Original Article
, Volume: 10( 3)Comparison of Cupric Ion Adsorption by Two Types of Raw Olive Solid Residues Issued from Artisanal and Modern Oil Mills
- *Correspondence:
- Besma Khiari Environment Science and Technology Research Laboratory High Institute of Environment Sciences and Technologies of Borj Cedria, Tunisia Tel: +21671749100 E-mail: besmakhiari@yahoo.com
Received: May 24, 2017; Accepted: June 21, 2017; Published: June 23, 2017
Citation: Besma K, Abdel-Moumen S, Khaoula E. Comparison of Cupric Ion Adsorption by Two Types of Raw Olive Solid Residues Issued from Artisanal and Modern Oil Mills. ChemXpress. 2017;10(2):126.
Abstract
Adsorption using biomaterials is a relatively new process for wastewater treatment which showed its evidence as an alternative to the conventional physicochemical methods, still expensive and difficult to implement. This paper falls within this framework and presents the results of the study of the adsorptive properties of two types of raw olive residues coming respectively from artisanal and modern oil mills, with respect to the cupric ions in solution in order to reveal the level of efficiency of this process as well as the optimal operating conditions to its realization for the two materials. To do this, this study is carried out along two axes. The first is devoted to the characterization of each bio adsorbent in order to determine its porosity, its chemical composition, its surface groups, etc. The second axis deals with the adsorption itself, its kinetic model, as well as optimal conditions with its realization. This study shows amongst other things that olive cakes are excellent adsorbents for copper with a maximum retained quantity of 370 mg/g for adsorption at room temperature and at medium pH (around 5), using a bio adsorbent of small particle size (less than 0.5 mm), preferentially obtained from a super-press artisanal oil mill.
Keywords
Olive solid wastes; Traditional oil mill; Modern oil mill; Cupric ions bio sorption; Kinetics
Introduction
The damaging effects of olive residues derive in large part from their content of polyphenols which are very difficult to (bio) degrade. The valorization of this oil mill waste can be twice useful since it makes it possible on the one hand to avoid their harmful effects on the environment and on the other hand to reuse them in various fields, including wastewater treatment.
For example, olive cakes were used for a long time to produce activated charcoal. However, the relatively high cost of these activation operations prompted the researchers to design the olive solid residue as a bio-adsorbent as such.
Indeed, oilcake showed properties of retention of several molecules, in addition to an excellent selectivity. Abundant, low-cost and harmless, oil extraction residues are very favorable and useful in the treatment of wastewaters. These may contain copper in solution since the latter is widely used in industry but also because it can be a result of leaching from agricultural soils or to rush by the rains of the atmospheric copper from the combustion of fossil fuels. In solution, copper is found in a predominant form (cationic, anionic or molecular) according to the imposed pH. Cupric ion Cu2+ is the species under study in this work. It is a metal cation, which can be combined with different counter-ions (Cl-, NO3-, SO42-, etc.) which make it easy to be transported and can therefore be found in the different links of the life chain.
However, this element can be bio-accumulated and thereby have harmful effects on health and environment (lower agricultural yields, slowing the decomposition of organic matter, disorders in animals and plants, groundwater contaminations, etc.).
Previous Work
It is only in the last two decades that researchers have begun to consider crude oils as bio-adsorbents. To do this, it is necessary to go through a characterization of this matter, something carried out for example by Veglio who conducted a microanalysis using a scanning electron microscope equipped with an energy dispersive X-ray analyzer to collect qualitative information on the elemental composition of olive pomace. It contains potassium, calcium, sulfur and chlorine. The presence of carbon and oxygen in this organic material is also demonstrated. The photographs show that an average fraction of olive cake has certain porosity, mainly in the mesopore domain (2nm-50 nm).
Infrared spectroscopy analysis by Pagnanelli shows the presence of carboxylic groups (elongation of the C=O bond at 1704 and 1643 cm-1) and phenolic groups (elongation of the C=C bond at 1507 cm-1) [1]. These would be the active sites mainly responsible for the retention of heavy metals. In order to better elucidate the acid-base character of olive pomace, carried out a potentiometric assay of this bio adsorbent [2]. The curve obtained (pH versus volume of poured NaOH) is typical of substances with numerous and various weakly acidic active sites.
Studies by Pagnanalli show that the metal best adsorbed on olive residue is copper with a retention percentage of up to 85% followed by mercury (63%), lead (62.5%), cadmium (25%) and zinc (16%) [3]. According to the same study, adsorption of copper is also the fastest since the adsorbate-adsorbent equilibrium is reached after 2 h against 3 h for zinc, 4 h for mercury and 6 h for lead.
Veglio have also carried out column adsorption tests. A mass of 80 g of olive cake is compacted and introduced into a column (diameter: 24 mm, length: 32 cm) [4]. A solution of cupric ions at 40 mg/l is introduced and the concentration of the eluate is measured as a function of time in order to determine the breakthrough curve. This reveals that the break point is reached after adding about 1 liter of solution. It also shows that the minimum amount of olive pomace required for column adsorption is 100 kg of adsorbent per 1 kg of adsorbed cupric ion.
