstrating the importance of nutritional resources to a successful pregnancy [2].
Hyperprolactinemia is the most common endocrine disorder of the hypothalamic-pituitary axis. The clinical presentations of hyperprolactinemia result from the hormonal influence on prolactin target tissue, which are predominantly the reproductive systems and breast tissue [19]. High prolactin levels are associated with anovulation or may directly or indirectly cause infertility. The prevalence of hyperprolactinemia varies in different patient populations, stays below 1% (0.4% in an unselected normal population) but can be as high as 17% in women with reproductive disorders [20]. Prolactin acts on the adipose tissue and increases leptin synthesis and secretion in rats. However, the relationship between prolactin and leptin has not been well established in humans. It is well documented that both hormones affect gonadotropins (FSH/LH) level in humans [21,22]. Several studies have found various levels of leptin, prolactin, FSH, LH and estradiol in women with infertility as discussed above but few of them have investigated their correlation [23,24]. This study was designed to look at the plasma level of leptin and its correlation with prolactin and fertility hormones in women with infertility in an effort to understand their roles in infertility.
Materials and Methods
Study participants
This study was a case-control study conducted in 2016 between January and May at Gynaecology and Family Planning clinics of LAUTECH Teaching Hospital, Ogbomoso and Osogbo. A total number of 106 women were recruited as cases and controls after categorizing them into infertile and fertile groups respectively using a multi-stage sampling technique (total sample size=212 respondents) (TABLE 1).
| Variables | Status (%) | X2 | df | p-value | ||
|---|---|---|---|---|---|---|
| Age group (years) | Subject (n=106) | Control (106) | Total (212) | 1.3 | 5 | 0.935 |
| 18-22 | 3 (2.8) | 2 (1.9) | 5 (2.4) | - | - | - |
| 23-27 | 11 (10.4) | 8 (7.5) | 19 (9.0) | - | - | - |
| 28-32 | 34 (32.1) | 37 (34.9) | 71 (33.5) | - | - | - |
| 33-37 | 34 (32.1) | 31 (29.2) | 65 (30.7) | - | - | - |
| 38-42 | 17 (16.0) | 19 (17.9) | 36 (17.0) | - | - | - |
| 43-45 | 7 (6.6) | 9 (8.5) | 16 (7.5) | - | - | - |
| Education | 9.594 | 4 | 0.048* | |||
| Primary | 11 (10.4) | 10 (9.4) | 21 (9.9) | - | - | - |
| Secondary | 17 (16.0) | 12 (11.3) | 29 (13.7) | - | - | - |
| Post-secondary | 15 (14.2) | 30 (28.3) | 45 (21.2) | - | - | |
| University | 55 (51.9) | 52 (49.1) | 107 (50.5) | - | - | - |
| Others | 8 (7.5) | 2 (1.9) | 10 (4.7) | - | - | - |
| Years of Marriage | 4.475 | 2 | 0.107 | |||
| 0-4 | 49 (46.2) | 36 (34.0) | 85 (40.1) | - | - | - |
| 5-9 | 41 (38.7) | 44 (41.5) | 85 (40.1) | - | - | - |
| 10 | 16 (15.1) | 26 (24.5) | 42 (19.8) | - | - | - |
| Occupation | 2.313 | 4 | 0.678 | |||
| Trader | 23 (21.7) | 25 (23.6) | 48 (22.6) | - | - | - |
| Civil servant | 51 (48.1) | 47 (44.3) | 98 (46.2) | - | - | - |
| Full house Wife | 5 (4.7) | 8 (7.5) | 13 (6.1) | - | - | - |
| Student | 9 (8.5) | 5 (4.7) | 14 (6.6) | - | - | - |
| Other | 18 (17.0) | 21 (19.8) | 39 (18.4) | - | - | - |
NB: *p<0.05 Significant
Table 1: Socio-demographic profile of the respondents.
The inclusion criteria were women aged 18¬49 years, regularly menstruating with a cycle length between 21 and 35 days. Also, women who presented for infertility treatment at both teaching hospitals and women attending infant welfare clinicswith their children and requesting for family planning services were recruited into the study. Women with previous history of myomectomy, oophorectomy, hysterectomy or any disease affecting the ovaries, biochemical or radiological findings suggestive of Polycystic Ovarian Syndrome (PCOS), diabetes mellitus and thyroid disease and those on hormone therapy or any drug that interferes with the menstrual cycle were excluded from the study.
