Research
, Volume: 21( 1) DOI: 10.37532/0974-7486.21.001Characterization of Corrosion properties of Aluminium 6013-Red Mud Metal Matrix Composites in Sodium Hydroxide Medium
- *Correspondence:
- Chandrashekara KN
Department of Chemistry,
SJC Institute of Technology,
Chikkballapur, Karnataka,
India
Tel: 9591549884
E-mail: chandrasjcit2014@gmail.com
Received: October 28, 2022, Manuscript No. TSMS-22-78526; Editor assigned: October 31, 2022, PreQC No. TSMS-22-78526 (PQ); Reviewed: November 14, 2022, QC No. TSMS-22-78526; Revised: January 09, 2023, Manuscript No. TSMS-22-78526 (R); Published: January 16, 2023, DOI: 10.37532/0974-7486.23.21.001
Citation:Chandrashekara KN, Sreenivasa K, Krupakara. Characterization of Corrosion properties of Aluminium 6013-Red Mud Metal Matrix Composites in Sodium Hydroxide Medium. Mater Sci Ind J. 2023;21(1):001.
Abstract
The current work is the concentrate of assembling and consumption portrayal of aluminum 6013/red mud particulate MMCs in basic medium. The MMCs are produced by utilizing vortex strategy where fluid soften metallurgical procedure was utilized which is additionally called stir casting technique. Composite materials containing red mud particulates with 2,4 and 6 weight rate were produced by the above strategy. Aluminum 6013 alloy was likewise casted so as to contrast the outcomes and composite materials. The samples are machined according to ASTM principles. The tests utilized for portrayal concerning corrosion properties were static weight reduction corrosion test and potentiodynamic polarization. Tests were done in sodium hydroxide arrangements with three distinct concentrate solutions. Polarization tests were directed utilizing electrochemical work station fabricated by CH instruments USA. The outcomes acquired for MMCs were contrasted and the outcomes got for network compound uncovered that the composite materials show improved corrosion opposition and diminishes corrosion rate. Hence MMCs containing red mud particulates are more reasonable in aviation, car and marine enterprises applications than the matrix compound.
Keywords
Aluminium 6013; Electrochemical workstation; Red mud; Polarization; Vortex
Introduction
Creators will get more alternatives for uses of materials in numerous fields by the utilization of composite materials as they have great quality at high temperature, great auxiliary inflexibility, dimensional strength and light weight [1]. These aluminum 6xxx arrangement amalgams have great formability, weld ability, machinability and consumption resistance [2]. Zaki Ahmed and Abdul Aleem contemplated the consumption conduct of aluminum 6013 fortified with red mud particulates in 3.5 percent sodium chloride and report that the corrosion rate diminishes with increment in season of presentation [3]. Broad writing study shows that the exploration work as for red mud fortified with aluminum 6061 and ZA-27 have been finished [4]. However, the examination work regarding aluminum 6013 composites is very panics. Henceforth this work has been taken up.
Materials and Methods
Material selection
Aluminum 6013 composite, which shows incredible casting properties and sensible quality, was utilized as base combination. This compound is most appropriate for large scale manufacturing of lightweight metal projecting. The compound organization of aluminum 6013 amalgam is given in Table 1. Red mud which is utilized as fortified as particulates contains oxides of aluminum, iron, calcium, sodium, titanium, zirconium and vanadium. It has a layered structure and is a three dimensional organization of oxygen silicon, tetrahedron sharing all the four corners. It has got explicit gravity 2.64 (2), with hardness of 9.8 on the VHN.
| Element | Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Al |
| Percentage | 0.6 | 0.5 | 1.1 | 0.2 | 0.8 | 0.1 | 0.25 | 0.1 | Bal. |
TABLE 1. Composition of aluminium 6013.
Specimen preparation
The castings are exposed to machining to fabricate sample for static weight loss test and potentiodynamic polarization test. The material was cut into 20 mm × 20 mm pieces utilizing a grating cutting wheel. The matrix composite likewise cast under indistinguishable conditions for examination. Round and hollow sample of the castings were progressively ground utilizing 240, 320, 400 and 600 SiC paper and were cleaned by standard metallographic strategies and degassed in (CH3)2CO and dried. The examples were weighed up to fourth decimal spot utilizing electronic parity and furthermore the example measurements were noted down utilizing vernier bandage. For polarization test examples of 2 cm length, 1 cm broadness and 1 mm thickness rectangular examples were produced by oppressing MMCs and framework to maching in CNC machine [5-7].
