Original Article
, Volume: 8( 2)Biomimetic Oxidation of Diphenyl Sulfide by Meso-Tetraphenyl-Porphyrinatochromium (3+) Chloride as an Electrochemical P-450 Model Compound
- *Correspondence:
- Takashi Michida, Faculty of Pharmaceutical Sciences, Kobe-Gakuin University, Minatojima, Kobe, Japan, Tel: 810789741551; E-mail: michida@pharm.kobegakuin.ac.jp
Received: August 30, 2017; Accepted: September 13, 2017; Published: September 15, 2017
Citation: Maeda M, Michida T. Biomimetic Oxidation of Diphenyl Sulfide by Meso-Tetraphenyl-Porphyrinatochromium (3+) Chloride as an Electrochemical P-450 Model Compound. Res Rev Electrochem. 2017;8(2):108
Abstract
Controlled potential electrolysis (CPE) of meso-tetraphenylporphyrinatochromium (3+) chloride (CrTPP, 1 mM) at -1.23 V (vs. Ag-AgCl) in acetonitrile containing diphenyl sulfide (1 mM) and sodium perchlorate (0.1 mM) as the, supporting electrolyte with a glassy carbon cathode gave diphenyl sulfoxide. The addition of acetic acid to the electrolyte solution increased the yield of diphenyl sulfoxide, as did the addition of KO2 to acetonitrile containing diphenyl sulfide. The catalytic cycle is thus similar to that of cytochrome P-450, an important drug-metabolizing enzyme.
Keywords
P-450 model; Meso-tetraphenylporphyrinatochromium (3+) chloride; Sulfide oxidation
Introduction
The molecular mechanism of biological oxidation by cytochrome P-450 is of great interest because cytochrome P-450 is an important drug-metabolizing enzyme that catalyzes a wide variety of oxygenation reactions and has a unique catalytic cycle [1]. In the generally accepted model for the catalytic cycle of cytochrome P-450, an oxo-iron (5+) species or oxo-iron (4+) porphyrin π-cation radical is produced by the transfer of two electrons and one dioxygen. Metalloporphyrins are often used as model compounds for P-450 and several reductants, such as NaBH4 or dihydrogen and colloidal platinum, are used as the electron source [2-13]. Metalloporphyrins are also effective in the oxidation of sulfides, the demethylation of dimethyl aniline, and the epoxidation of alkenes [14-21]. In the oxidation of sulfides, it is particularly important to choose the reductant carefully because the oxidation products of sulfides, sulfoxides, can be easily reduced to sulfides again. Since the applied potential can be changed freely, controlled potential electrolysis (CPE) is suitable for such studies. Other researchers, in particular Murry and co-workers, have used meso-tetraphenylporphyrinatochromium (3+) chloride (CrTPP) to catalyze oxygen-atom transfer reactions or as P-450 model compounds [22].
In our previous paper we described the epoxidation of olefins by Cr (3+) TPP, used as an electrochemical P-450 model compound [23]. The aim of this paper is to clarify the biomimetic oxidation of diphenyl sulfide using CrTPP as an electrochemical P450 model.
Materials and Methods
Materials acetonitrile was HPLC grade and the other chemicals were reagent grade. All reagents were purchased from Nacalai Tesque, Kyoto, Japan, and used without purification.
Apparatus A Hokutodenko (Tokyo, Japan) HF-102 coulometer and HA-501 potentiostat were used for controlled potential electrolysis. Gas chromatography was carried out with a Shimadzu (Kyoto, Japan) GC-14A equipped with a C-R6A Chromatopac.
Controlled potential electrolysis (CPE)
A representative procedure is as follows:
Acetonitrile (30 ml) containing CrTPP (21.33 mg), diphenyl sulfide (0.508 ml), imidazole (102 mg), acetic acid (0.3 ml) and NaClO4 (0.37 g) used as the supporting electrolyte was placed in an undivided cell. A glassy carbon plate, a Pt plate, and an Ag/AgCl electrode were used as the working electrode, counter electrode, and reference electrode, respectively. CPE was carried out at -1.27 V for 4 h. Aliquots of the electrolyte (0.1 ml) were diluted with acetonitrile containing diphenyl as an internal standard to 0.2 ml and then analyzed. The products were determined by GC (capillary column, GL Sciences (Tokyo, Japan) Inert Cap (0.25 mm × 30 m, df 0.25 μm)).
Reaction with potassium superoxide acetonitrile (30 ml) containing CrTPP (21.48 mg), diphenyl sulfide (0.508 ml), NaClO4 (0.37 g), imidazole (0.1 g) and acetic acid (0.3 ml) was placed in a flask and KO2 (0.213 g) was added to the solution. The reaction was carried out for 1 h. Aliquots (0.1 ml) were diluted with acetonitrile containing diphenyl as an internal standard to 0.2 ml and then analyzed. The products were determined by GC as described above.