The nature of the study was explained to the respondents and signed consent forms were obtained before semi-structured questionnaires were administered to them. Arrangement was made thereafter for collection of day three menstrual cycle sample for measurement of leptin, FSH, LH, estradiol and prolactin.
Five milliliters of venous blood was collected aseptically in eligible women on day three of the menstrual cycle into a well labelled serum separator gel bottle and taken to the laboratory. The blood was allowed to clot and retract. At room temperature, serum was separated by centrifugation at 2000 g-3000 g for 20 minutes to remove the supernatant. Then, the sample was stored at -20°C.
Leptin, FSH, LH, estradiol and prolactin assays
The samples were processed in duplicates at the Metabolic Research Laboratory of Ladoke Akintola University of Technology Teaching Hospital, Ogbomoso. Control sera and calibrator were included in each batch. Serum leptin was quantified with human leptin, Enzyme Linked Immunosorbent Assay (ELISA) kit manufactured by Span Biotech Ltd., Hong Kong using a double-antibody sandwich ELISA according to the manufacturer’s manual. The sensitivity of the assay was 0.01 ng/ml.
FSH, LH, estradiol and prolactin were quantified using ACCU-BIND IMMUNOASSAY ELISA KIT manufactured from Monbind Inc. Lake Forest, CA 92630, USA. The assay principles for FSH, LH and prolactin followed; two step capture or sandwich according to manufacturer’s manual. The estradiol assay principle was three steps and a delayed competitive enzyme immunoassay. After incubation, the absorbance was read at 450 nm within 30 minutes using micro-plate ELISA reader (LT 4000).
Statistical analysis
Statistical analysis was performed by using the IBM SPSS software version 20.0. Results were expressed as mean ± SD; p-values<0.05, was considered statistically significant. Relationships among different variables of the test groups were compared using pearson correlation coefficient.
Results
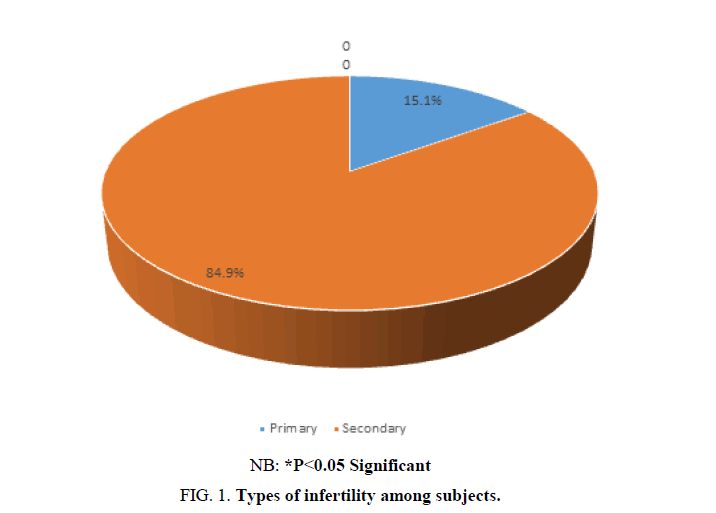
The distribution of respondents according to age (p=0.935), years of marriage (p=0.107) and occupation (p=0.678) were not significantly different between the subjects and the controls. There was a statistically significant difference between the levels of education (p=0.048) of the cases and the controls. The study also found that the prevalence of secondary infertility was higher than primary infertility among the cases (FIG. 1).
TABLE 2 shows the weight and BMI were higher in the subjects (74.49 ± 17.9 and 28.9 ± 7.29) than controls (69.96 ± 11.54 and 27.13 ± 4.46) and the differences were statistically significant p-value<0.05 while the difference in their heights were not statistically significant (p=0.120). FSH, estradiol and prolactin were significantly higher in subjects (13.25 ± 18.41, 66.50 ± 85.37, and 31.55 ± 57.16 respectively) compared with controls (8.1 ± 1.99, 29 ± 18.51, and 16.18 ± 7.55 respectively), p-value<0.05. The plasma level of leptin was lower in subjects compared to controls and the difference was statistically significant (p-value<0.001) except for LH levels whose difference was not statistically significant.