Corrosion testing
Inundation technique was utilized to do the weight loss test as per ASTM standard G69-80. 20 mm breadth and 20 mm tallness sample of round and hollow shape were produced from the castings. At first the sample was made to go through standard metallographic strategies with emery paper and diamond glue so a mirror finish surface is acquired. At that point samples were exposed to exhaustive cleaning by washing in refined water. At that point dunked in acetone and air dried. At that point weights of the samples were resolved precisely by utilizing electronic parity which shows weight up to fourth decimal spot. Subtleties of the strategy for testing utilized for discovering the consumption rate is as given beneath. ASTM guidelines were embraced for getting the mirror finish on MMCs and matrix composite. The corrodents utilized were 0.25, 0.5 and 1.0 molar solutions of sodium hydroxide. Static or ordinary weight reduction strategy was utilized to contemplate the consumption property. The test span was changed from 24 hours to 96 hours, in time frames hours. The samples were plunged in beakers containing 200 cm3 sodium hydroxide solutions. According to ASTM norms the proportion of corrodent to uncovered zone of sample was kept up at 50 cm3 of HCl to 1 mm2 of surface zone. To limit the sullying of the fluid arrangement and misfortune because of dissipation, the flasks were secured with paraffin during the whole trial. After the predefined time the example were taken out from the measuring utencils and cleaned with a brush to eliminate saved erosion item and washed with refined water. At that point plunged in acetone and air dried. At the point when the sample gets dried the final weight after corrosion is found so the distinction in starting and final weight gives the weight reduction. At that point corrosion rates were dictated by utilizing the formula, corrosion rate=534 W/DAT mpy where W is the weight reduction in grams, D is thickness of the example (g cm-3), an is the region of the example (inch) and T is the presentation time in hours.
Electrochemical estimations were done utilizing electrochemical work station model CHI 608E series fabricated by CH instruments, USA which associated with cell with a reference cathode, counter terminal and an arrangement for interfacing the made example as working anode. Figure 1 shows the electrochemical work station. The electrochemical examinations were completed in a 100 cm3 container which is utilized as cell containing an Ag/AgCl terminal as the reference anode and a platinum wire as the Counter Cathode (CE). 1 cm2 region of the example was presented to the destructive condition. Figure 2 shows the cell utilized for the test.
Results and Discussion
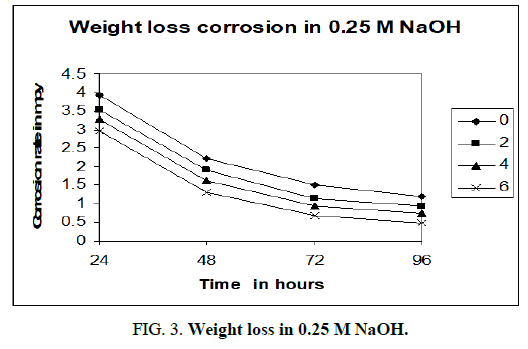
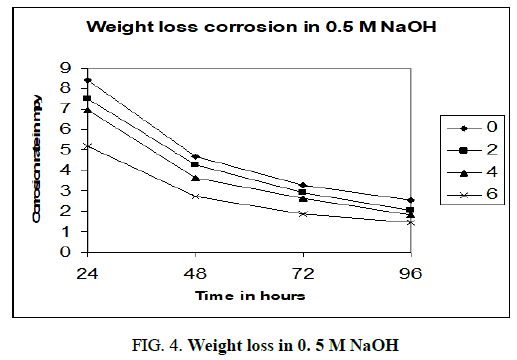
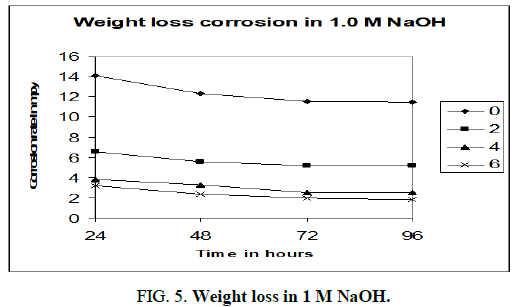
Figures 3-5 shows the computer simulations of the results obtained for the static weight loss corrosion tests of aluminium 6013/red mud particulate composites and matrix alloy. The graphs are drawn by taking time of exposure on x axis and corrosion rate in mpy on y axis.