As for the mechanism of retention of cupric ions on olive cakes, speculate with caution an exchange of ions between the moving protons of the acid groups and Cu2+ in solution [5]. Indeed, bioadsorption is characterized by different physico-chemical mechanisms operating simultaneously and the demonstration of a particular mechanism remains particularly difficult. But the decrease of the adsorbent power in acidic media (pH<5) suggests a competition between the protons in solution and the cupric ions, which makes us think that the adsorption mechanism is an ion exchange.
In addition to the effect of the operating parameters on biosorption, the effect of rinsing the olive cake on the adsorbent power was elucidated by Veglio Indeed; the adsorption tests reveal the presence of a slight yellow color in solution which may be associated with the increase of the COD in the liquid phase [6]. This is probably due to the soluble organic compounds present in the solid.
To avoid that COD of the liquid phase exceeds the limit indicated by the Italian standard (which amounts to creating a new pollution problem by trying to eliminate another one) the same Italian authors proceeded to rinse the bio adsorbent with distilled water before use. When the solid is rinsed only once, the COD increases from 1250 mg/l at the start of the operation to 2250 mg/l after 3 h of adsorption. If the solid is rinsed twice, the COD stabilizes at a lower level: 600 mg/l. It is evident that the rinsing solution must be specifically treated before being discharged into public pipelines or into a natural environment. This simple or double rinsing has no effect on the absorbent properties of olive pomace because the quantity of Cu2+ retained remains unchanged.
Contact times ranging from 1 min to 5 days have been cited in the literature. However, the maximum adsorption yields are generally reached after a short contact time. For example, Fiol showed that the maximum adsorption efficiency of copper is reached within 1 hour [7].
The study of the efficiency of the adsorption of copper on olive wastes at different pH shows that the optimum pH at retention is 5. Indeed, Pagnanalli postulated that for a pH less than 5, protons abundant in solution compete with the cupric ions, thus preventing them from being retained on the surface [8]. For pH above 5, on the other hand, the active sites are probably in molecular (non-ionized) form and copper may also be precipitated.
Fiol studied the adsorption capacity of copper (initial concentration of 100 mg/l) by olives (1.5 10-4 mol/l) in solutions adjusted to pH ranging from 1.5 to 6 [9]. The results obtained after 1 hour of contact time show an increase in the adsorption yield with the pH increase, from 0% at pH between 1.5 and 2.5 to more than 85% at pH 4 to 7. The same phenomenon was noted by other authors with other biomasses. The decrease of the adsorption efficiency with the acidification of the medium is explained by the increasing competition of the H+ ions with the metal cations for the adsorption sites of the adsorbent [10,11].
Work has shown that the rate of adsorption increases with increasing temperature for agricultural products. However, there is a temperature around 60°C. At which the adsorption level may decrease. This phenomenon is attributable to the solubilization at this temperature of low molecular weight organic compounds present in olive pomace and involved in the adsorption of cupric ions [12].
The adsorption yields of heavy metals increase with the increase in the concentration of olives. This fact can be explained directly by the increase in the number of available adsorption sites. Veglio also studied the effect of the amount of adsorbent used on the efficiency of the operation [13]. It is then evident that the higher is the concentration of olive pomace, the better is the cupric ions retention. The adsorption capacity thus moves from 20% for a concentration of 1 g/l to 85% for 100 g/l.
The grain size of the solid also has a great influence on the adsorptive properties of olive pomace. Indeed, the finer the particle size, the more efficient the adsorption. According to Pagnanalli, the difference in the adsorbed amount can reach 45% between a fraction larger than 355 μm and another smaller than 212 μm [14]. Finally, three regeneration trials were conducted by Veglio in the three regeneration solutions (HCl, CaCl2 and EDTA), there is good desorption since the cupric ions are retained during a second adsorption operation carried out on the regenerated cake [15]. However, the adsorbed amounts remain well below that of the first adsorption on virgin olive residues.
Experimental Study Protocol
The olive residues studied are of two types:
1. Trad type: these are crude olives supplied by an artisanal oil mill and the corresponding olives (traditional type)
2. Mod type: these are raw cakes provided by a modern oil mill with continuous system (modern type).
The water content is determined by steaming at 105°C, to a constant mass, that of ash is obtained by heating in an oven at 550°C for 15 min. The percentage of the fat is obtained thanks to Soxhlet extraction method. Several sizes were obtained by different sieves (2.8 mm, 1.7 mm, 1.5 mm and 0.5 mm).
The crystalline compounds are detected by X-ray diffraction. The infrared spectrum of olive pomace produces information on the functional groups which constitute them and thus provides information on the active sites involved in the phenomenon of adsorption of heavy metals [16]. A few milligrams of olive pomace were then crushed with a few milligrams of potassium bromide (KBR) and then pelleted. The two pellets obtained (corresponding to the two types of olive cake) are introduced into the infrared spectrophotometer.
The BET method was used to study the texture of our samples and determine their specific surface area, porosity, porous and micro porous volume. An automated device (Micrometrics ASAP 2000) was used to do this. First, degassing of the sample under vacuum and at appropriate temperature (taking care to respect the physical properties of the sample) is carried out in order to evacuate the molecules of water or other gases which have settled in the sample [17]. The mass of the degassed sample is noted in order to introduce it into the final calculation of the specific surface area. When the sample is prepared, the sample holder is placed on the analysis station and is immersed in a cooling bath (liquid nitrogen).