Results and Discussion
As noted earlier, the epoxidation of olefins by CrTPP in acetonitrile as an electrochemical P-450 model compound was previously reported. In the present study, similar solutions were tested for the biomimetic oxidation of diphenyl sulfide. Four electrolytic solutions were prepared: acetonitrile containing CrTPP (1 mM), diphenyl sulfide (100 mM) and NaClO4 (0.1 M) (SA); acetonitrile containing CrTPP (1 mM), imidazole (5 mM), diphenyl sulfide (100 mM) and NaClO4 (0.1 M) (SB); acetonitrile containing CrTPP (1 mM), acetic acid (1%), diphenyl sulfide (100 mM) and NaClO4 (0.1 M) (SC); and acetonitrile containing CrTPP (1 mM), imidazole (5 mM), acetic acid (1%), diphenyl sulfide (100 mM) and NaClO4 (0.1 M) (SD).
The results of cyclic voltammetry of CrTPP (1 mM) in dry acetonitrile containing NaClO4 have been reported [23]. The addition of acetic acid (1%) to the electrolyte resulted in fusion of the two cathodic waves in the potential range from 0 V to -1.6 V: the first cathodic wave shifted approximately 200 mV lower and the ip (peak current) of the cathodic wave increased more than sevenfold. The addition of imidazole (5 mM) to the electrolyte resulted in the cathodic wave shifting approximately 400 mV lower, in a manner similar to that observed following the addition of acetic acid.
When both acetic acid (1%) and imidazole (5 mM) were added to the electrolyte solution, a new oxidation wave appeared at -1.24 V and was a counterpart of the first cathodic wave.
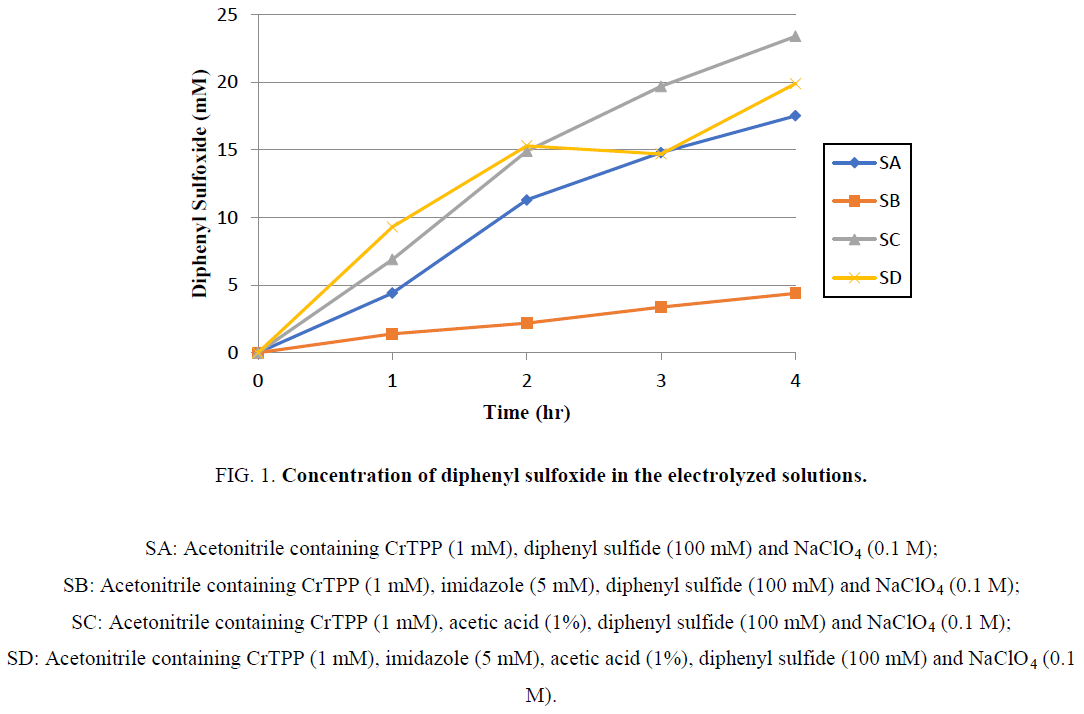
CPE was performed in an undivided cell using a glassy carbon electrode and an Ag/AgCl electrode. The electrolytic solution was stirred during electrolysis under atmospheric conditions. At hourly intervals, 100 μl of electrolytic solution was withdrawn and analyzed by gas chromatography. The electrolytic experiments were stopped after 4 h and the results are summarized in Figure 1.
Figure 1: Concentration of diphenyl sulfoxide in the electrolyzed solutions.