| Variables | Subject | Controls | t-test | p-value |
|---|---|---|---|---|
| Weight (Kg) | 74.49 ± 17.9 | 69.96 ± 11.54 | 2.195 | 0.029* |
| Height (m) | 1.59 ± 0.11 | 1.61 ± 0.05 | -1.56 | 0.12 |
| BMI (Kg/m2) | 28.90 ± 7.29 | 27.13 ± 4.46 | 2.135 | 0.034* |
| FSH (mlu/ml) | 13.25 ± 18.41 | 8.18 ± 1.99 | 2.817 | 0.005* |
| LH (mlu/ml) | 8.27 ± 10.32 | 6.63 ± 1.95 | 1.609 | 0.109 |
| Estradiol (pg/ml) | 66.50 ± 85.37 | 29.25 ± 18.51 | 4.391 | 0.000** |
| Leptin (ng/ml) | 361.05 ± 377.68 | 712 ± 504.15 | -5.737 | 0.000** |
| Prolactin (ng/ml) | 31.55 ± 57.16 | 16.18 ± 7.55 | 2.745 | 0.007** |
NB: *p<0.05 Significant, **p<0.001 Highly significant
Table 2: Comparison of anthropometric measurements and biochemical parameters.
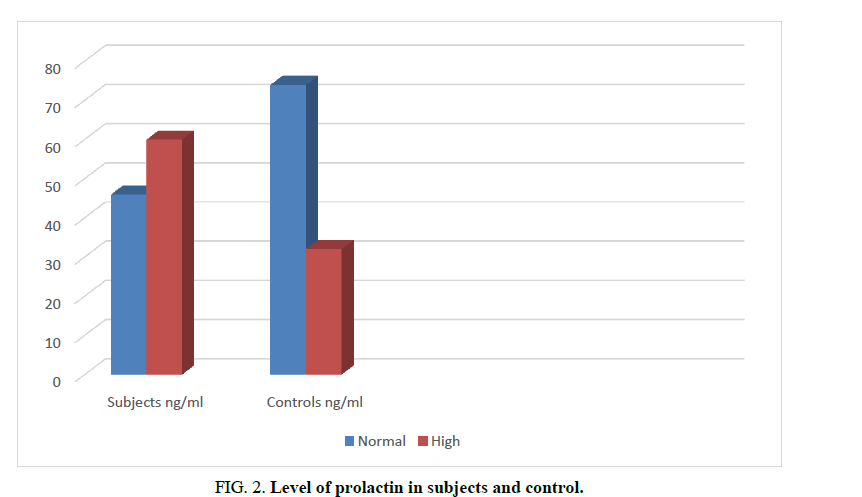
The study also revealed that 56.6% of cases and 30.2% of the control had abnormal prolactin levels respectively (Normal range 5-20 ng/mL; (FIG. 2) [20].
Figure 2. Level of prolactin in subjects and control.
TABLE 3 and 4 shows BMI had negative correlation with plasma level of estradiol which was significant (r=-0.197, p<0.05). FSH had strong and highly significant positive correlation with LH (r=0.875, p=0.000), while leptin showed a weak and negative significant correlation with both FSH (r=-0.246, p=0.011) and LH (r=-0.229, p=0.018). There were no significant associations between prolactin level and other parameters.
| BMI | FSH | LH | Estradiol | Leptin | Prolactin | |
|---|---|---|---|---|---|---|
| BMI (kg/m2) r p | 1 | -0.003 | 0.192 | -0.197 | 0.023 | 0.077 |
| - | 0.974 | 0.097 | 0.044* | 0.814 | 0.430 | |
| FSH (mlU/mL)r p | -0.003 | 1 | 0.875 | 0.176 | -0.246 | 0.155 |
| 0.974 | - | 0.000** | 0.070 | 0.011* | 0.113 | |
| LH (mlU/mL)r p | 0.162 | 0.675 | 1 | 0.047 | -0.229 | 0.119 |
| 0.097 | 0.000* | - | 0.630 | 0.018* | 0.223 | |
| Estradiol (pg/ml)r p | -0.196 | 0.202 | 0.047 | 1 | -0.104 | 0.043 |
| 0.044* | 0.038* | 0.630 | - | 0.289 | 0.658 | |
| Leptin (ng/ml)r p | 0.023 | -0.248 | -0.229 | -0.104 | 1 | -0.047 |
| 0.814 | 0.011* | 0.018* | 0.289 | - | 0.634 | |
| Prolactin (ng/ml)r p | 0.077 | 0.155 | 0.119 | 0.043 | -0.047 | 1 |
| 0.430 | 0.113 | 0.223 | 0.658 | 0.634 | - |
NB:*p<0.05 Significant, **p<0.001 Highly significant
Table 3: Correlation of anthropometric measurements and biochemical parameters in subjects.