The dead volume, that is to say the volume of the sample holder not occupied by the sample itself is determined by means of helium (the adsorption of helium at the surface of the sample at low temperature is considered negligible).
The method used nitrogen as an adsorbate at the boiling point of liquid nitrogen under atmospheric pressure. It employs volumetric measurements to determine the quantities of adsorbed gas as a function of the equilibrium pressure. Concretely, the isotherm is determined by the sequential introduction of known quantities of adsorption gas into the sample holder [18]. At each step the adsorption of the gas by the sample occurs and the pressure in the isolated volume drops until the adsorbate and the remaining gas are in equilibrium. The quantity of nitrogen adsorbed for each equilibrium pressure is determined by the difference between the quantity of gas introduced initially and that remaining actually.
For the adsorption tests, preliminary steps are necessary. The olive residues are rinsed by contact, with stirring, with distilled water for 3 h [19]. The whole is filtered under vacuum. The cakes recovered and steamed at 70°C for 24 h are thus ready for use.
To determine the contact times, one liter of an olive-pomace suspension (10 g/l) was prepared in distilled water. The chronometer is triggered as soon as 200 mg of CuCl2 (200 mg/l) are added, the pH is then rapidly adjusted to 5 by addition of a few drops of HCl (0.1 N). A volume of 1 ml of solution is then recovered at different time intervals and then centrifuged in order to separate the liquid phase from the adsorbent.
The effect of pH and more particularly the determination of the optimum pH are achieved by contacting a mass of 20 mg of CuCl2 with 100 ml of an olive residue suspension of 10 g/l concentration. The pH is adjusted to 3, 5, 7 and 9 by addition of HCl (0.1N) or NaOH (0.1N). Two sets of samples are thus prepared, each corresponding to one type of olive solid residue. The mixtures are then stirred for 2 h and then centrifuged.
For each of the five particle sizes studied (?> 2.8 mm, 1.7 mm <? <2.8 mm, 1.5 mm <? <1.7 mm, 0.5 mm <? <?1.5 mm and ? <0.5 mm), 100 ml of a suspension of olive cake with a concentration of 10 g/l is prepared and brought into contact with 20 mg of cupric chloride. The pH is adjusted to 5 by adding a few drops of HCl (0.1 N). The suspension is stirred for 2 h at room temperature before being centrifuged.
To establish the adsorption isotherm, different CuCl2 samples are contacted with 100 ml of an olive residue suspension (10 g/l) so as to have several adsorbate concentrations ranging from 20 mg/l to 300 mg/l. The samples were stirred at room temperature (18°C) for 2 h before being centrifuged.
In all these experiments, the concentration of the liquid phase in cupric ions is measured by flame atomic absorption using an AAS VARIO 6 spectrometer. This apparatus is controlled by software which makes it possible to obtain directly the calibration curves and to deduce the values of the concentrations of the samples. It should be noted that this device is equipped with a non-specific absorber corrector (deuterium corrector) and a dual-beam monochromator. The wavelength used for the determination of copper is 324 nm. The flame, on the other hand, is generated by an air/acetylene mixture.
Results of Characterization and Discussions
Olive residues from an artisanal oil mill have a water content of 10.25% close to the 11.70% of the cakes provided by the modern oil mill. This is consistent with other work that states that olive residues from a continuous centrifugal crushing system are richer in water than those from a discontinuous press system. The ash rate is 3.42% and 3.75%, respectively [18]. These low values of mineral matter are easily foreseen since the product studied is essentially organic. This does not prevent us from elucidating their nature, using X-ray diffractometry, for a better characterization of the bio adsorbent.
The olive residues from traditional oil mills contain slightly more fat (11.4%) than those from modern oil mill (10%). Since the olive crushing is in the first case, is manual, the remaining oil fraction is slightly larger.
The presence of oil in the olive pomace, also gives information on the chemical groups found there. Indeed, since the oil is composed of triglycerides, the presence of ester groups and of carboxyl groups can already be asserted. The latter come from free acidity (hydrolysis of esters and release of fatty acids due to time and heat) observed when the oil rancid a little, which is probably the case since our olives date from previous harvests.