SA: Acetonitrile containing CrTPP (1 mM), diphenyl sulfide (100 mM) and NaClO4 (0.1 M);
SB: Acetonitrile containing CrTPP (1 mM), imidazole (5 mM), diphenyl sulfide (100 mM) and NaClO4 (0.1 M);
SC: Acetonitrile containing CrTPP (1 mM), acetic acid (1%), diphenyl sulfide (100 mM) and NaClO4 (0.1 M);
SD: Acetonitrile containing CrTPP (1 mM), imidazole (5 mM), acetic acid (1%), diphenyl sulfide (100 mM) and NaClO4 (0.1 M).
Diphenyl sulfoxide was detected in each electrolyzed solution. There were 17.5 catalytic cycle turnovers in SA over a 4 h period. The presence of acetic acid in the electrolyte solution (SC, SD) increased the turnover of the catalytic cycles, whereas the presence of imidazole (a base) reduced turnover.
In the catalytic cycle of cytochrome P-450, the iron peroxo species Fe3+O22- is generated through two-electron reduction and dioxygen binding. Heterolysis of the O-O bond in the iron peroxo species, with concomitant generation of the reactive oxidant [FeO]5+ and a molecule of water, requires two protons [1]. The addition of a large excess of imidazole to a solution of metalloporphyrin results in the exchange of the fifth ligand to imidazole. The fifth ligand of metalloporphyrins plays an important role in binding oxygen to the metalloporphyrin.
Our results suggest that the rate limiting step of the catalytic cycle is the heterolysis of the O-O bond in the chromium peroxo species.
The applied potential was -1.27 V and thus there was reduction of O2 dissolved in the electrolyte solution. Potassium superoxide (0.213 g), a reduced product of dioxygen, was added to SD and the solution was stirred. The reaction was conducted for 1 h; GC analysis showed diphenyl sulfoxide (19.1%) and diphenyl sulfone (12.1%) (yields calculated based on the initial amount of diphenyl sulfide).
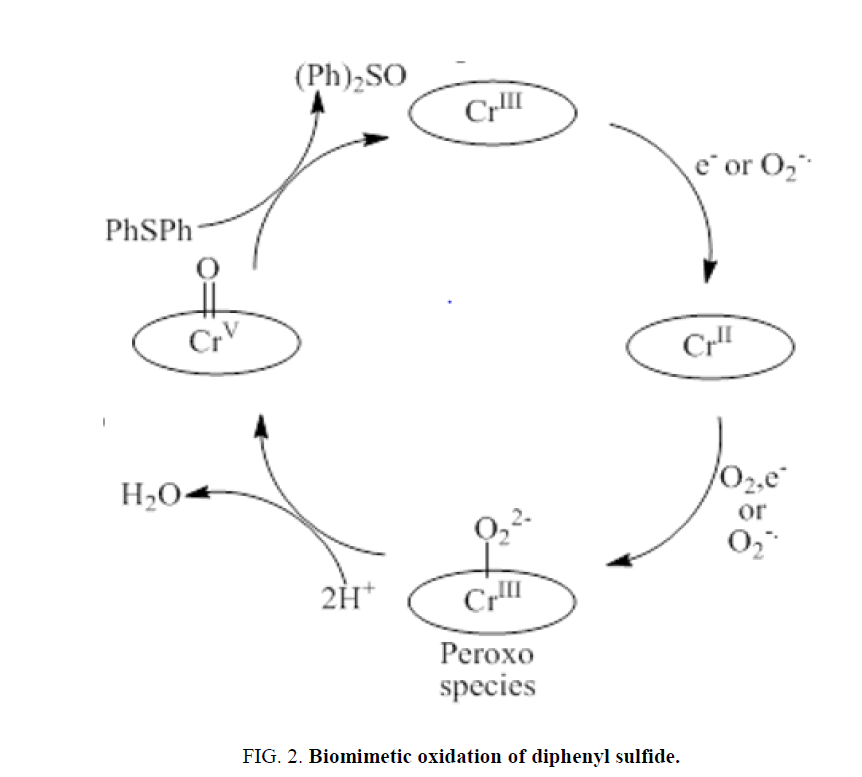
Shirazi pointed out that two moles of superoxide produce one mole of metallo peroxo species from metalloporphyrins [24]. The catalytic cycle may therefore include the generation of superoxide, and was proposed in Figure 2. Details of this reaction are currently under study.
Conclusion
Electrochemical reduction of acetonitrile containing CrTPP (1 mM) and diphenyl sulfide (100 mM) in air selectively gave diphenyl sulfoxide. The rate limiting step of the catalytic cycle was the heterolysis of the O-O bond in the chromium peroxo species.
References
- Oriz de Montellano PR. Cytochrome P-450. New York:Plenum. 1986.