| BMI | FSH | LH | Estradiol | Leptin | Prolactin | |
|---|---|---|---|---|---|---|
| BMI (kg/m2) r p | 1 | -0.003 | 0.162 | -0.196 | 0.023 | 0.077 |
| - | 0.974 | 0.097 | 0.044* | 0.814 | 0.430 | |
| FSH (mlU/mL)r p | -0.003 | 1 | 0.675 | 0.176 | 0.246 | 0.155 |
| 0.974 | - | 0.000* | 0.070 | 0.011 | 0.133 | |
| LH (mlU/mL)r p | 0.162 | 0.675 | 1 | 0.047 | -0.229 | 0.119 |
| 0.097 | 0.000 | - | 0.630 | 0.018 | 0.223 | |
| Estradiol (pg/ml)r p | -0.196 | 0.176 | 0.047 | 1 | -0.104 | 0.043 |
| 0.044 | 0.070 | 0.630 | - | 0.289 | 0.658 | |
| Leptin (ng/ml)r p | 0.023 | -0.246 | -0.229 | -0.104 | 1 | -0.047 |
| 0.814 | 0.011 | 0.018 | 0.289 | - | 0.634 | |
| Prolactin (ng/ml)r p | 0.077 | 0.155 | 0.119 | 0.043 | -0.047 | 1 |
| 0.430 | 0.133 | 0.223 | 0.658 | 0.634 | - |
NB: *p<0.05 Significant
Table 4: Correlation of anthropometric measurements and biochemical parameters in control.
Discussion
This study found significant association between level of education and fertility, with the subjects more educated than the controls. Several studies have also shown that the level of education has negative impact on fertility [25-27].
Suggested reason for our result may be the advanced maternal age observed particularly among women with higher educational attainments, which may also contribute to the reduction in their fertility levels [28,29]. A reflection of this finding is the definition of infertility that put six months duration of regular unprotected sexual intercourse for women above 35 years as against one year for women less than 35 years of age [1,30]. The prevalence of secondary infertility 84.9% was found to be higher, in this study than primary infertility 15.1%. This is similar to results obtained in Enugu, South-Eastern, Nigeria and in Lagos, South-Western Nigeria. Where secondary infertility accounted for 76.8% and 80% respectively [31,32]. Though, this is different from the previous studies conducted in Morocco, North Africa and Saudi Arabia where the prevalence (67.37% and 78.99%) of primary infertility was found to be higher than that in secondarAy infertility [33,34]. This variation may be due to regional socio-cultural differences.
Weight and BMI were found to be significantly high in subjects when compared with control group. Abnormal weight and other anthropometric profiles have been described in both men and women with infertility [35,36]. The mean values of FSH and estradiol were found to be significantly high in the study population. Several studies have found similar high level of FSH and estradiol in women with infertility. The elevated level of these hormones showed a late decline in ovarian reserves and is integral parts of assessment tools for ovarian reserves [37-39].
In this study, the mean plasma level of prolactin was found to be significantly high in subjects compared with controls, which is similar to findings from other studies [19,40]. Conversely, Ugwa et al. from the North-West of Nigeria found low level of prolactin in women with infertility [41]. The regional variations in causes of infertility among Nigerian women may account for the difference in the mean plasma level of prolactin obtained in our study [30]. Our study also found that over half of the infertile women and nearly one-third of the controls had a high level of prolactin. Endocrine disorder is one of the causes of infertility and hyperprolactinaemia is the commonest cause of hypothalamic-pituitary axis in the reproductive age group [1,19]. The high level of prolactin level seen in the current study may be because majority of the controls used were breast feeding mothers. Increased prolactin levels have been established among women during lactation [42].
In this study, pearson’s correlation was used to find if there were any relationships between biochemical parameters and anthropometric measurements in subjects and in controls.
A dissimilar finding of positive correlation between BMI and estradiol was reported in another study, which was conducted among apparently healthy individuals and this may account for the difference observed [12].