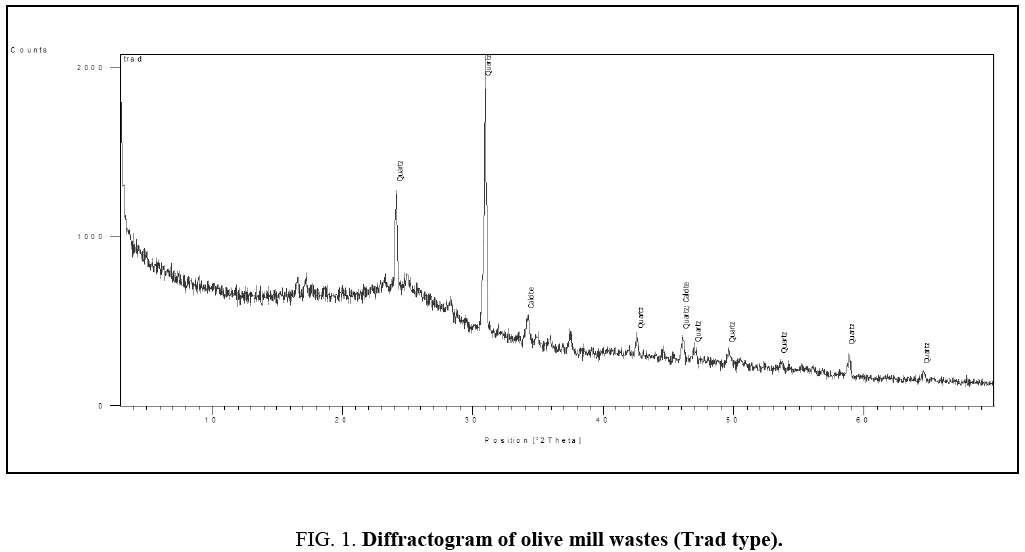
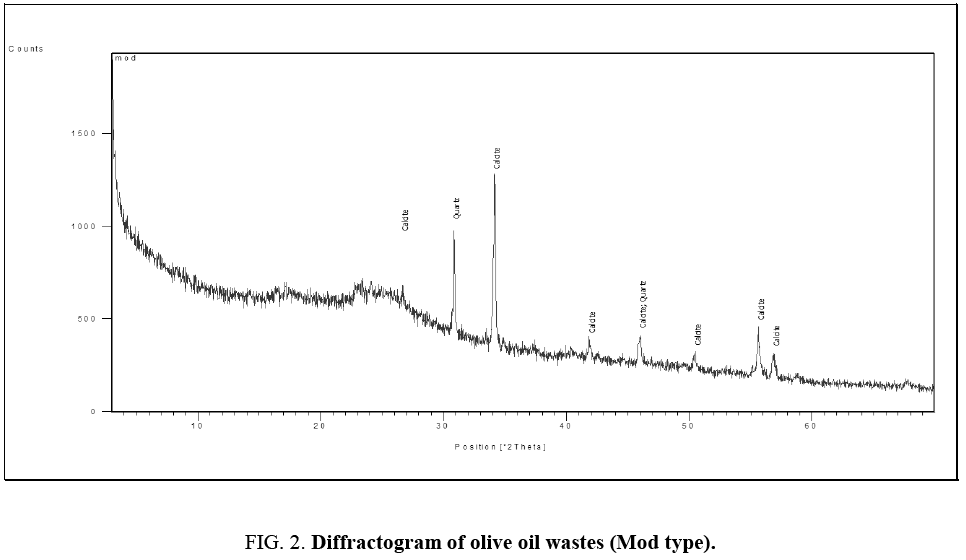
Figure 1 and 2 show the diffractograms of the two types of olive mill wastes.
Figure 1: Diffractogram of olive mill wastes (Trad type).
Figure 2: Diffractogram of olive oil wastes (Mod type).
The presence of characteristic peaks of calcite CaCO3 and quartz SiO2 is observed for both types of olive. Calcite, crystallized in the rhombohedral structure, is one of the most abundant carbonates in nature. Quartz, of hexagonal crystalline structure, is a very common mineral since it constitutes more than 12% wt of the lithosphere.
It is therefore very probable that these elements, present in the soil, have been assimilated by the roots of the olive tree and then found, as is the case, in the form of traces in the olive-pomace.
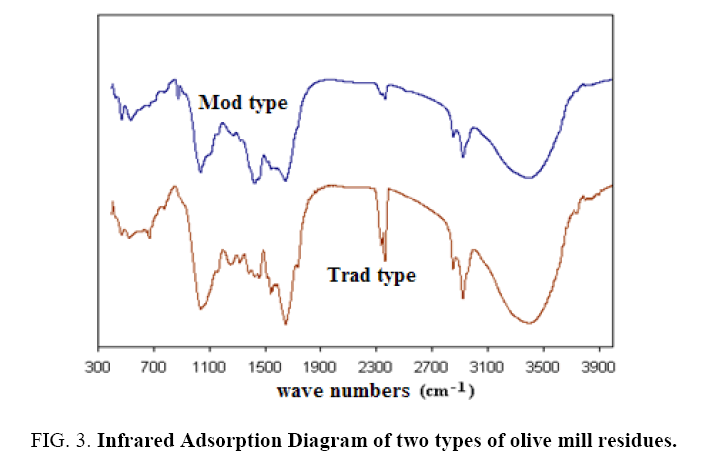
The analysis by infrared spectroscopy (Figure 3) shows the presence of carboxylic groups (elongation C=O at 1704 cm-1) and phenol groups (elongation of the C=C bond around 1500 cm-1). We also find the alcohol groups around 2900 cm-1. The mass of adsorption towards 3420 cm-1 is characteristic of the vibrations OH of hydration water.
Figure 3: Infrared Adsorption Diagram of two types of olive mill residues.
The existence of these groups is perfectly foreseeable insofar as they are characteristic of the lignin, fatty acids and cellulose constituting the olive pomace. These functional groups can already be suspected of being directly involved in the adsorption process.
The two types of olives gave a very small specific surface area not exceeding 2 m2/g. The pore volume is also very small. It is 0.0128 cm3/g for the Trad type and 0.0213 cm3/g for the Mod type.