- Michida T, Kasuya Y, Sayo H. Biomimetic oxidation of diphenyl sulfide with metalloporphyrin-?O2-?NaBH4 system. Chem Pharm Bull. 1993;41(8):1462-4.
- Nishiki M, Satoh T, Sakurai H. Biomimetic models of cytochrome P-450 monooxygenases: Studies on cyclohexene oxygenation in metalloporphyrin-sodium borohydride-microcrystalline cellulose-oxygen systems. J Mol Catal. 1990;62(1):79-91.
- Sakurai H, Mori Y, Shibuya M. A chemical model of cytochrome P-450 with electron-transfer activity. Inorg Chim Act. 1989:162:23-5.
- Sakurai H, Mizu M. Ethyl oleate oxygenation by cytochrome P-450 models. J Mol Catal.1988:48(2-3):175-82.
- Shimizu M, Orita H, Hayakawa T, et al. The oxidation of olefins with oxygen and sodium borohydride catalyzed by manganese meso-?tetrakis (p-?sulfonatophenyl) ?porphyrin. J Mol Catal. 1988:45(1):85-90.
- Santa T, Mori T, Hirobe M. Chemical studies on drug metabolism. Oxygen activation and olefin oxygenation by iron (III) porphyrin as a model of cytochrome P-?450. Chem Pharm Bull. 1985;33(5):2175-8.
- Tabushi I, Koga N. P-?450 type oxygen activation by porphyrin-?manganese complex. J Am Chem Soc. 1979:101(21):6456-8.
- Tabushi I, Kodera M. Flavin-?catalyzed reductive dioxygen activation with N-?methyldihydronicotinamide. J Am Chem Soc. 1986:108(5):1101-3.
- Tabushi I, Morimitsu K. Catalytic hydroxylation on aromatic rings by use of an artificial P-?450 system assisted by acid and acid anhydride. Tetrahedron Lett. 1986;27(1):51-4.
- Tabushi I, Kodera M, Yokoyama M. Kinetics and mechanism of reductive dioxygen activation catalyzed by P-?450 model system. Iron picket fence as a catalytic center. J Am Chem Soc. 1985:107(15):4466-73.
- Tabushi I, Morimitsu K. Stereospecific, regioselective, and catalytic monoepoxidation of polyolefins by the use of a P-?450 model, dihydrogen-?dioxygen-?TPP·Mn-?colloidal platinum. J Am Chem Soc. 1984;106(22):6871-2.
- Tabushi I, Yazaki A. P-?450-?type dioxygen activation using hydrogen?/colloidal platinum as an effective electron donor. J Am Chem Soc.1981;103(24):7371-3.
- Michida T, Osawa E, Yamaoka Y. Electrochemical P-450 model system using organic acids and Metalloporphyrins. porphyrins, 1999;8(1):1-7.
- Michida T, Osawa E, Yamaoka Y. Biomimetic oxidation of diphenyl sulfide with electrochemical P-?450 model system in CH2Cl2 treated with alkaline solution. Yakugaku Zasshi.1999;119(10):780-5.
- Michida T, Sakai E, Himeno M, et al. Phenazine linked porphyrine as an electrochemical P-450 model compound. Porphyrins. 2000;9(3,4):287-90.
- Michida T, Mori C, Yamazaki C, et al. Demethylation of N, N-dimethylaniline with an electrochemical P-450 model system. Porphyrins.2004;13(2):55-8.
- Michida T, Saiki C, Yamaszaki C,et al. Epoxidation with an electro catalytic P-?450 model system in an acidic solution. Chem Pharm Bull.2005;53(2):151-2.
- Michida T, Saiki C. The roles of the imidazole in the electrochemical P-450 model system. Porphyrins. 2008;17(1):11-6.
- Murai A, Michida T. Studies on an anthraquinone-linked porphyrin as an electrochemical P450 model. Porphyrins.2010;19(1),15-21.
- Murai A, Michida T. Synthesis and electrochemistry of manganese porphyrin with a carboxy group in the vicinity of the metal. Porphyrins.2010;19(2-3):7-10.
- Creager SE, Murray RW. Electrochemical studies of oxo (meso-?tetraphenylporphinato) ?chromium (IV)?. Direct evidence for epoxidation of olefins by an electrochemically generated formal Chromium (V) state. Inorg Chem. 1985;24(23):3824-8.
- Michida T, Omichi T. The epoxidation of the olefins by meso-tetraphenylporphyrinato-chromium (3+) chloride as an electrochemical P-450 model compound. Chem Pharm Bull. 2013;61(8):799-801.
- Shirazi A, Goff HM. Characterization of superoxide-?metalloporphyrin reaction products: effective use of deuterium NMR spectroscopy. J Am Chem Soc.1982;104(23):6318-22.