The above charts (Figure 3-5) are PC re-enactments of the consequences of customary weight loss test. In all the three charts MMCs and matrix show diminished corrosion rate with increment in season of presentation. Unmistakably the matrix alloy compound and MMCs go through passivation consequently corrosion rate diminishes. Dark film covering the surfaces of MMCs and lattice amalgam was found after the expulsion of samples from corrosion medium. The inactive layer, most likely comprising of Al(OH)3, shields the surface from further corrosion. As indicated by McCafferty, the solution gets exhausted in hydrogen ions as the response continues and stops when all the H+ ions are taken out [8]. Gireesha who got comparable outcomes in Al 6061/red mud MMCs, underpins the perspective on McCafferty and our outcomes [9]. It very well may be obviously seen that as the level of fortification like red mud particulates increments in the combination the corrosion rate diminishes. Matrix compound aluminium 6013 shows higher corrosion rate than the MMCs in all convergences of sodium hydroxide. Aluminium 6013 goes through high corrosion in the mediums. The fortification red mud particulates are not influenced by the corrosion medium during the investigation because of its latency and ceramic nature. Red mud particulates won’t viably have engaged with the electrochemical mechanism of the MMCs. Subsequently according to the above charts the corrosion obstruction increments with increment in fortified content. Naddoni Saachin Govind, et al., got comparative outcomes when they tried aluminium 7075 combination strengthened with beryl particulates in sodium hydroxide medium by static weight reduction technique [10]. They report that the ceramic idea of beryl particulates will forestall corrosion because of the way that it won’t be assaulted by the alkaline solution.
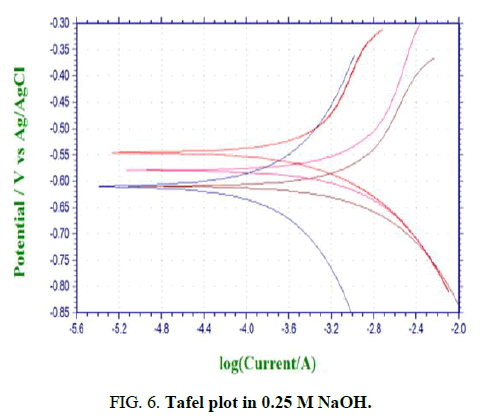
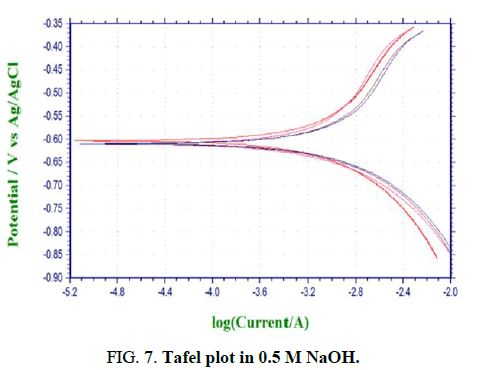
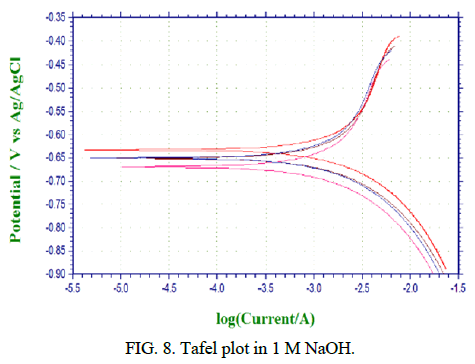
Figures 6-8 shows the PC simulations of the outcomes got for the polarization trial of aluminium 6013/red mud particulate composites and matrix alloys. The charts are legitimately given by the PC connected to the electrochemical work station.