These low values certainly do not mean the absence of porosity for olive cake. Indeed, the literature reports values of the specific surface area between 10 and 20 m²/g for this olive waste [20]. Although N2 is the gas most commonly used in BET, it may not be appropriate for our biomass, and more logical results could be obtained with another gas (such as helium with a smaller atomic radius) that would diffuse throughout the available surface of the pores, and which is used in many biomass adsorption works. Measurement errors are unlikely since each of the tests has been repeated twice and the more so as the adsorption of copper by the pomace is good, as we will see in next sections.
Results of the Sorption and Discussions
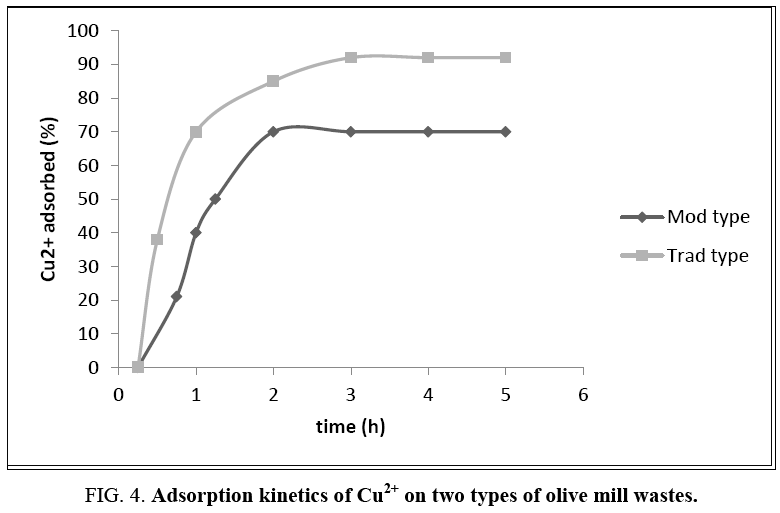
By varying, as described above, the contact time between the metal cations and the solid substrate, the curve showing the quantity of cupric ions adsorbed as a function of time is plotted (Figure 4).
Figure 4: Adsorption kinetics of Cu2+ on two types of olive mill wastes.
It is observed that the sorption kinetics is relatively rapid since the adsorbate-adsorbent equilibrium is reached within 2 h, whereupon the residues become saturated. The maximum adsorbed quantity is then 92% for the Trad type olives (conventional oil mill) and 70% for Mod type (modern oil mill). These satisfying retention rates as well as rapid kinetics prove that these olive wastes are as efficient for the elimination of this heavy metal as the activated carbon [21]. Their use in industrial pretreatment stations is therefore quite feasible, and even economically profitable.
On the other hand, the difference in the quantity retained between the two samples may have several reasons, which may be combined: The chemical composition of the two types of pomace could be the primary cause. Indeed, according to a previous study in the laboratory of these same olives, elemental analysis revealed that the olives from traditional oil mills have a carbon content of 58% and hydrogen content of 8% against 47% and 5% respectively for olive residue from modern oil mills. This directly influences the number of organic active sites. Since the remaining residual oil content in the spent oils after centrifugation is slightly less than that of the olives obtained by a press system, it could also be thought that more active sites are available in the Trad type. The heterogeneity of these pellets and the difficulty observed in the filtration phases suggest that additional adsorption mechanisms may come into play, thus improving their adsorption power.
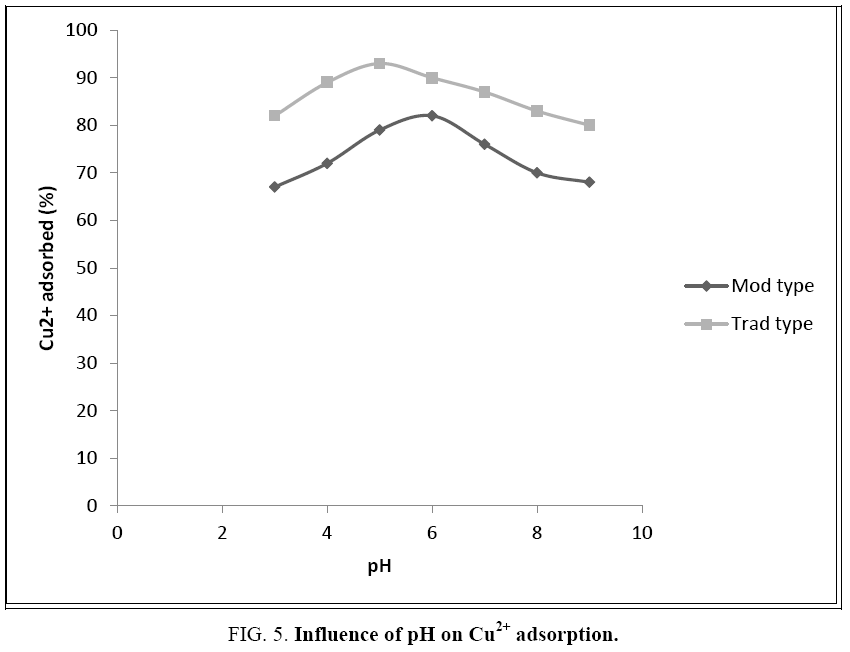
The pH plays a very important role in the mobility of copper (Figure 5). The adsorbent power reaches a maximum at pH=5 for the Trad type and at pH=6 for the Mod type. It could be concluded that an acidic pH leads to the solubilization of the metal salt, the desorption of the protons and the adsorption of the metal ions. The solubility thus decreases when pH increases, goes through a minimum and then increases when the element is found in anionic form. Copper is therefore more or less mobile according to its oxidation-reduction state. To ensure maximum retention, it is therefore necessary to impose these two optimal pHs on the adsorbent-adsorbate system. Nevertheless, we note that for this entire pH range, the removal of copper exceeds 50%.