The current study found a weak negative correlation between leptin level, FSH and LH and this relationship was significant. Few studies have also linked plasma level of leptin with fertility hormones [10,43], and the correlation depends on the underlying causes of infertility, BMI and the phase of menstrual cycle in the woman [13]. This may account for no correlation between leptin level and body fat observed in this study. A study by Mukhtiar et al showed an increase in leptin level at low BMI<24.9 while at high BMI there was reduction in leptin level [2]. However, BMI stratification was not done in our study.
No significant correlation was observed between plasma level of prolactin and other hormones. Although, high level FSH and LH have been observed in hyperprolactinaemia [44].
Conclusion
In conclusion, there is correlation between plasma level of leptin, prolactin and fertility hormones in women with infertility but the relationship is non-linear and depends largely on the underlying causes of the infertility.
Significance Statement
The study found a non-linear association among plasma level of leptin, prolactin, estradiol, FSH and LH in women with infertility. In management of infertility, emphasis should be laid on the primary causes of infertility. Molecular studies may be required to establish a direct relationship between leptin level and other fertility hormones in the reproductive processes of infertile women.
Acknowledgements
We acknowledge and appreciate the contributions of the staff of Gynaecology and Family Planning clinics of LAUTECH Teaching Hospital, Ogbomoso and Osogbo.
Conflict of Interest
Authors declared no conflict of interest.
References
- Weiss RV, Clapauch R. Female infertility of endocrine origin. Arq Bras Endocrinol Metab. 2014;58;144-52.
- Baig M, Azhar A, Rehman R, et al. Relationship of serum leptin and reproductive hormones in unexplained infertile and fertile females. Cureus. 2019;11:e6524.
- Okohue JE, Onuh SO, Ikimalo JI. Comparison of IVF/ICSI outcome in patients with polycystic ovarian syndrome or tubal factor infertility. Niger J Clin Pr. 2013;16:207-10.
- Omoaregba JO, James BO, Lawani AO, et al. Psychosocial characteristics of female infertility in a tertiary health institution in Nigeria. Ann Afr Med. 2011;10:19-24.
- Menuba IE, Ugwu EO, Obi SN, et al. Clinical management and therapeutic outcome of infertile couples in Southeast Nigeria. Ther Clin Risk Manag. 2014;10:763-8.
- Tafvizi F, Masomi M. Comparison of Serum leptin level in women with unexplained infertility and fertile women in Iran. J Obs Gynaecol India. 2016;66:466-70.
- Münzberg H, Morrison CD. Structure, production and signaling of leptin. Metabolism. 2015;64:13-23.
- Cammisotto PG, Bendayan M. Leptin secretion by white adipose tissue and gastric mucosa. Histol Histopathol. 2007;22:199-210.
- Ramos-Lobo AM, Donato J Jr. The role of leptin in health and disease. Temperature. 2017;4:258-91.
- Ajala OM, Ogunro PS, Elusanmi GF, et al. Changes in serum leptin during phases of menstrual cycle of fertile women: relationship to age groups and fertility. Int J Endocrinol Metab. 2013;11:27-33.
- Takle ZJ, Legesse TG. The Effect of leptin on the hypothalamic-pituitary gonadal axis and puberty. Int J Heal Sci Res. 2017;7:156-64.
- Ziomkiewicz A, Ellison PT, Lipson SF, et al. Body fat, energy balance and estradiol levels a study based on hormonal profiles from complete menstrual cycles Human Reproduction Oxford Academic. Hum Reprod. 2008;23:2555-63.
- Farooq R, Ullah SL, Ishaq H. Relation of serum leptin with sex hormones of obese infertile men and women. J Appl Pharm Sci. 2013;3:60-5.
- Catteau A, Caillon H, Barrière P, et al. Leptin and its potential interest in assisted reproduction cycles. Hum Reprod Update. 2016;22:320-41.
- Hou N, Luo JD. Leptin and cardiovascular diseases. Clin Exp Pharmacol Physiol. 2011;38:905-13.
- Nirav RS, Braverman ER. Measuring adiposity in patients: the utility of body mass index (BMI), percent body fat, and leptin. PLoS One. 2012;7:e33308.
- Elias CF, Purohit D. Leptin signaling and circuits in puberty and fertility. Cell Mol Life Sci. 2013;70:841-62.
- Chojnowska K, Czerwinska J, Kaminski T, et al. Leptin/leptin receptor system in the regulation of reproductive functions and stress response in the european beaver. Curr Zool. 2019;65:197-203.