The purpose of crossing point between the cathodic and anodic bend gives Icorr. The corrosion rate in mpy is determined by the product joined to electrochemical work station. The polarization bends for both the base matrix alloy and the Al 6013/red mud composites where potential is plotted versus galvanic current are introduced in Figures 6-8. Diminishing in the grouping of the corrodent brings about reduction in consumption obstruction for MMCs and matrix alloy (Table 2).
| Percentage reinforcement normality of NaOH | Corrosion rate in 105 mpy | |||
|---|---|---|---|---|
| 0 | 2 | 4 | 6 | |
| 1 | 3.1772 | 3.1518 | 2.7212 | 2.479 |
| 0.5 | 3.0689 | 2.8332 | 2.6204 | 2.4568 |
| 0.25 | 2.7516 | 2.6895 | 2.4538 | 2.356 |
TABLE 2. Corrosion rate in mpy of matrix and composites in sodium hydroxide.
Figures 6-8 shows the polarization bends for both the base matrix alloy and the Al 6013/redmud MMCs where potential is plotted versus galvanic current in three concntrations of sodium hydroxide. Thus there will be a foundation of higher electrical obstruction between sodium hydroxide and saamples of MMCs and matrix alloy. Tafel polarization curves for both the unreinforced and fortified aluminum alloys show that the cathodic and anodic curves of both alloy and composites are comparable, however cathodic bend of composites is marginally moved to the more positive side, which is credited to the reduction of hydrogen gas advancement.
Concentrations of sodium hydroxide will remarkably affect the outcomes concerning corrosion rates of MMCs and matrix alloy. Corrosion rate of MMCs and matrix alloy increments with increment in centralization of sodium hydroxide. Hydrogen gas advancement during the test will create corrosion current proportionately. Concentration and afrea of response surface will finaly chose the system of corrosion. The expansion in rate of corrosion is because of increment in concentration. Some researchers clarify that the pattern of increment in corrosion rate is because of expanded concetration and attck by sodium hydroxide.
Conclusion
Aluminum 6013 alloy containing red mud particulates as fortification were effectively created by liquid melt metallurgy strategy utilizing vortex technique. MMCs and matrix alloy were exposed to customary weight loss and potentiodynamic polarization tests to comprehend the corrosion conduct in sodium hydroxide solutions. Results show that MMCs and matrix alloy go through expanded corrosion with increment in concentration. In all the concentrations corrosion rate composites diminishes with increment in fortified content. Red mud particulates utilized as fortified assume a key function to decrease the corrosion rate of MMCs concerning matrix alloy. Accordingly utilization of MMCs in numerous applications is suggested rather than matrix alloy.
References
- Ahmad Z, Aleem BA. Degradation of aluminum metal matrix composites in salt water and its control. Mater Des. 2002;23(2):173-180.
- Chandrashekara KN, Narasimhamurthy B, Krupakara PV. Stress corrosion studies of aluminium 6013 red mud metal matrix composites. J Chem Chem Sci. 2017;7:640-646.
- Krupakara PV, Kulkarni SN. Studies on the stress corrosion behaviour of aluminium 6061/red mud metal matrix composites. Mater Today: Proc. 2022;59:1225-1230.
[Crossref]
- Jayaprakash HV, Krupakara PV. Potentiodynamic polarization studies of ZA-27/red mud metal matrix composites in hydrochloric acid solutions. J Chem Chem Sci. 2017.
- Hua Y, Heal KV, Friesl-Hanl W. The use of red mud as an immobiliser for metal/metalloid contaminated soil: A review. J Hazard Mater. 2017;325:17-30.
[Crossref] [Google Scholar] [PubMed]
- Samal S. Utilization of red mud as a source for metal ions-A review. Materials. 2021;14(9):2211.
[Crossref] [Google Scholar] [PubMed]
- Jayaprakash HV, Krupakara PV. Corrosion studies of ZA-27/red mud metal matrix composites in sodium chloride and sodium hydroxide medium by static weight loss method. Ind J Adv Chem Sci. 2017;5(4):280-284.
- Heinz B, Skrotzki B. Characterization of a friction stir welded aluminum alloy 6013. Metall Mater Trans. 2002;33:489-498.
- Kaneko RS, Bakow L, Lee EW. Aluminium alloy 6013 sheet for new US navy aircraft. Jom. 1990;42(5):16-18.
- Lei G, Wang B, Lu J, et al. Effects of solid solution temperature on the microstructure and properties of 6013 aluminum alloy. Mater Chem Phys. 2022;280:125829.
[Crossref]