The granulometry is also a parameter influencing the adsorptive power of olive cakes. Indeed, this one is more important for the small sizes. Thus, the percentage of retention of cupric ions decreases by about 30% from a fine powder smaller than 0.5 mm to particles with a diameter exceeding 2.8 mm. In fact, the more the solid is divided, the greater is its specific surface area and the better is the adsorption.
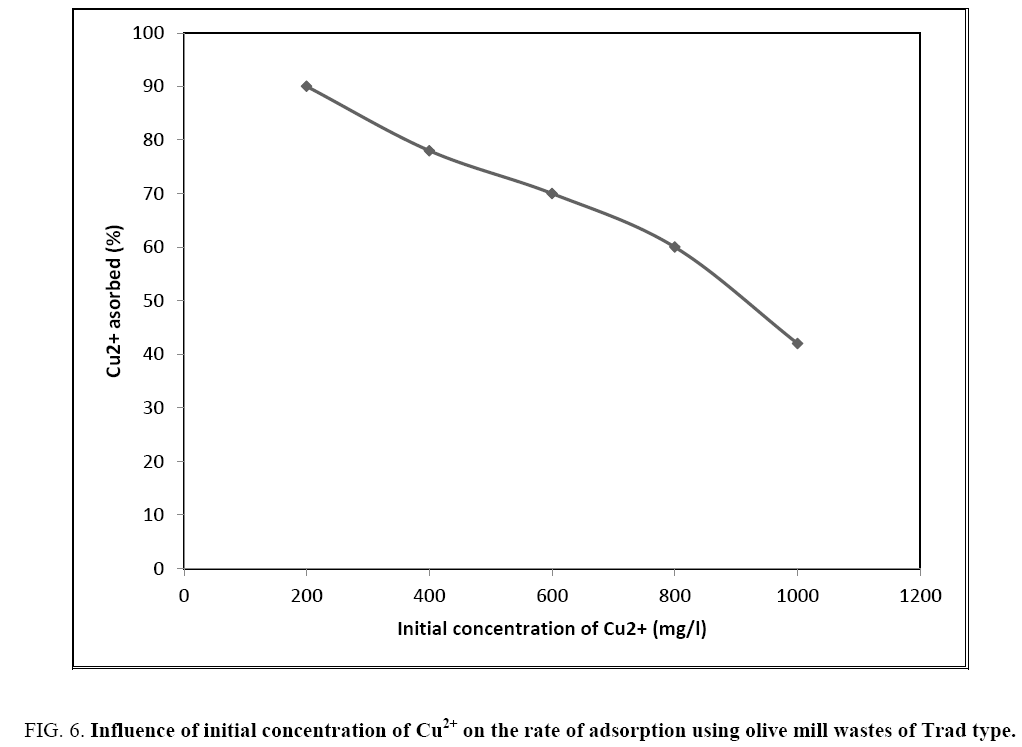
The influence of the initial concentration of copper on the adsorption percentage of Trad-type residues is also shown in Figure 6. The same pace is obtained for the Mod type.
Figure 6: Influence of initial concentration of Cu2+ on the rate of adsorption using olive mill wastes of Trad type.
It is found that the higher the initial copper concentration, the less efficient the adsorption. Indeed, biomass has, as already stated, a well-defined retention capacity beyond which there is saturation. If the effluent is slightly or moderately loaded with copper, the latter will be totally or predominantly retained. If, on the other hand, the Cu2+ concentration is very large, only a small fraction will be retained. The effluent remains sufficiently loaded with heavy metal to damage the environment or public sewers.
This does not mean, however, that adsorption is a limited-efficiency depollution technique because industrial used waters are rarely highly concentrated in copper. By way of example, the average Cu2+ concentration of an effluent from a tannery is between 10 and 30 mg/l whereas the curve shows a drop in the adsorption power beyond 50%, starting from a copper concentration of 800 mg/l.