- Isah I, Aliyu I, Yusuf R, et al. Hyperprolactinemia and female infertility: Pattern of clinical presentation in a tertiary health facility in Northern Nigeria. Sahel Med J. 2018;21:1-5.
- Heller ME, Jacobs HS. Prolactin and infertility. Fertil Contracept. 1978;2:33-8.
- Moschos S, Chan JL, Mantzoros CS. Leptin and reproduction: A review. Fertil Steril. 2002;77:433-44.
- Odle AK, Akhter N, Syed MM, et al. Leptin regulation of gonadotrope gonadotropin-releasing hormone receptors as a metabolic checkpoint and gateway to reproductive competence. Front Endocrinol (Lausanne). 2018;8:367.
- Geber S, Brandão AHF, Sampaio M. Effects of estradiol and FSH on leptin levels in women with suppressed pituitary. Reprod Biol Endocrinol. 2012;10:45.
- Levine S, Muneyyirci-Delale O. Stress-induced hyperprolactinemia pathophysiology and clinical approach. Obstet Gynecol Int. 2018;4:1-6.
- Swift BE, Liu KE. The effect of age, ethnicity, and level of education on fertility awareness and duration of infertility. J Obstet Gynaecol Canada. 2014;36:990-6.
- Testa MR. On the positive correlation between education and fertility intentions in Europe: Individual-and country-level evidence. Adv Life Course Res. 2014;21:28-42.
- Kim J. Female education and its impact on fertility. IZA World Labor. 2016;228:1-10.
- Sauer MV. Reproduction at an advanced maternal age and maternal health. Fertil Steril. 2015;103:1136-43.
- Monstad K, Propper C, Salvanes KG. Education and fertility: evidence from a natural experiment. Scand J Econ. 2008;110:827-52.
- Mascarenhas MN, Flaxman SR, Boerma T, et al. National, regional, and global trends in infertility prevalence since 1990 a systematic analysis of 277 health surveys. PLoS Med. 2012;9:e1001356.
- Ugwu EO, Onwuka CI, Okezie OA. Pattern and outcome of infertility in Enugu: the need to improve diagnostic facilities and approaches to management. Niger J Med. 2012;21:180-4.
- Adegbola O, Akindele MO. The pattern and challenges of infertility management in Lagos, Nigeria. Afr Health Sci. 2013;13:1126-9.
- Benksim A, Elkhoudri N, Ait AR, et al. Difference between primary and secondary infertility in morocco: Frequencies and associated factors. Int J Fertil Steril. 2018;12:142-6.
- Al-Turki HA. Prevalence of primary and secondary infertility from tertiary center in eastern Saudi Arabia. Middle East Fertil Soc J. 2015;20:237-40.
- Chitme HR, Al Azawi EAK, Al Abri AM, et al. Anthropometric and body composition analysis of infertile women with polycystic ovary syndrome. J Taibah Univ Med Sci. 2017;12:139-45.
- Nguyen RH, Wilcox AJ, Skjaerven R, et al. Men's body mass index and infertility. Hum Reprod. 2007;22:2488-93.
- de Carvalho BR, Sobrinho DBG, Vieira ADD, et al. Ovarian reserve assessment for infertility investigation. ISRN Obstet Gynecol. 2012;2012:1-10.
- Jirge PR. Ovarian reserve tests. J Hum Reprod. 2011;4:108-13.
- Mutlu MF, Erdem A. Evaluation of ovarian reserve in infertile patients. J Turkish Ger Gynecol Assoc. 2012;13:196-203.
- Akande T, Adesiyun A, Aliyu S, et al. Prevalence of hyperprolactinemia among infertile patients with menstrual abnormalities and/or galactorrhea at a University Teaching Hospital, North West Nigeria. Arch Med Surg. 2017;2:55-9.
- Ugwa AE, Ashimi AO, Abubakar MY, et al. An assessment of serum prolactin levels among infertile women with galactorrhea attending a gynecological clinic North-West Nigeria. Niger Med J. 2016;57:178-81.
- Mennella JA, Pepino MY. Breastfeeding and prolactin levels in lactating women with a family history of alcoholism. Pediatr. 2010;125: e1162-70.
- Sharon HCM. 20 years of leptin role of leptin in human reproductive disorders in Journal of Endocrinology. Soc Endocrinol. 2014;223:49-62.
- Bheem P, Parmar D, Sharma NC. A study on serum FSH, LH and prolactin levels among infertile women. Int J Med Res Health Sci. 2015;4:876-8.