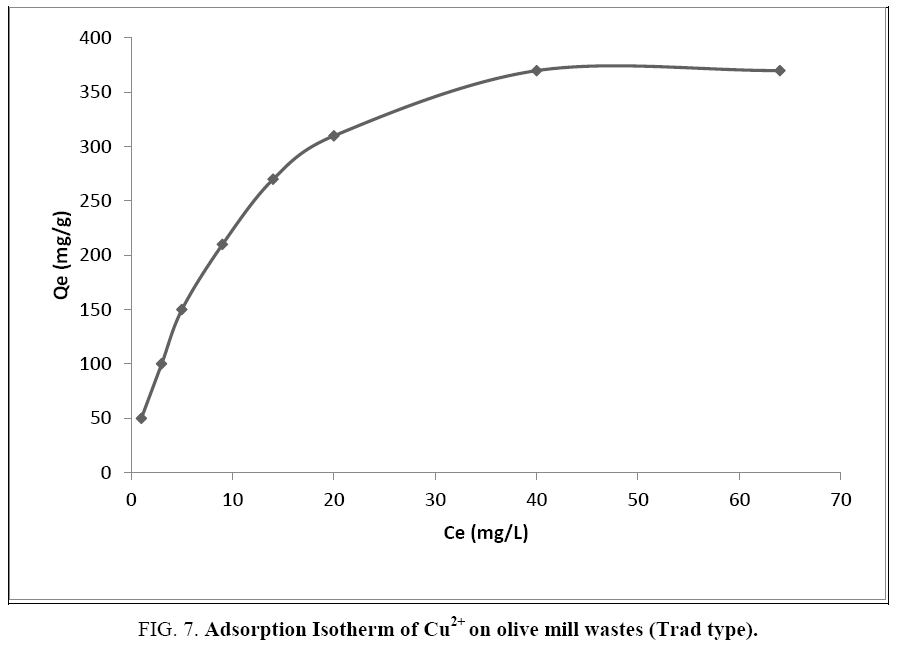
The amount of adsorbate on the present qe adsorbent (expressed in mg per g of adsorbent) is plotted against the amount of adsorbate remaining in solution Ce (expressed in mg/l) (Figure 7).
Figure 7: Adsorption Isotherm of Cu2+ on olive mill wastes (Trad type).
The obtained shape corresponds to a type 1 isotherm. This is characteristic of an adsorbent whose microporous volume is particularly high. Saturation of adsorption sites occurs gradually from low concentrations. The shape of the isotherm is characterized by a long plateau indicating a weak formation of multilayers. This statement must, however, be given with great caution because the shape of the isotherm gives precise information on the texture of the solid only in the case of gaseous adsorbates. In solution, the surface functional groups are of greater importance than the porosity of the adsorbent, which can therefore only be elucidated with the BET method.
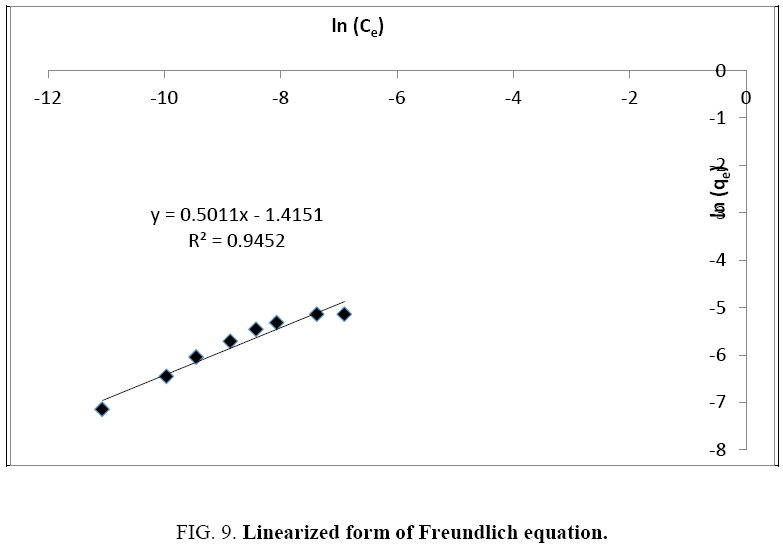
Two mathematical models can describe such an isotherm. Indeed, although this form is to be compared to the Langmuir model, the Freundlich model is not excluded.
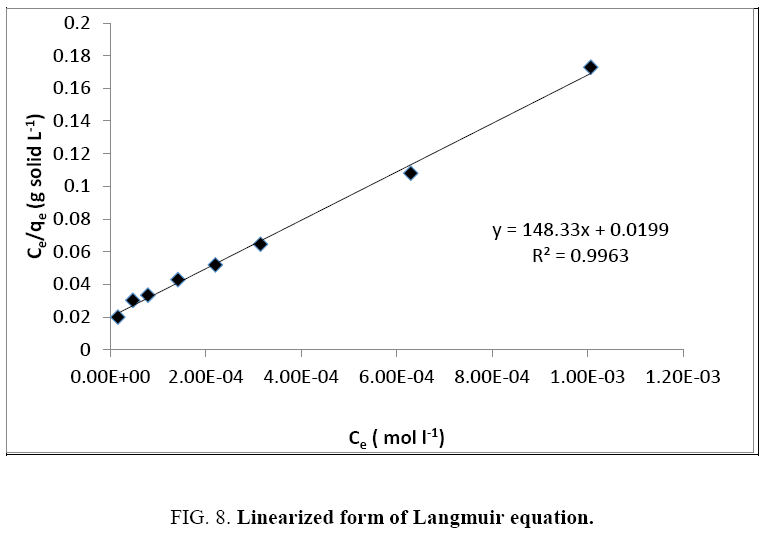
To decide which one of the two theories better describes the adsorption of Cu2+ on olive cake, we perform a linearization followed by a comparison of the regression coefficients (R²).
The data deduced from these two linearizations (Figure 8 and 9) are listed in Table 1.
| Langmuir model | Freundlichmoel | ||||
|---|---|---|---|---|---|
| qmax | KL (lmol-1) | R2 | KF | n | R2 |
| 6,74.10-3 mol.g-1 428 mg.g-1 |
7456 | 0,9963 | 0.24 | 2 | 0,9452 |
Table 1: Constants and regression coefficients of the two models.
A better regression coefficient is obtained for the Langmuir isotherm. It is therefore the model that best describes the adsorption of Cu2+ on olive solid residue, and its approximations are therefore valid to simplify the unfolding of the phenomenon. We can therefore consider that:
• The adsorption sites on the surface of the biomass are homogeneous from an energy point of view.
• Each of these sites can adsorb a single molecule, and a single layer of molecules can be formed
• Each site has the same affinity for the molecules in solution;
• There are no interactions between the adsorbed molecules.
On the other hand, the maximum quantity of copper that can be adsorbed by olive cake, according to this model, is 370 mg/g of solid residue. This is an excellent retention power compared to that of activated carbon. Indeed, according to a previous study, 1 g of activated carbon modified by various chemical agents retains 212 mg of copper. It can be seen then that the retention capacity of this biomass is greater than that of the activated carbon and this without any prior treatment.
Conclusion
This study demonstrates the efficiency of olive residue in the removal of copper present in liquid effluents from various industries. Indeed, this oleic waste has been found to be highly adsorbing to cupric ions with a maximum adsorbed amount up to 370 mg/g under the optimum conditions, namely medium acid pH (around 5) and solid fine powder (Diameter <0.5 mm) preferentially obtained from an artisanal oil mill. The possibility of regenerating this biomass makes it possible to use it in several successive adsorption-desorption cycles, thus further reducing the cost of the depollution operation.
References
- Barett E, Joyner L, Halenda P. The determination of pore volume and area distributions in porous substances. I. computations from nitrogen isotherms.J Am Chem Soc. 1951;73(1):373-380.
- Braunuer S, Emmet H, Teller E. Adsorption of gases in multimolecular layers. J Am Chem Soc. 1938;60(2): 309-19.
- Caputo AC, Scacchia F, Pelagagge PM. Disposal of by-products in olive oil industry: Waste-to-energy solutions. ApplTherm Eng. 2003;23(2):197-214.
- Zhou Y, Zhang L, Cheng Z. Removal of organic pollutants from aqueous solution using agricultural wastes: A review. J Mol Liq. 2015;212:739-62.
- Chouchene A, Jeguirim M, Khiari B, et al. Study on the emission mechanism during devolatilization/char oxidation and direct oxidation of olive solid waste in a fixed bed reactor. J Anal ApplPyrol. 2010;87(1):168-74.
- Chouchene A, Jeguirim M, Favre-Reguillon A, et al. Energetic valorization of olive mill wastewater impregnated on low cost absorbent: Sawdust versus olive solid waste. Energy. 2012;39(1):74-81.
- Defay F, Pringorine C, Bellemans A.Surface tension and adsorption, London, 1966.
- Dubinin M. Progress in surface and membrane science.Chemicals and Environment.1965;60:235.
- Fiol N, Villaescusa I, Martínez M, et al. Sorption of Pb (II), Ni (II), Cu (II) and Cd (II) from aqueous solution by olive stone waste. Sep Purif Technol. 2006;50(1):132-40.
- Fiset JF, Blais JF, Ben Cheikh R, et al. Review on metal removal from effluents by adsorption on sawdusts and wood barks. Revue des sciences de l’eau 13. 2000;pp:325-349.
- Galli EF, Di Mario F, Rapana P, et al. Copper biosorption by Auriculariapolytricha. LettApplMicrobiol. 2003;37(2):133-7.
- Hammad M, Badarneh D, Tahboub K. Evaluating variable organic waste to produce methane. Energ Convers Manage. 1999;40(13):1463-75.
- Hawari A, Rawajfih Z, Nsour N. Equilibrium and thermodynamic analysis of zinc ions adsorption by olive oil mill solid residues. J Hazard Mater. 2009;168(2):1284-9.
- Masghouni M, Hassairi M. Energy applications of olive-oil industry by-products:—I. The exhaust foot cake. Biomass and Bioenergy. 2000;18(3):257-62.
- Pagnanelli F, Mainelli S, Vegliò F, et al. Heavy metal removal by olive pomace: Biosorbentcharacterisation and equilibrium modelling. ChemEng Sci. 2003;58(20):4709-17.
- Pagnanelli F, Toro L, Vegliò F. Olive mill solid residues as heavy metal sorbent material: A preliminary study. Waste Manage. 2002;22(8):901-7.
- Reddy BR, Mirghaffari N, Gaballah I. Removal and recycling of copper from aqueous solutions using treated Indian barks. Resources. Conservation and Recycling. 1997;21(4):227-45.
- Sun LM, Meunier F. Adsorption. Aspects théoriques. Techniques de l'ingénieur. Génie des procédés. 2003;2(J2730):1.
- Veglio F, Beolchini F, Prisciandaro M. Sorption of copper by olive mill residues. Water Research. 2003;37(20):4895-903.
- West S, Analytique C.In: De Boech,Larcier, editors.Bruxelles. Paris;1997.
- Yacoub Y. Valorisation des sous produits. L’investisseuragricole. 1997;19:17-8.